Abstract
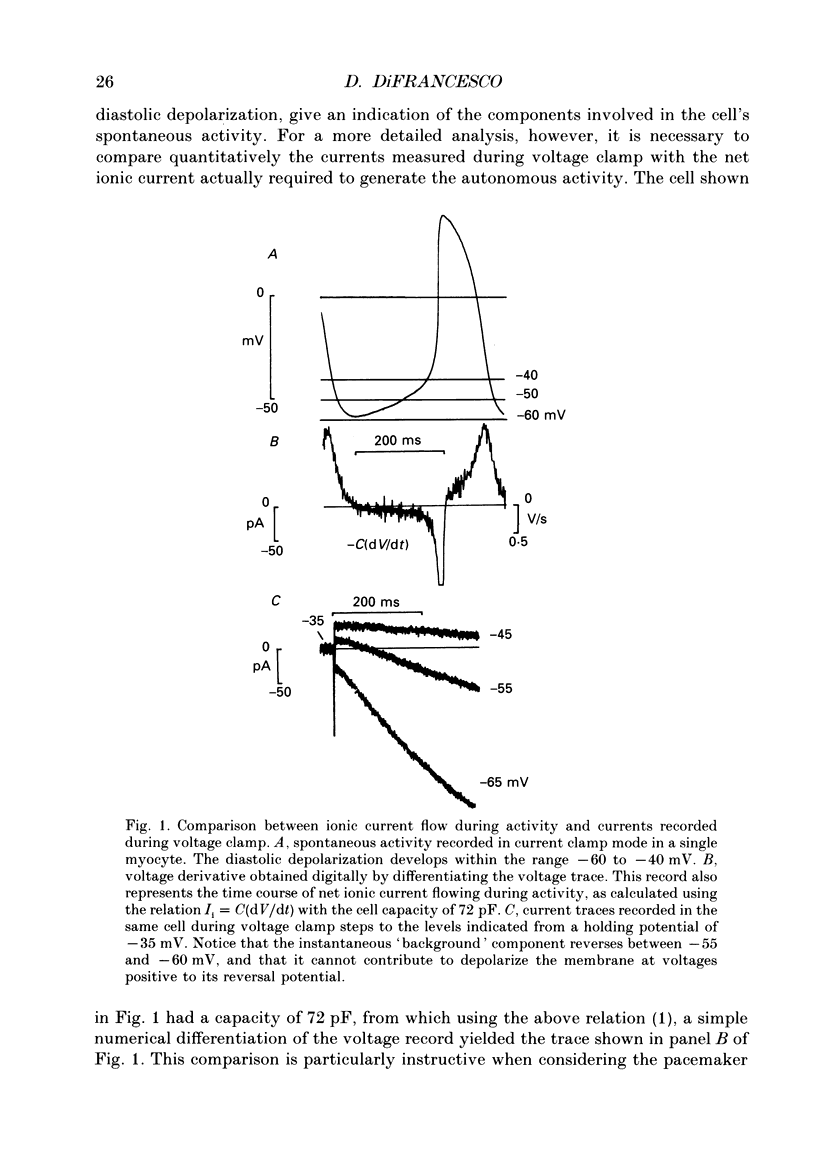
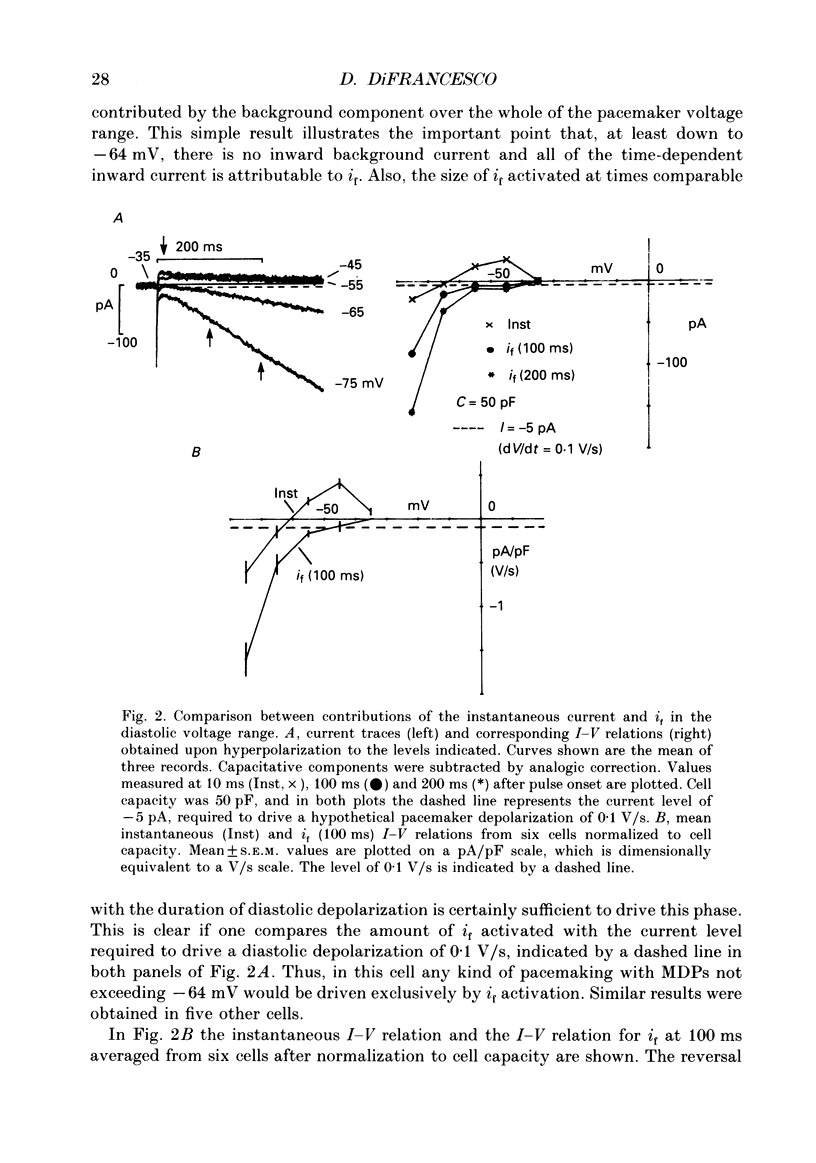
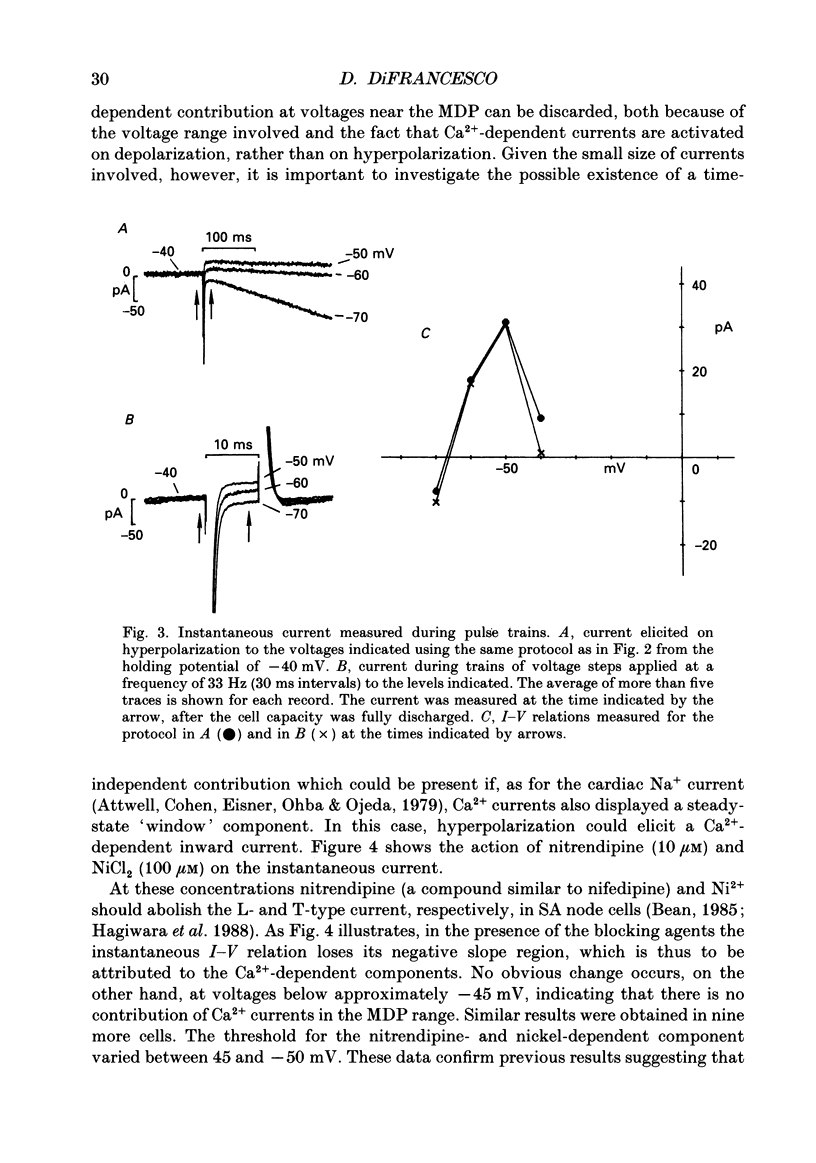
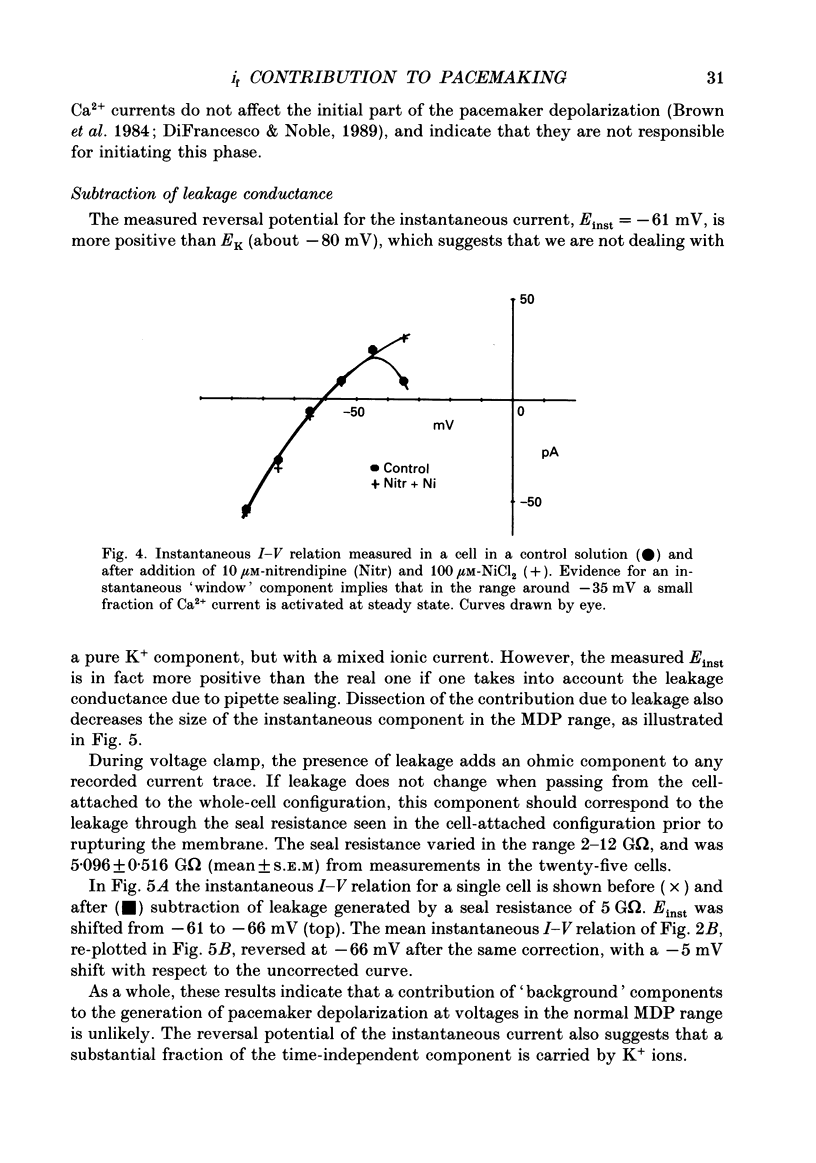
1. The contribution to the diastolic depolarization of the hyperpolarization-activated current, if, relative to other components was investigated in isolated rabbit sino-atrial (SA) node myocytes. 2. During the diastolic phase the membrane potential depolarized by 0.1096 +/- 0.014 V/s, which requires only about 3 pA of inward current in a cell with an average capacity of 30 pF. The problem of which ionic component is responsible for initiating the diastolic depolarization was investigated by analysing the composition and the properties of the net inward current in the diastolic range of voltages. 3. The measured instantaneous 'background' current activated during voltage clamp steps from a holding potential of -35 mV was outward positive to approximately -61 mV, and had a region of negative slope conductance from -45 to -35 mV. 4. The