Abstract
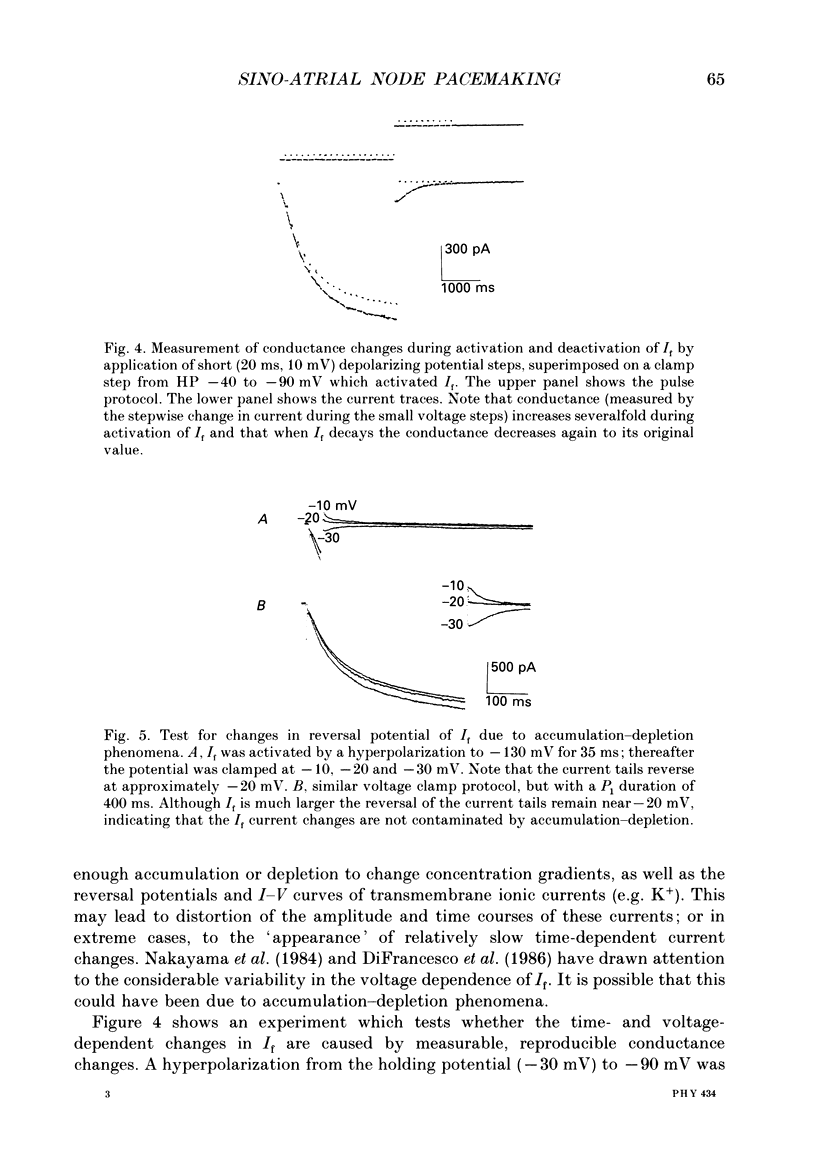
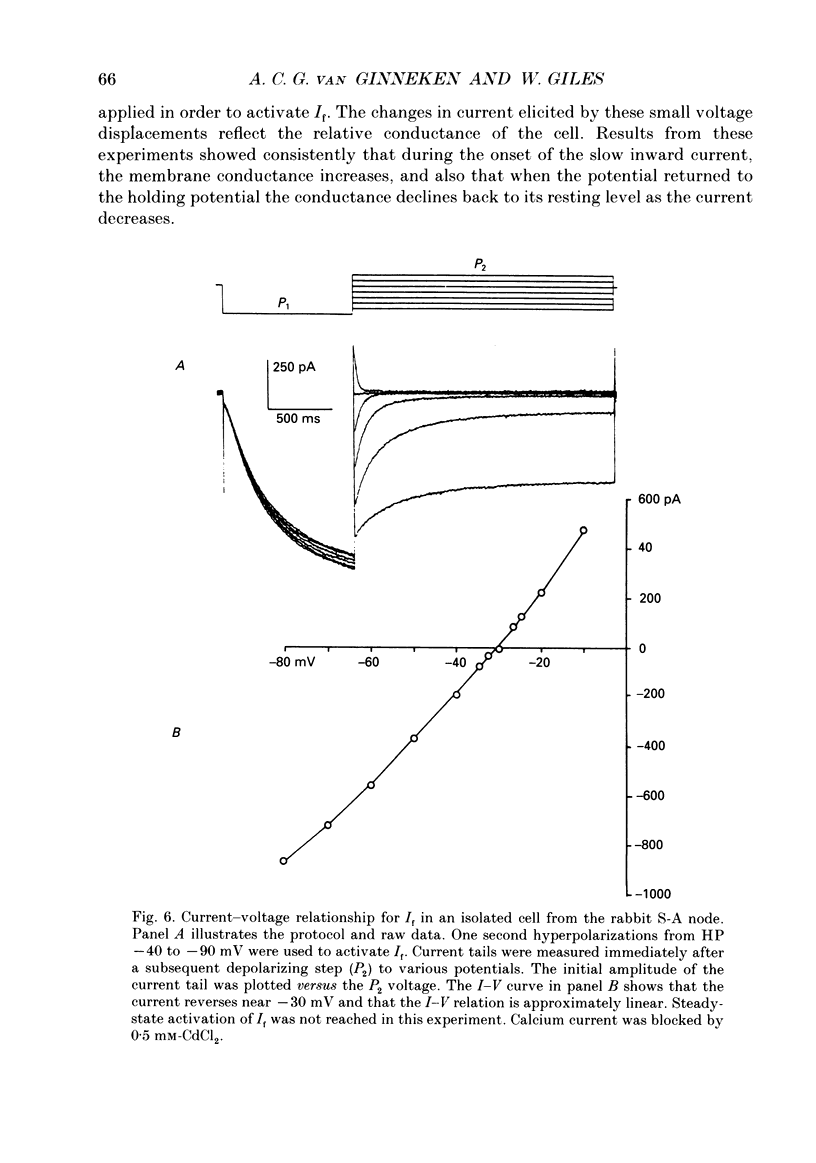
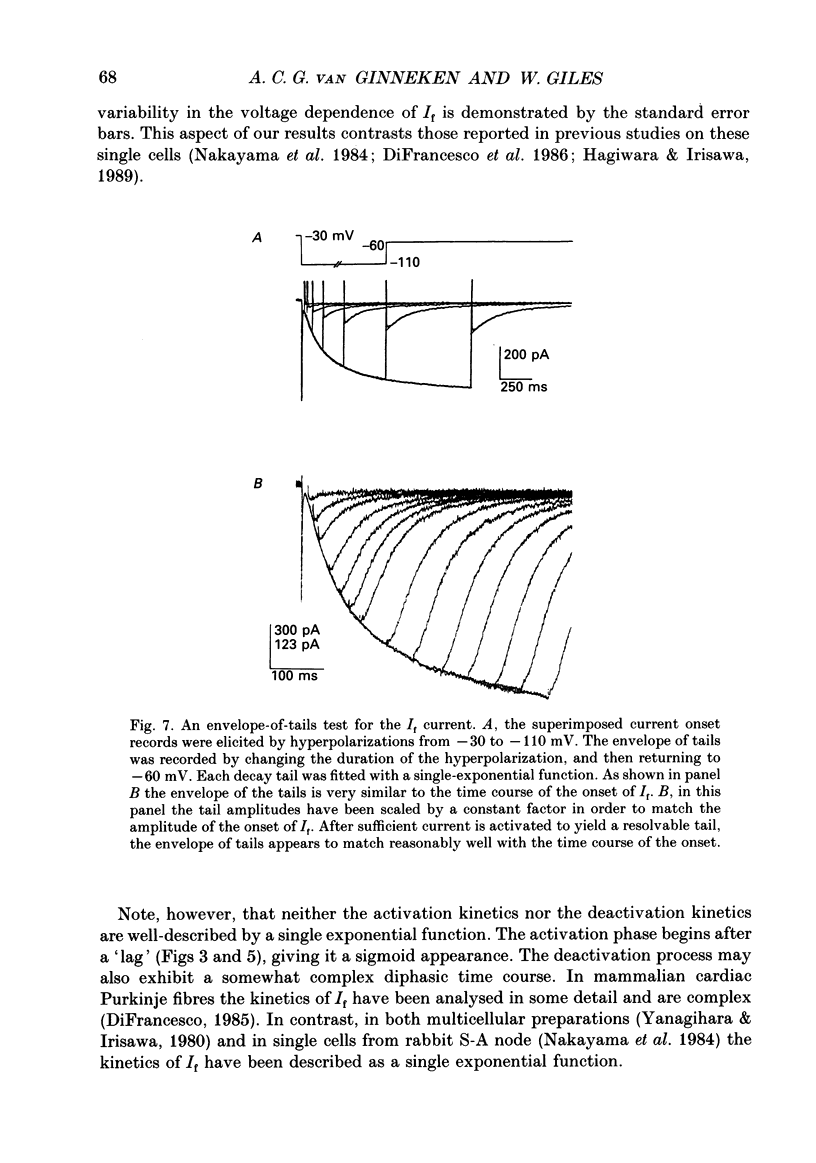
1. The kinetics and ion transfer characteristics of the hyperpolarization-activated inward current, I(f), have been studied in single cells obtained by enzymatic dispersion from the rabbit sino-atrial (S-A) node. These experiments were done to assess the role of I(f) in the generation of the pacemaker depolarization in the S-A node. 2. The activation and the deactivation of I(f) in these single cells are accompanied by significant conductance increases and decreases respectively, confirming earlier findings from multicellular man-made strips of rabbit S-A node, and from mammalian Purkinje fibres. 3. The steady-state activation of I(f) lies between -40 and -120 mV, and its voltage dependence can be described by a Boltzmann relation with the half-activation point at approximately -70 mV. 4. The delay or sigmoidicity in both the onset of I(f) and the deactivation of the tail currents can be accounted for semi-quantitatively by using a second-order Hodgkin-Huxley kinetic scheme. 5. The reversal potential for I(f) is -24 +/- 2 mV (mean +/- S.E.M., n = 6). It does not change significantly as a function of the amount of I(f) which is activated, indicating that ion accumulation or depletion phenomena are not important variables controlling the time course of I(f), or its selectivity. 6. The fully-activated current-voltage relationship for I(f) is approximately linear with a slope conductance of 12.0 +/- 0.88 nS per cell (mean +/- S.E.M., n = 6). 7. A simple mathematical model based on the measured values of maximum conductance, reversal potential, and kinetics of I(f) has been developed to simulate the size and time course of I(f) during typical spontaneous pacemaker activity in rabbit sino-atrial node cells. The calculations show that I(f) can change significantly during pacing and suggest that this current change is, at least in part, responsible for the pacemaker depolarization.
Full text
PDF























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belardinelli L., Giles W. R., West A. Ionic mechanisms of adenosine actions in pacemaker cells from rabbit heart. J Physiol. 1988 Nov;405:615–633. doi: 10.1113/jphysiol.1988.sp017352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F. Gating of sodium and potassium channels. J Membr Biol. 1985;88(2):97–111. doi: 10.1007/BF01868424. [DOI] [PubMed] [Google Scholar]
