Abstract
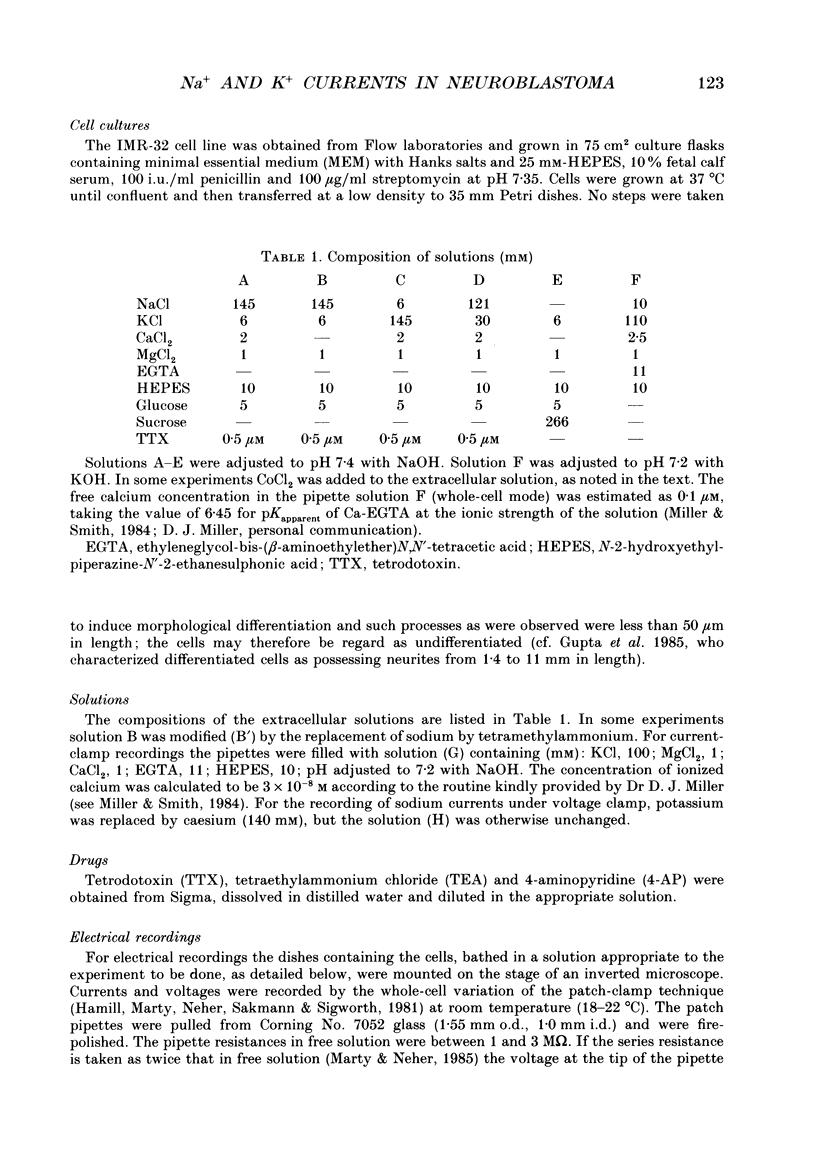
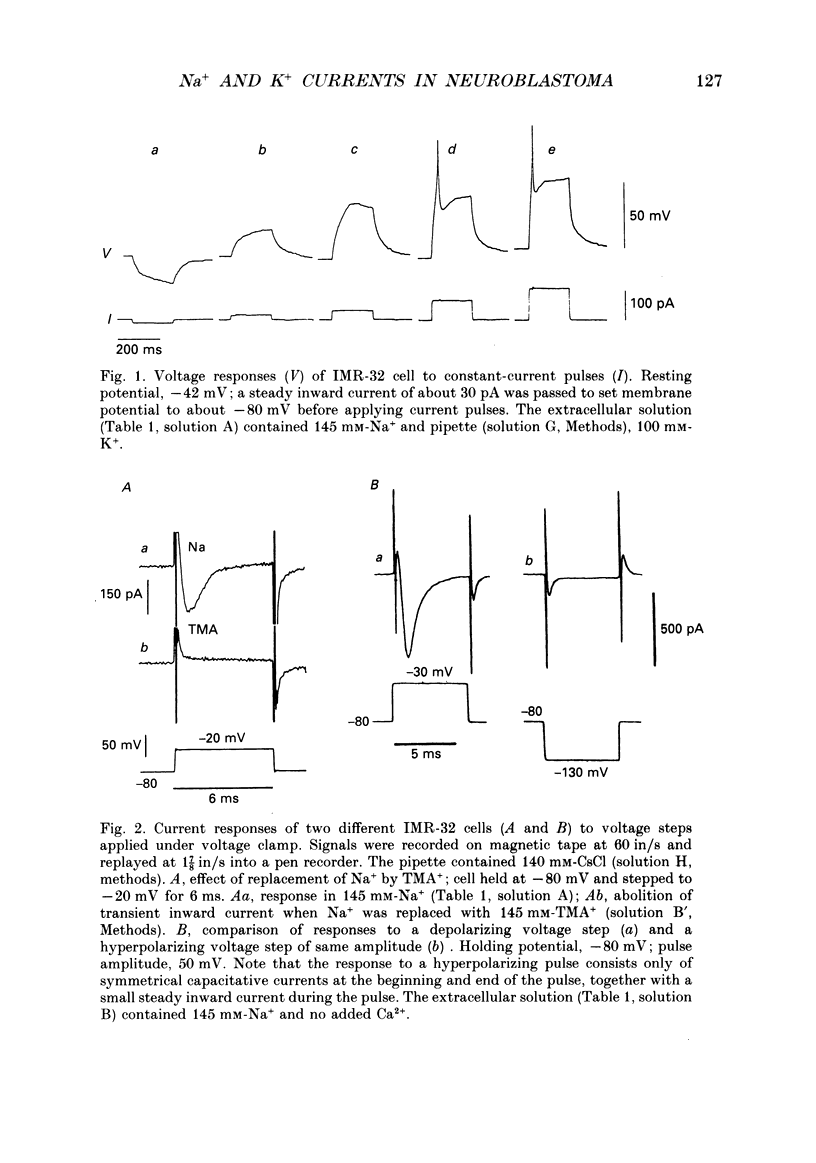
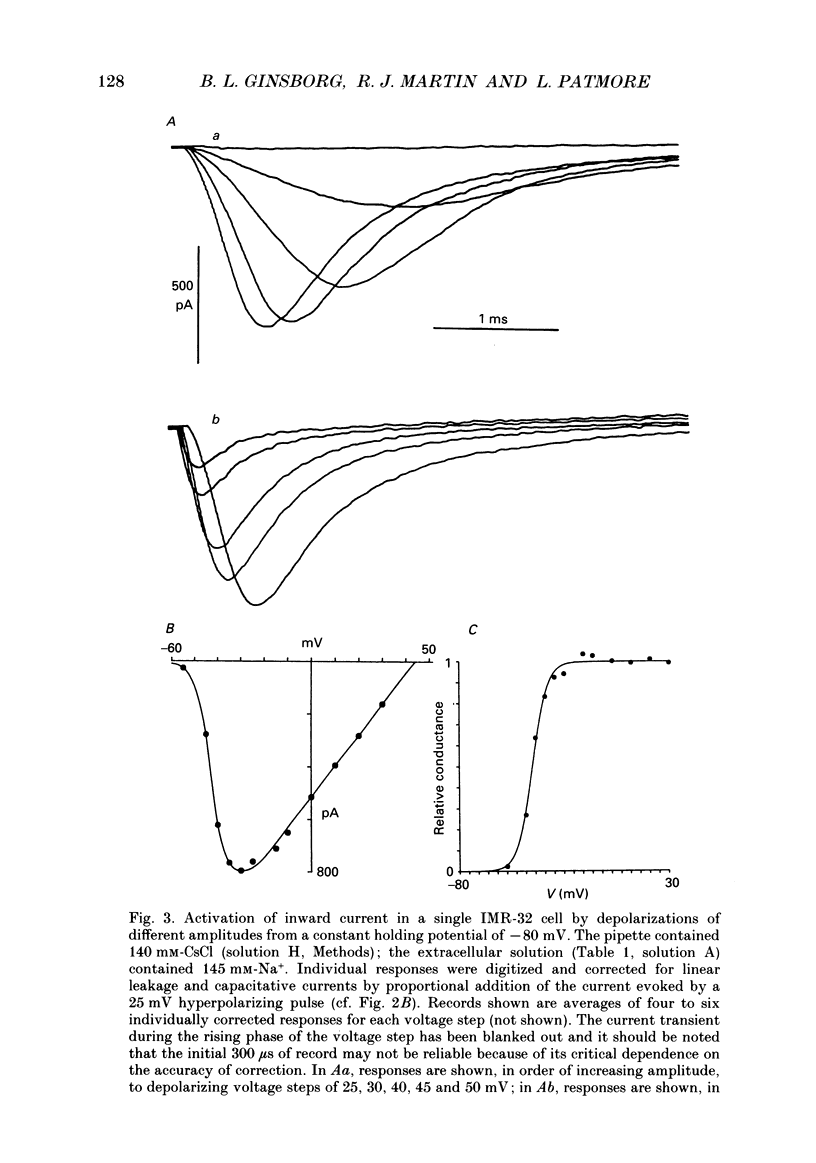
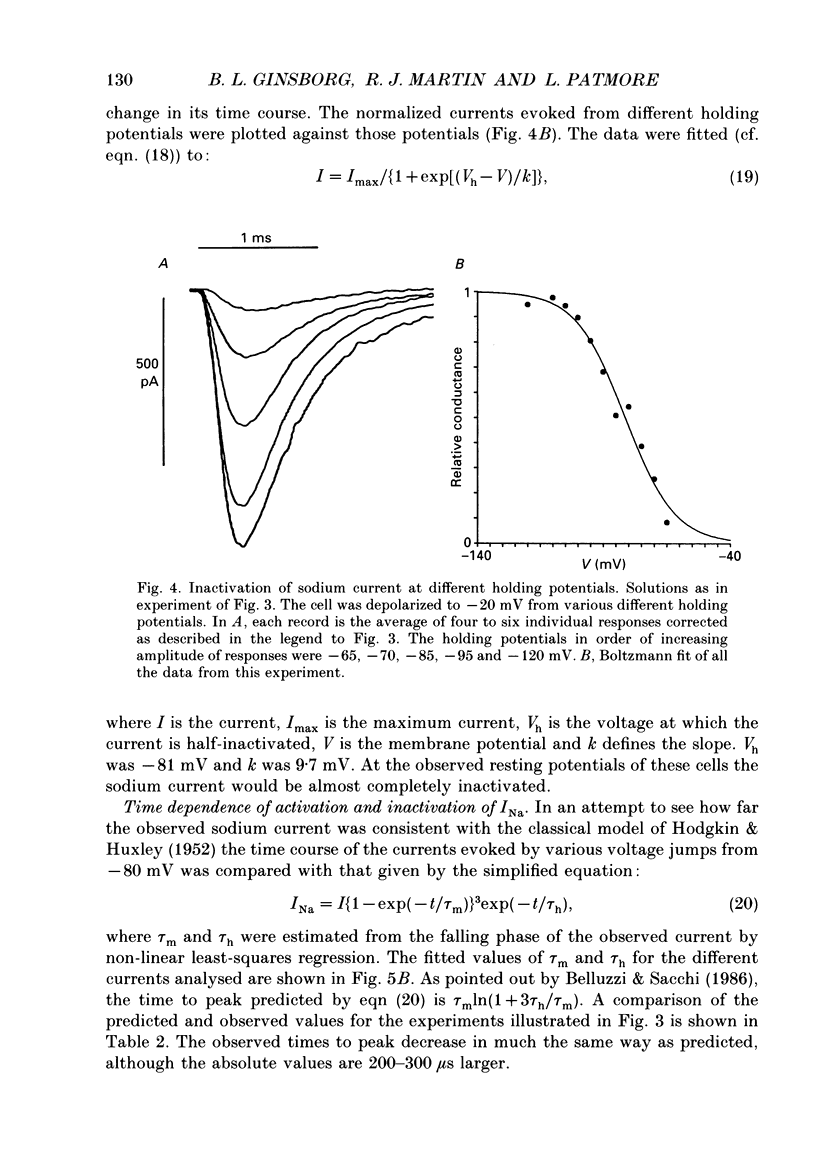
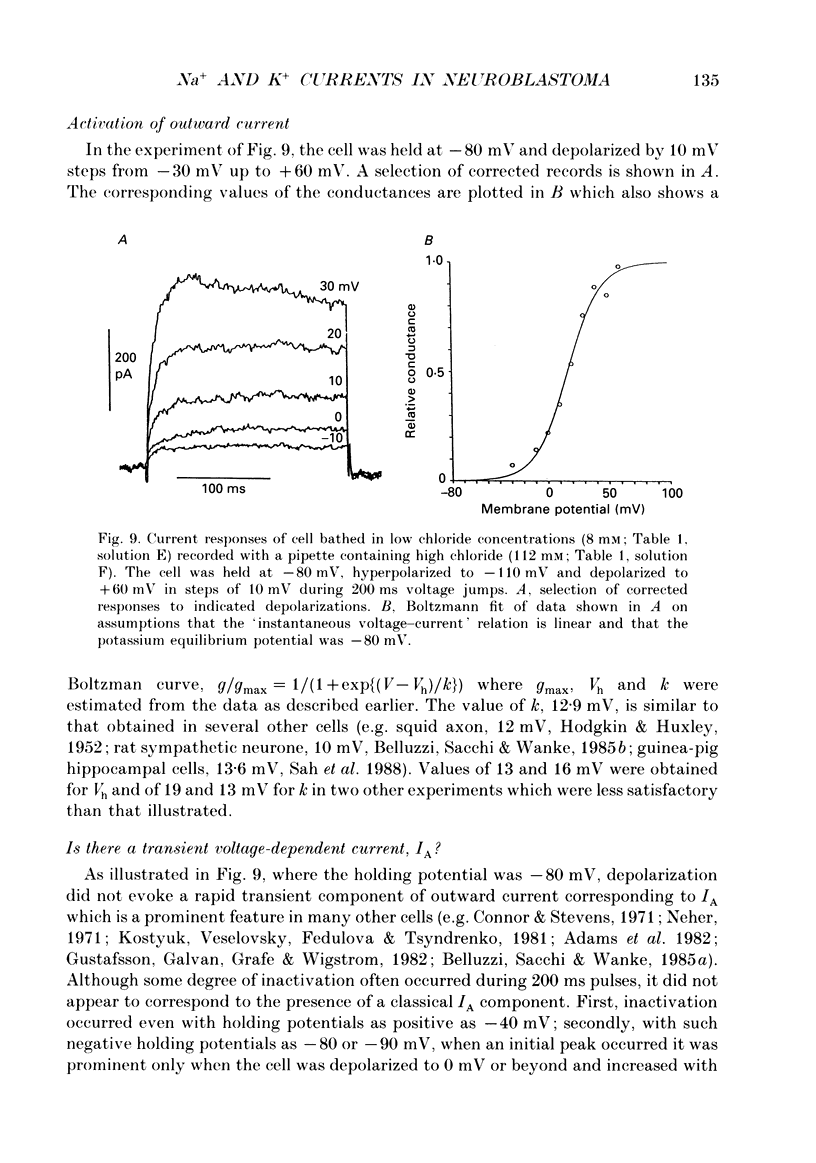
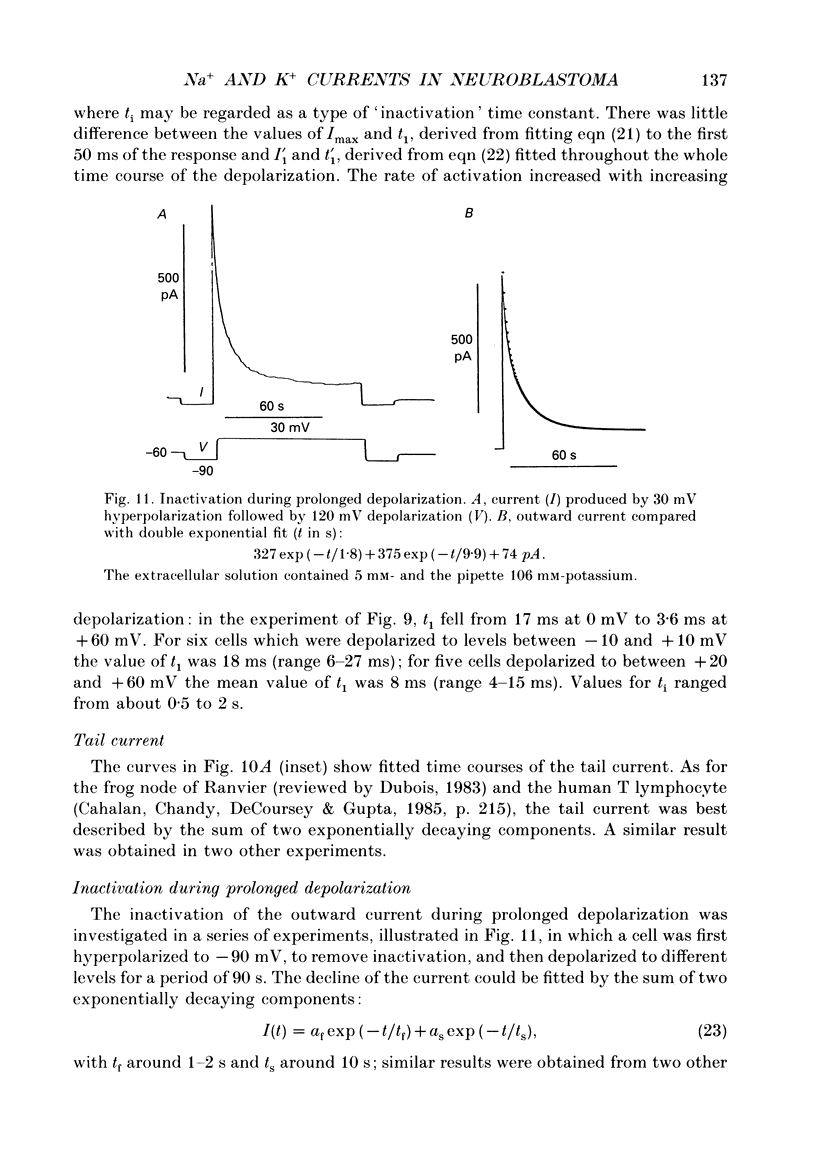
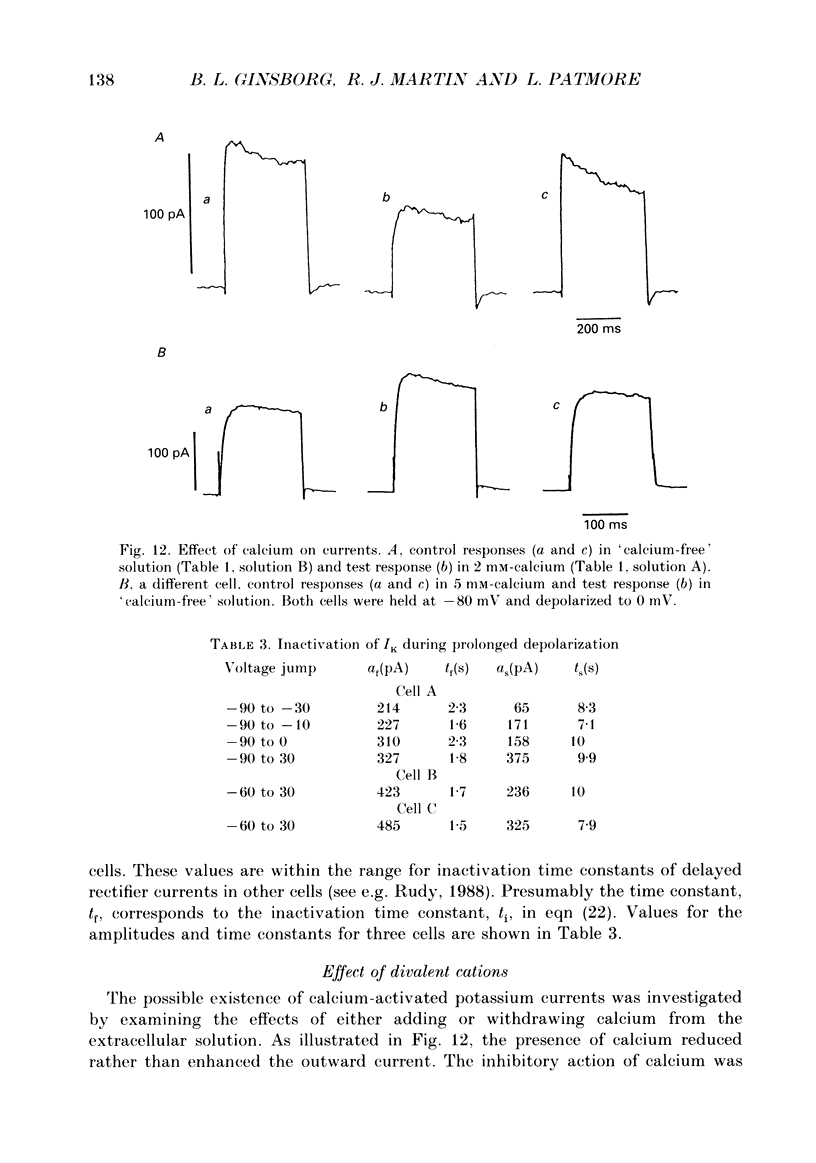
1. The patch-clamp method was applied to the study of ionic