instantaneous component lost its rectifying behaviour in the presence of Ni2+ (100 microM) and nitrendipine (10 microM). These blockers of Ca(2+)-dependent currents modified the instantaneous I-V relation at voltages positive to -45 to -50 mV, thus implying that Ca2+ currents become important at less negative potentials than -50 mV, towards the very end of diastolic depolarization. 5. Possible errors introduced by voltage clamp analysis with the whole-cell method on the instantaneous current and on if measurement were evaluated. Leakage through the seal resistance caused the instantaneous I-V relation to be displaced in the inward direction at negative voltages. Correction for the seal leakage moved the reversal potential for the instantaneous current toward the negative direction from -61 to approximately -66 mV. Thus, no depolarization can be driven by the background current beyond -66 mV. 6. During voltage clamp analysis, lack of series-resistance compensation led to lack of intracellular voltage control, as was apparent using a second pipette on the same cell. This slowed activation of if and led to a 1.5- to 2-fold reduction of if size in the range -55 to -115 mV. Thus, uncorrected measurements of the instantaneous component and of if may concur to underestimate the role of if in pacemaking. 7. These results lead to the conclusion that in the SA node cells analysed, pacemaker activity is generated with the essential contribution of the hyperpolarization-activated current, if. Numerical computation of SA node cell activity using an extension of the DiFrancesco-Noble model shows that the if-activation hypothesis can account for the presence of spontaneous action potentials and their sensitivity to if changes.
Full text
PDF









Selected References
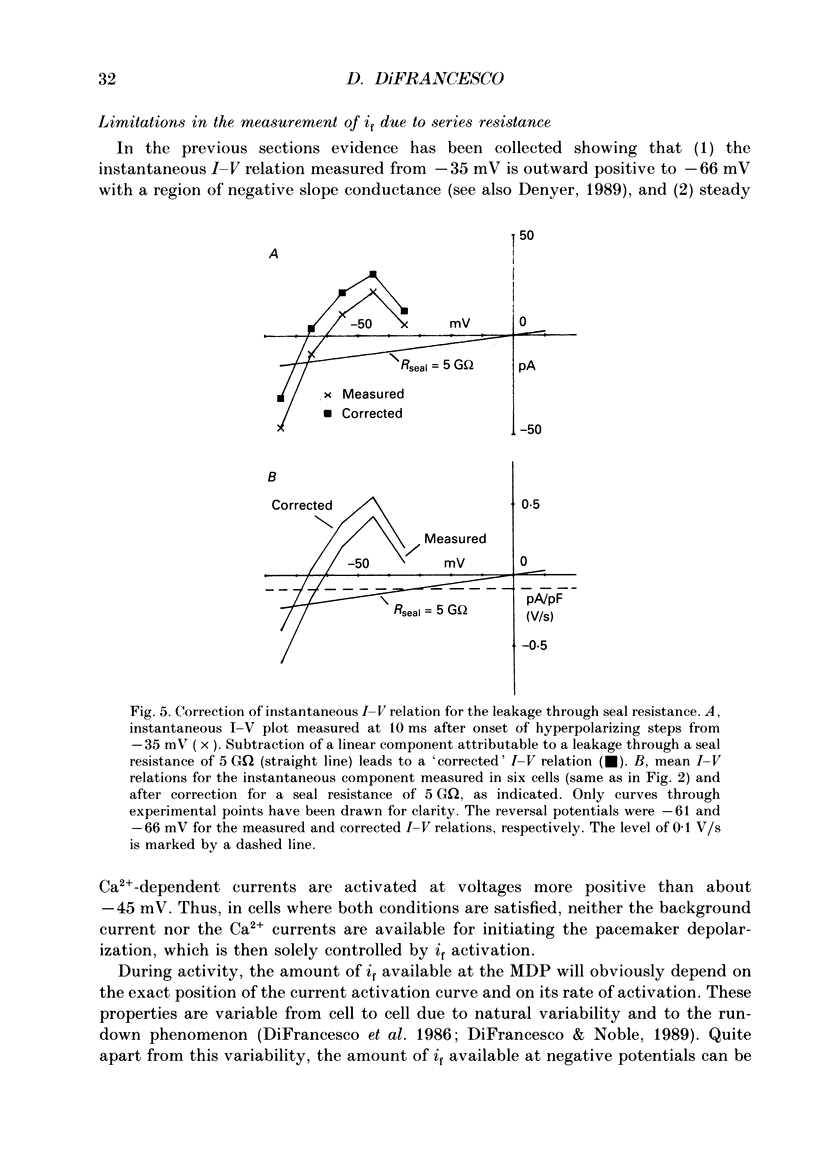
These references are in PubMed. This may not be the complete list of references from this article.
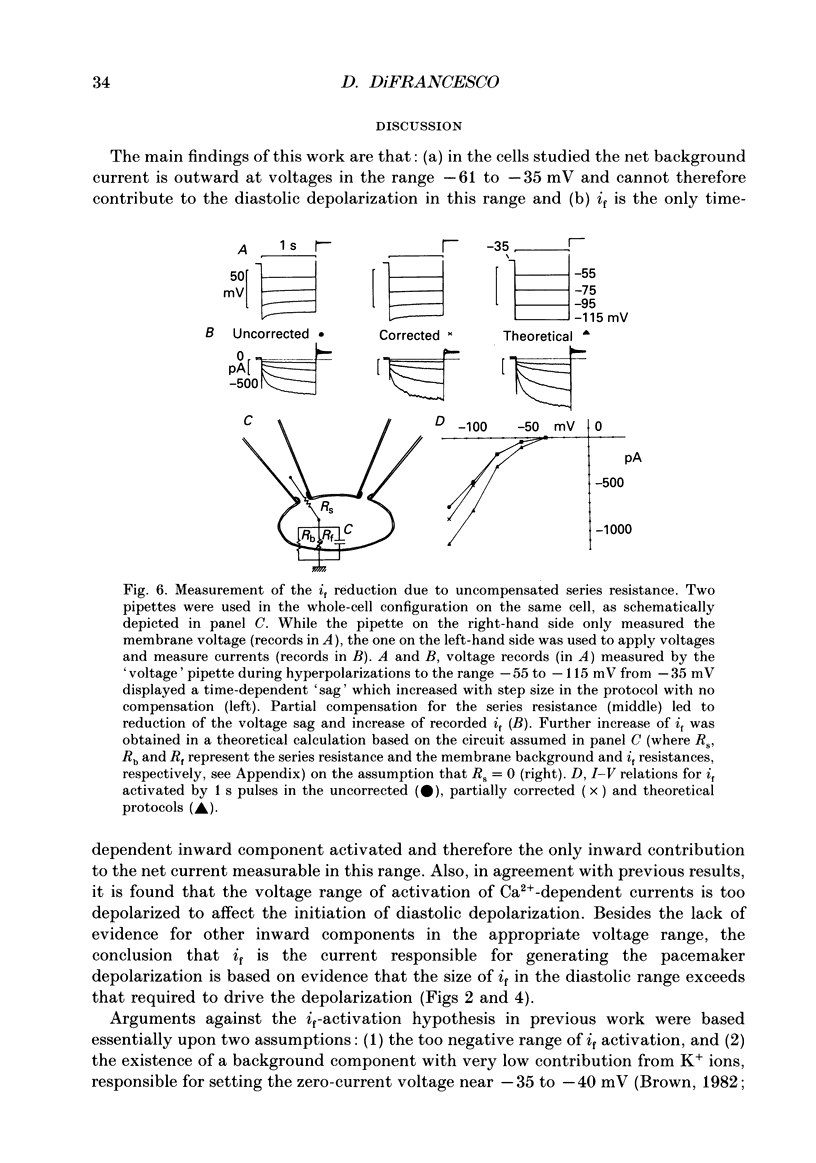