- Bleeker W. K., Mackaay A. J., Masson-Pévet M., Bouman L. N., Becker A. E. Functional and morphological organization of the rabbit sinus node. Circ Res. 1980 Jan;46(1):11–22. doi: 10.1161/01.res.46.1.11. [DOI] [PubMed] [Google Scholar]
- Bois P., Lenfant J. Isolated cells of the frog sinus venosus: properties of the inward current activated during hyperpolarization. Pflugers Arch. 1990 May;416(3):339–346. doi: 10.1007/BF00392071. [DOI] [PubMed] [Google Scholar]
- Brown H. F. Electrophysiology of the sinoatrial node. Physiol Rev. 1982 Apr;62(2):505–530. doi: 10.1152/physrev.1982.62.2.505. [DOI] [PubMed] [Google Scholar]
- Brown H. F., Giles W., Noble S. J. Membrane currents underlying activity in frog sinus venosus. J Physiol. 1977 Oct;271(3):783–816. doi: 10.1113/jphysiol.1977.sp012026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H., Difrancesco D. Voltage-clamp investigations of membrane currents underlying pace-maker activity in rabbit sino-atrial node. J Physiol. 1980 Nov;308:331–351. doi: 10.1113/jphysiol.1980.sp013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F., Gao J., Tromba C., Cohen I., DiFrancesco D. Acetylcholine reverses effects of beta-agonists on pacemaker current in canine cardiac Purkinje fibers but has no direct action. A difference between primary and secondary pacemakers. Circ Res. 1990 Mar;66(3):633–636. doi: 10.1161/01.res.66.3.633. [DOI] [PubMed] [Google Scholar]
- Denyer J. C., Brown H. F. Pacemaking in rabbit isolated sino-atrial node cells during Cs+ block of the hyperpolarization-activated current if. J Physiol. 1990 Oct;429:401–409. doi: 10.1113/jphysiol.1990.sp018264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denyer J. C., Brown H. F. Rabbit sino-atrial node cells: isolation and electrophysiological properties. J Physiol. 1990 Sep;428:405–424. doi: 10.1113/jphysiol.1990.sp018219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. A new interpretation of the pace-maker current in calf Purkinje fibres. J Physiol. 1981 May;314:359–376. doi: 10.1113/jphysiol.1981.sp013713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. A study of the ionic nature of the pace-maker current in calf Purkinje fibres. J Physiol. 1981 May;314:377–393. doi: 10.1113/jphysiol.1981.sp013714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. Characterization of single pacemaker channels in cardiac sino-atrial node cells. Nature. 1986 Dec 4;324(6096):470–473. doi: 10.1038/324470a0. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Ducouret P., Robinson R. B. Muscarinic modulation of cardiac rate at low acetylcholine concentrations. Science. 1989 Feb 3;243(4891):669–671. doi: 10.1126/science.2916119. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Ferroni A., Mazzanti M., Tromba C. Properties of the hyperpolarizing-activated current (if) in cells isolated from the rabbit sino-atrial node. J Physiol. 1986 Aug;377:61–88. doi: 10.1113/jphysiol.1986.sp016177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Noble D. A model of cardiac electrical activity incorporating ionic pumps and concentration changes. Philos Trans R Soc Lond B Biol Sci. 1985 Jan 10;307(1133):353–398. doi: 10.1098/rstb.1985.0001. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. The cardiac hyperpolarizing-activated current, if. Origins and developments. Prog Biophys Mol Biol. 1985;46(3):163–183. doi: 10.1016/0079-6107(85)90008-2. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. The pacemaker current in the sinus node. Eur Heart J. 1987 Dec;8 (Suppl 50):19–23. doi: 10.1093/eurheartj/8.suppl_l.19. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Tromba C. Acetylcholine inhibits activation of the cardiac hyperpolarizing-activated current, if. Pflugers Arch. 1987 Sep;410(1-2):139–142. doi: 10.1007/BF00581906. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Tromba C. Inhibition of the hyperpolarization-activated current (if) induced by acetylcholine in rabbit sino-atrial node myocytes. J Physiol. 1988 Nov;405:477–491. doi: 10.1113/jphysiol.1988.sp017343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerr T., Denger R., Trautwein W. Calcium currents in single SA nodal cells of the rabbit heart studied with action potential clamp. Pflugers Arch. 1989 Apr;413(6):599–603. doi: 10.1007/BF00581808. [DOI] [PubMed] [Google Scholar]
- Doerr T., Trautwein W. On the mechanism of the "specific bradycardic action" of the verapamil derivative UL-FS 49. Naunyn Schmiedebergs Arch Pharmacol. 1990 Apr;341(4):331–340. doi: 10.1007/BF00180659. [DOI] [PubMed] [Google Scholar]
- Eaton D. C., Brodwick M. S. Effects of barium on the potassium conductance of squid axon. J Gen Physiol. 1980 Jun;75(6):727–750. doi: 10.1085/jgp.75.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C. The Na/K pump of cardiac cells. Annu Rev Biophys Bioeng. 1984;13:373–398. doi: 10.1146/annurev.bb.13.060184.002105. [DOI] [PubMed] [Google Scholar]