currents activated by depolarization of undifferentiated IMR-32 human neuroblastoma cells. Whole-cell sodium and potassium currents and single potassium ion channel currents from cell-attached patches were investigated. 2. Cells had a mean resting potential of -38 mV and mean input resistance of 1.6 G omega. Single action potentials were evoked under current clamp during the injection of depolarizing currents. 3. A voltage-dependent inward sodium current was observed which reversed at +44 mV. A Boltzmann fit to the activation curve gave a half-maximal activation voltage of -41.6 mV and a 'slope' of 3.9 mV. The steady-state inactivation curve had a half-maximal inactivation voltage of -81 mV and a 'slope' of 9.7 mV. 4. The time-dependent activation and inactivation of the current displayed classical Hodgkin-Huxley kinetics. Values for the time constants tau m and tau h of 0.16 and 0.63 ms were calculated for a voltage jump from -80 to -10 mV; tau m and tau h decreased as the step potential was changed from -30 to +20 mV. 5. Outward currents were activated in bathing solutions substantially free of anions and could thus be attributed to potassium ions. The tail current reversed in direction on repolarization to -60 mV when the potassium concentration in the bathing solution was increased from 6 to 30 mM. When the bathing solution contained 145 mM-potassium, and the patch pipette, 95 mM, a depolarization to -10 mV from a holding potential of -60 mV evoked an inward current. 