- Attwell D., Cohen I., Eisner D., Ohba M., Ojeda C. The steady state TTX-sensitive ("window") sodium current in cardiac Purkinje fibres. Pflugers Arch. 1979 Mar 16;379(2):137–142. doi: 10.1007/BF00586939. [DOI] [PubMed] [Google Scholar]
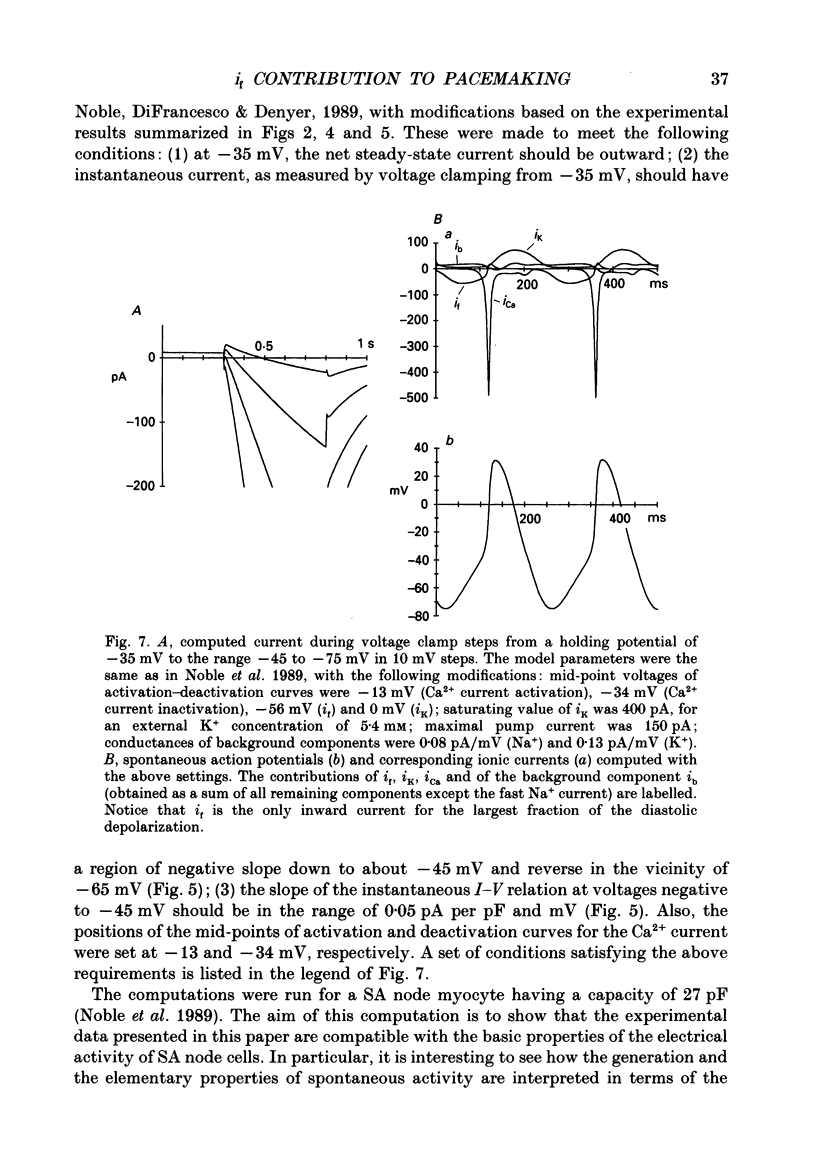
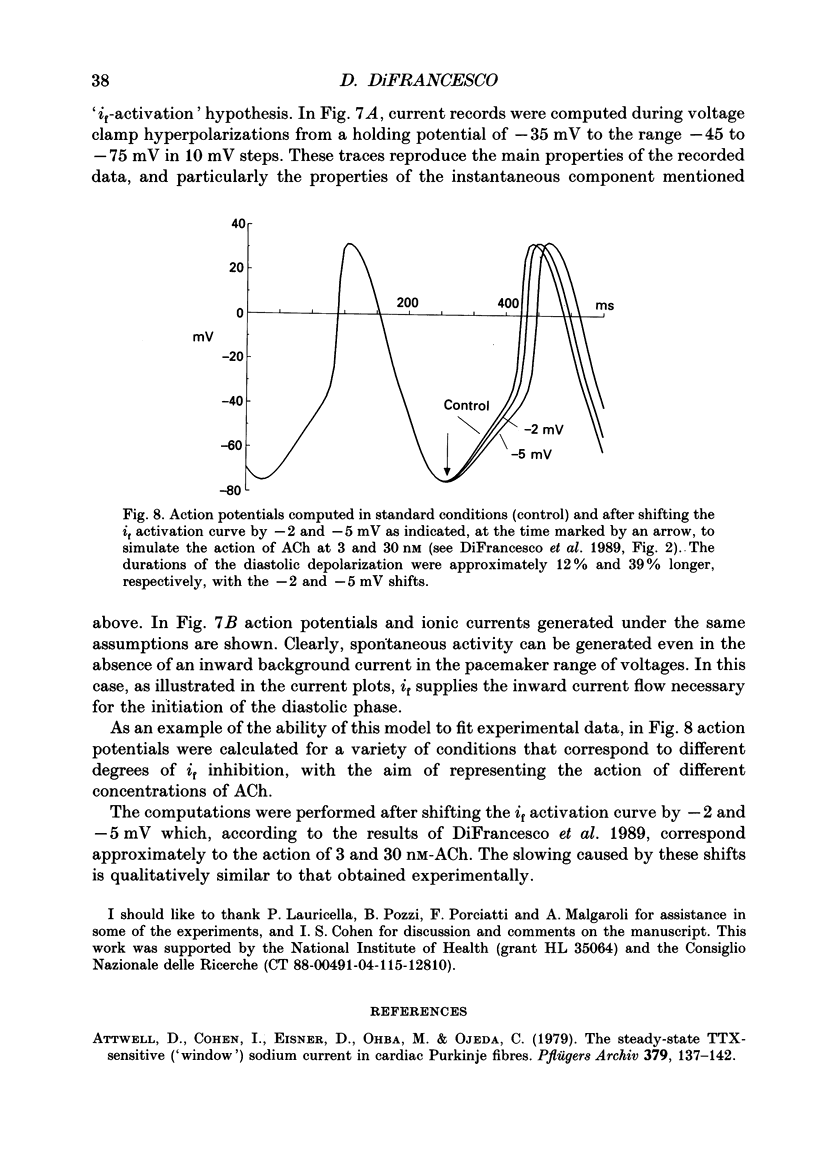
- Bean B. P. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J Gen Physiol. 1985 Jul;86(1):1–30. doi: 10.1085/jgp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett P., McKinney L., Begenisich T., Kass R. S. Adrenergic modulation of the delayed rectifier potassium channel in calf cardiac Purkinje fibers. Biophys J. 1986 Apr;49(4):839–848. doi: 10.1016/S0006-3495(86)83713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. F., DiFrancesco D., Noble S. J. How does adrenaline accelerate the heart? Nature. 1979 Jul 19;280(5719):235–236. doi: 10.1038/280235a0. [DOI] [PubMed] [Google Scholar]
- Brown H. F. Electrophysiology of the sinoatrial node. Physiol Rev. 1982 Apr;62(2):505–530. doi: 10.1152/physrev.1982.62.2.505. [DOI] [PubMed] [Google Scholar]