- Giles W. R., Shibata E. F. Voltage clamp of bull-frog cardiac pace-maker cells: a quantitative analysis of potassium currents. J Physiol. 1985 Nov;368:265–292. doi: 10.1113/jphysiol.1985.sp015857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles W. R., van Ginneken A. C. A transient outward current in isolated cells from the crista terminalis of rabbit heart. J Physiol. 1985 Nov;368:243–264. doi: 10.1113/jphysiol.1985.sp015856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch H. G., Pusch H., Verdonck F. The contribution of Na and K ions to the pacemaker current in sheep cardiac Purkinje fibres. Pflugers Arch. 1986 May;406(5):464–471. doi: 10.1007/BF00583368. [DOI] [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H., Kameyama M. Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J Physiol. 1988 Jan;395:233–253. doi: 10.1113/jphysiol.1988.sp016916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H. Modulation by intracellular Ca2+ of the hyperpolarization-activated inward current in rabbit single sino-atrial node cells. J Physiol. 1989 Feb;409:121–141. doi: 10.1113/jphysiol.1989.sp017488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hume J. R., Giles W. Ionic currents in single isolated bullfrog atrial cells. J Gen Physiol. 1983 Feb;81(2):153–194. doi: 10.1085/jgp.81.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irisawa H. Electrophysiology of single cardiac cells. Jpn J Physiol. 1984;34(3):375–388. doi: 10.2170/jjphysiol.34.375. [DOI] [PubMed] [Google Scholar]
- Irisawa H. Membrane currents in cardiac pacemaker tissue. Experientia. 1987 Dec 1;43(11-12):1131–1135. doi: 10.1007/BF01945510. [DOI] [PubMed] [Google Scholar]
- Kreitner D. Electrophysiological study of the two main pacemaker mechanisms in the rabbit sinus node. Cardiovasc Res. 1985 May;19(5):304–318. doi: 10.1093/cvr/19.5.304. [DOI] [PubMed] [Google Scholar]
- Masson-Pévet M. A., Bleeker W. K., Besselsen E., Treytel B. W., Jongsma H. J., Bouman L. N. Pacemaker cell types in the rabbit sinus node: a correlative ultrastructural and electrophysiological study. J Mol Cell Cardiol. 1984 Jan;16(1):53–63. doi: 10.1016/s0022-2828(84)80714-2. [DOI] [PubMed] [Google Scholar]
- Masson-Pévet M., Gros D., Besselsen E. The caveolae in rabbit sinus node and atrium. Cell Tissue Res. 1980;208(2):183–196. doi: 10.1007/BF00234869. [DOI] [PubMed] [Google Scholar]
- Masson-Pévet M., Jongsma H. J., Bleeker W. K., Tsjernina L., van Ginneken A. C., Treijtel B. W., Bouman L. N. Intact isolated sinus node cells from the adult rabbit heart. J Mol Cell Cardiol. 1982 May;14(5):295–299. doi: 10.1016/0022-2828(82)90208-5. [DOI] [PubMed] [Google Scholar]
- Maylie J., Morad M. Ionic currents responsible for the generation of pace-maker current in the rabbit sino-atrial node. J Physiol. 1984 Oct;355:215–235. doi: 10.1113/jphysiol.1984.sp015415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maylie J., Morad M., Weiss J. A study of pace-maker potential in rabbit sino-atrial node: measurement of potassium activity under voltage-clamp conditions. J Physiol. 1981 Feb;311:161–178. doi: 10.1113/jphysiol.1981.sp013579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao M., Gadsby D. C. [Na] and [K] dependence of the Na/K pump current-voltage relationship in guinea pig ventricular myocytes. J Gen Physiol. 1989 Sep;94(3):539–565. doi: 10.1085/jgp.94.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T., Kurachi Y., Noma A., Irisawa H. Action potential and membrane currents of single pacemaker cells of the rabbit heart. Pflugers Arch. 1984 Nov;402(3):248–257. doi: 10.1007/BF00585507. [DOI] [PubMed] [Google Scholar]
- Nathan R. D. Two electrophysiologically distinct types of cultured pacemaker cells from rabbit sinoatrial node. Am J Physiol. 1986 Feb;250(2 Pt 2):H325–H329. doi: 10.1152/ajpheart.1986.250.2.H325. [DOI] [PubMed] [Google Scholar]
- Noble D., Noble S. J. A model of sino-atrial node electrical activity based on a modification of the DiFrancesco-Noble (1984) equations. Proc R Soc Lond B Biol Sci. 1984 Sep 22;222(1228):295–304. doi: 10.1098/rspb.1984.0065. [DOI] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. The kinetics and rectifier properties of the slow potassium current in cardiac Purkinje fibres. J Physiol. 1968 Mar;195(1):185–214. doi: 10.1113/jphysiol.1968.sp008454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A., Morad M., Irisawa H. Does the "pacemaker current" generate the diastolic depolarization in the rabbit SA node cells? Pflugers Arch. 1983 May;397(3):190–194. doi: 10.1007/BF00584356. [DOI] [PubMed] [Google Scholar]
- Noma A., Nakayama T., Kurachi Y., Irisawa H. Resting K conductances in pacemaker and non-pacemaker heart cells of the rabbit. Jpn J Physiol. 1984;34(2):245–254. doi: 10.2170/jjphysiol.34.245. [DOI] [PubMed] [Google Scholar]
- Provencher S. W. A Fourier method for the analysis of exponential decay curves. Biophys J. 1976 Jan;16(1):27–41. doi: 10.1016/S0006-3495(76)85660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson R. L., Clark J. W., Giles W. R., Shibata E. F., Campbell D. L. A mathematical model of a bullfrog cardiac pacemaker cell. Am J Physiol. 1990 Aug;259(2 Pt 2):H352–H369. doi: 10.1152/ajpheart.1990.259.2.H352. [DOI] [PubMed] [Google Scholar]
- Roberts L. A., Slocum G. R., Riley D. A. Morphological study of the innervation pattern of the rabbit sinoatrial node. Am J Anat. 1989 May;185(1):74–88. doi: 10.1002/aja.1001850108. [DOI] [PubMed] [Google Scholar]