- Brown H. F., McNaughton P. A., Noble D., Noble S. J. Adrenergic control of cardian pacemaker currents. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):527–537. doi: 10.1098/rstb.1975.0029. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. Characterization of single pacemaker channels in cardiac sino-atrial node cells. Nature. 1986 Dec 4;324(6096):470–473. doi: 10.1038/324470a0. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Ducouret P., Robinson R. B. Muscarinic modulation of cardiac rate at low acetylcholine concentrations. Science. 1989 Feb 3;243(4891):669–671. doi: 10.1126/science.2916119. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Ferroni A., Mazzanti M., Tromba C. Properties of the hyperpolarizing-activated current (if) in cells isolated from the rabbit sino-atrial node. J Physiol. 1986 Aug;377:61–88. doi: 10.1113/jphysiol.1986.sp016177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Noble D. A model of cardiac electrical activity incorporating ionic pumps and concentration changes. Philos Trans R Soc Lond B Biol Sci. 1985 Jan 10;307(1133):353–398. doi: 10.1098/rstb.1985.0001. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Noma A., Trautwein W. Kinetics and magnitude of the time-dependent potassium current in the rabbit sinoatrial node: effect of external potassium. Pflugers Arch. 1979 Sep;381(3):271–279. doi: 10.1007/BF00583259. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. The cardiac hyperpolarizing-activated current, if. Origins and developments. Prog Biophys Mol Biol. 1985;46(3):163–183. doi: 10.1016/0079-6107(85)90008-2. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Tromba C. Inhibition of the hyperpolarization-activated current (if) induced by acetylcholine in rabbit sino-atrial node myocytes. J Physiol. 1988 Nov;405:477–491. doi: 10.1113/jphysiol.1988.sp017343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Tromba C. Muscarinic control of the hyperpolarization-activated current (if) in rabbit sino-atrial node myocytes. J Physiol. 