- Robinson K., Giles W. A data acquisition, display and plotting program for the IBM PC. Comput Methods Programs Biomed. 1986 Dec;23(3):319–327. doi: 10.1016/0169-2607(86)90067-2. [DOI] [PubMed] [Google Scholar]
- Scheid C. R., Fay F. S. Control of ion distribution in isolated smooth muscle cells. I. Potassium. J Gen Physiol. 1980 Feb;75(2):163–182. doi: 10.1085/jgp.75.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyama I. Characteristics of the anion channel in the sino-atrial node cell of the rabbit. J Physiol. 1979 Sep;294:447–460. doi: 10.1113/jphysiol.1979.sp012940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki T. Conductance and kinetics of delayed rectifier potassium channels in nodal cells of the rabbit heart. J Physiol. 1987 Jun;387:227–250. doi: 10.1113/jphysiol.1987.sp016571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata E. F., Drury T., Refsum H., Aldrete V., Giles W. Contributions of a transient outward current to repolarization in human atrium. Am J Physiol. 1989 Dec;257(6 Pt 2):H1773–H1781. doi: 10.1152/ajpheart.1989.257.6.H1773. [DOI] [PubMed] [Google Scholar]
- Shibata E. F., Giles W. R. Ionic currents that generate the spontaneous diastolic depolarization in individual cardiac pacemaker cells. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7796–7800. doi: 10.1073/pnas.82.22.7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata E. F., Giles W., Pollack G. H. Threshold effects of acetylcholine on primary pacemaker cells of the rabbit sino-atrial node. Proc R Soc Lond B Biol Sci. 1985 Jan 22;223(1232):355–378. doi: 10.1098/rspb.1985.0006. [DOI] [PubMed] [Google Scholar]
- Taniguchi J., Kokubun S., Noma A., Irisawa H. Spontaneously active cells isolated from the sino-atrial and atrio-ventricular nodes of the rabbit heart. Jpn J Physiol. 1981;31(4):547–558. doi: 10.2170/jjphysiol.31.547. [DOI] [PubMed] [Google Scholar]
- Yanagihara K., Irisawa H. Inward current activated during hyperpolarization in the rabbit sinoatrial node cell. Pflugers Arch. 1980 May;385(1):11–19. doi: 10.1007/BF00583909. [DOI] [PubMed] [Google Scholar]
- Yanagihara K., Noma A., Irisawa H. Reconstruction of sino-atrial node pacemaker potential based on the voltage clamp experiments. Jpn J Physiol. 1980;30(6):841–857. doi: 10.2170/jjphysiol.30.841. [DOI] [PubMed] [Google Scholar]
- van Ginneken A. C., Bouman L. N., Jongsma H. J., Duivenvoorden J. J., Opthof T., Giles W. R. Alinidine as a model of the mode of action of specific bradycardic agents on SA node activity. Eur Heart J. 1987 Dec;8 (Suppl 50):25–33. doi: 10.1093/eurheartj/8.suppl_l.25. [DOI] [PubMed] [Google Scholar]