1988 Nov;405:493–510. doi: 10.1113/jphysiol.1988.sp017344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerr T., Denger R., Trautwein W. Calcium currents in single SA nodal cells of the rabbit heart studied with action potential clamp. Pflugers Arch. 1989 Apr;413(6):599–603. doi: 10.1007/BF00581808. [DOI] [PubMed] [Google Scholar]
- Duchatelle-Gourdon I., Hartzell H. C., Lagrutta A. A. Modulation of the delayed rectifier potassium current in frog cardiomyocytes by beta-adrenergic agonists and magnesium. J Physiol. 1989 Aug;415:251–274. doi: 10.1113/jphysiol.1989.sp017721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles W., Nakajima T., Ono K., Shibata E. F. Modulation of the delayed rectifier K+ current by isoprenaline in bull-frog atrial myocytes. J Physiol. 1989 Aug;415:233–249. doi: 10.1113/jphysiol.1989.sp017720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H., Kameyama M. Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J Physiol. 1988 Jan;395:233–253. doi: 10.1113/jphysiol.1988.sp016916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irisawa H. Comparative physiology of the cardiac pacemaker mechanism. Physiol Rev. 1978 Apr;58(2):461–498. doi: 10.1152/physrev.1978.58.2.461. [DOI] [PubMed] [Google Scholar]
- Irisawa H. Membrane currents in cardiac pacemaker tissue. Experientia. 1987 Dec 1;43(11-12):1131–1135. doi: 10.1007/BF01945510. [DOI] [PubMed] [Google Scholar]
- Noble D. The surprising heart: a review of recent progress in cardiac electrophysiology. J Physiol. 1984 Aug;353:1–50. doi: 10.1113/jphysiol.1984.sp015320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A., Nakayama T., Kurachi Y., Irisawa H. Resting K conductances in pacemaker and non-pacemaker heart cells of the rabbit. Jpn J Physiol. 1984;34(2):245–254. doi: 10.2170/jjphysiol.34.245. [DOI] [PubMed] [Google Scholar]
- Pappano A. J., Carmeliet E. E. Epinephrine and the pacemaking mechanism at plateau potentials in sheep cardiac Purkinje fibers. Pflugers Arch. 1979 Oct;382(1):17–26. doi: 10.1007/BF00585899. [DOI] [PubMed] [Google Scholar]