6. Outward currents were examined by using voltage pulses which depolarized the cell to -20 mV, or more positive values, from a holding potential of -80 mV and by pulses which depolarized the cell to 0 mV, or to positive values, from a holding potential of -30 mV. A Boltzmann fit of typical activation data gave a half-maximal activation voltage of 17 mV and a 'slope' of 14 mV. 7. The time course of the rising phase of the current was described by a function of the form A(1-exp[-(t-delta t)/tau]), where delta t varied between 1 and 4 ms and tau varied between 4 and 27 ms, decreasing with increasing depolarization. There was no evidence for a fast transient component. 8. The amplitude of outward currents was reduced by extracellular calcium ions, cobalt ions, tetraethylammonium and 4-aminopyridine.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





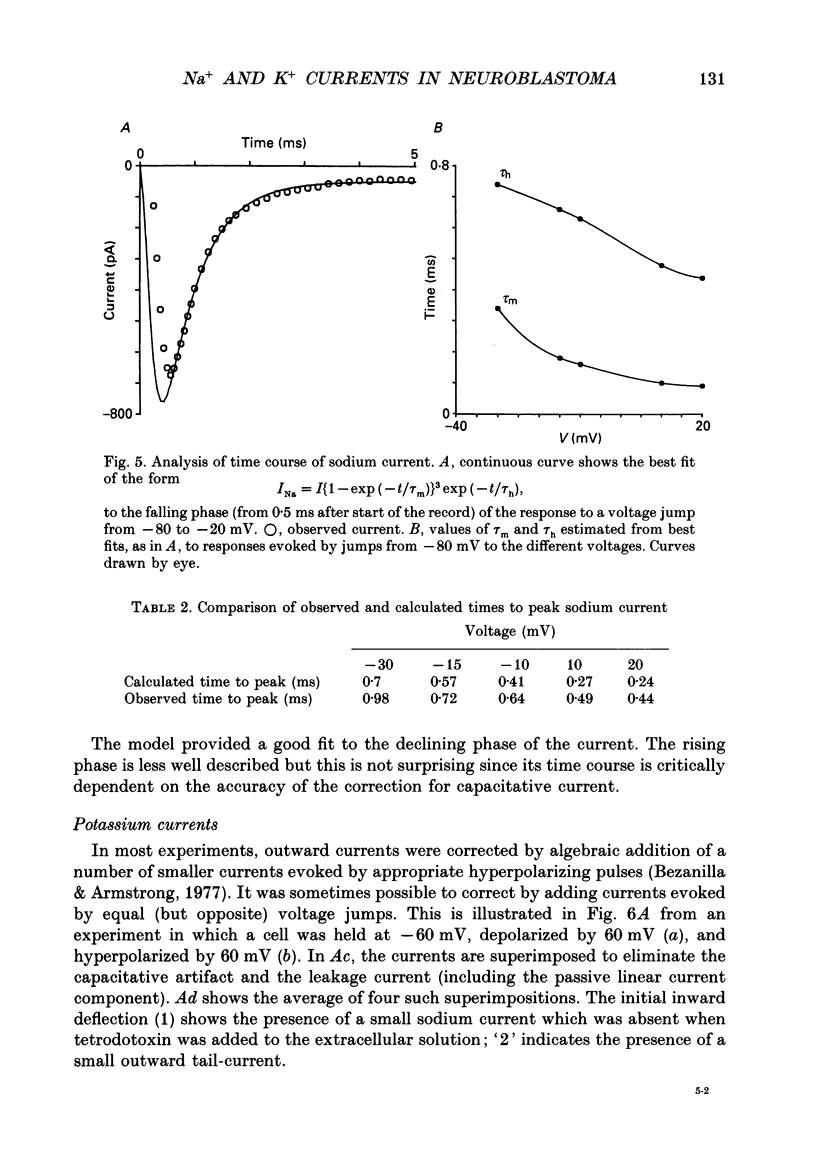
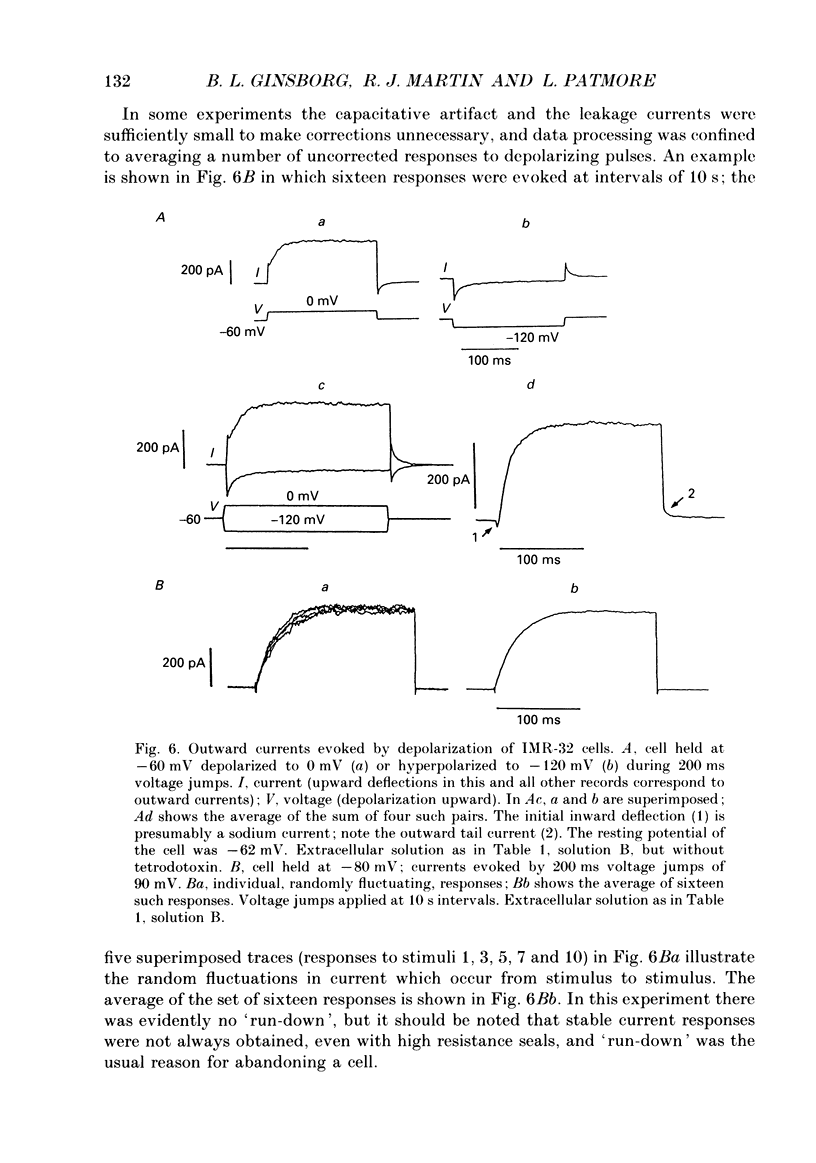
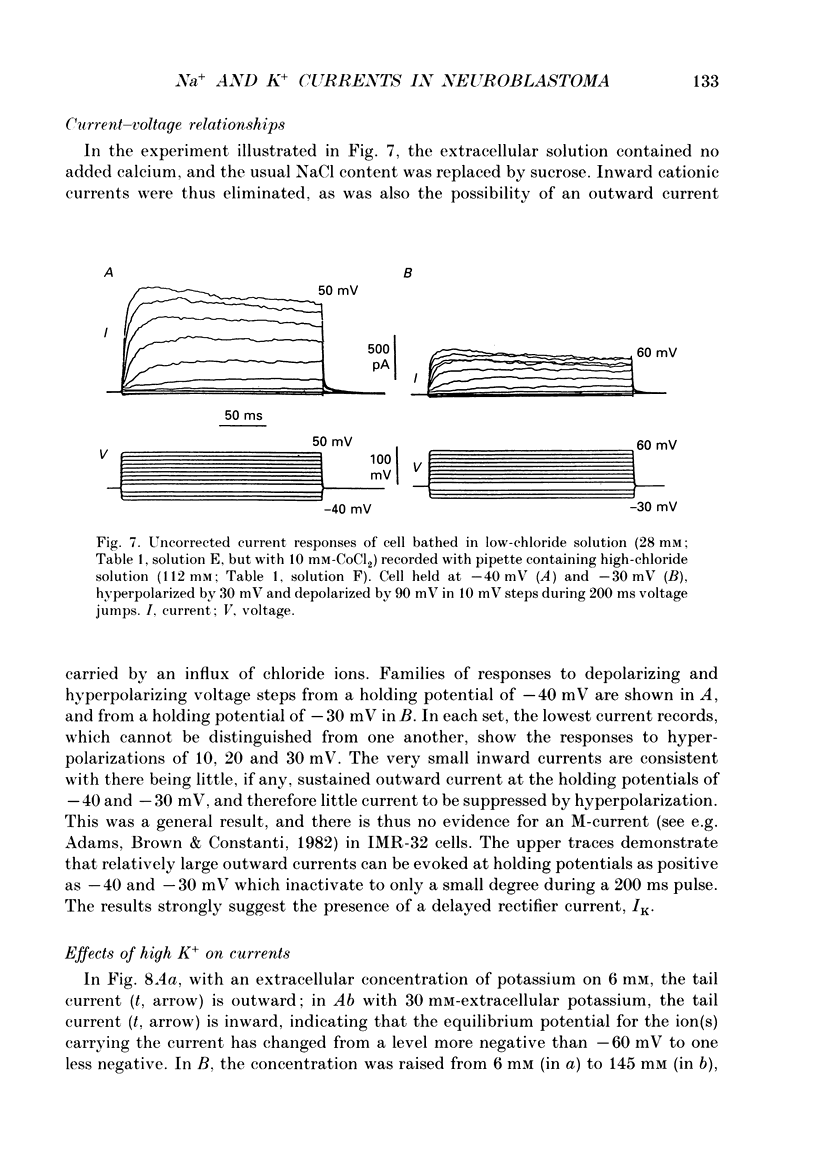
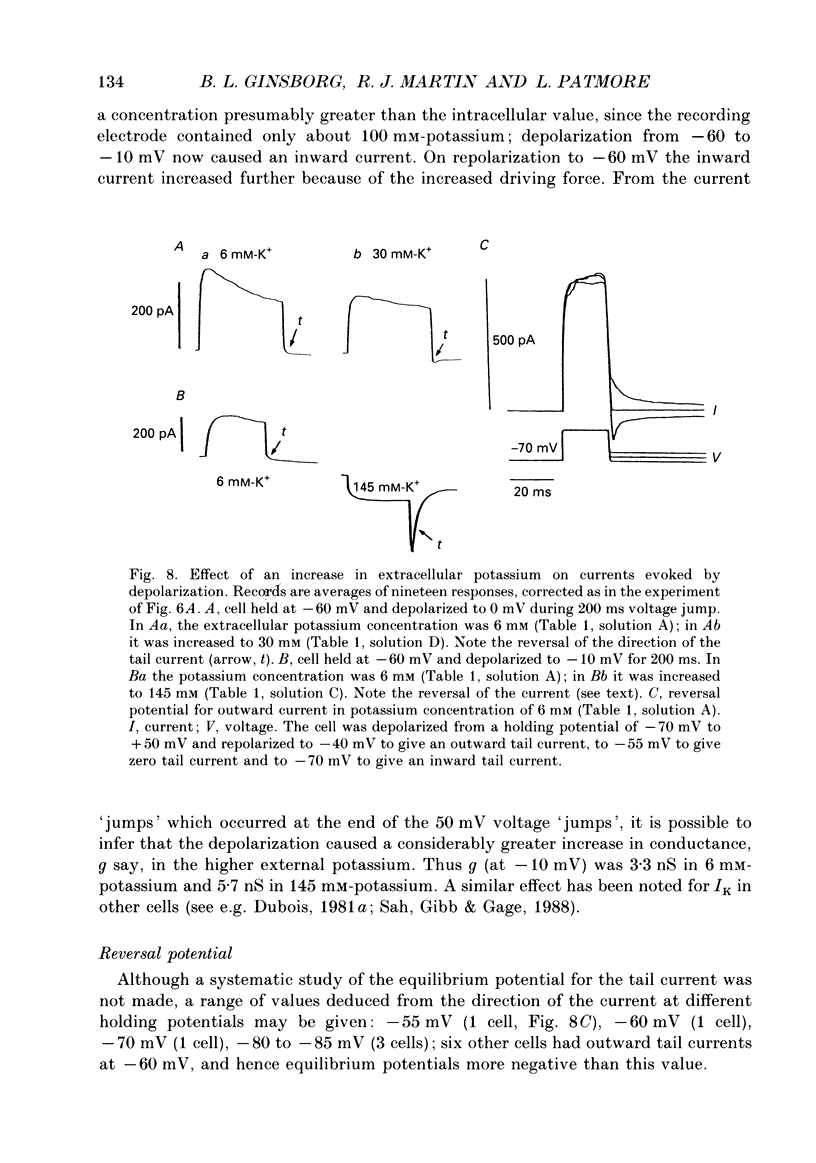
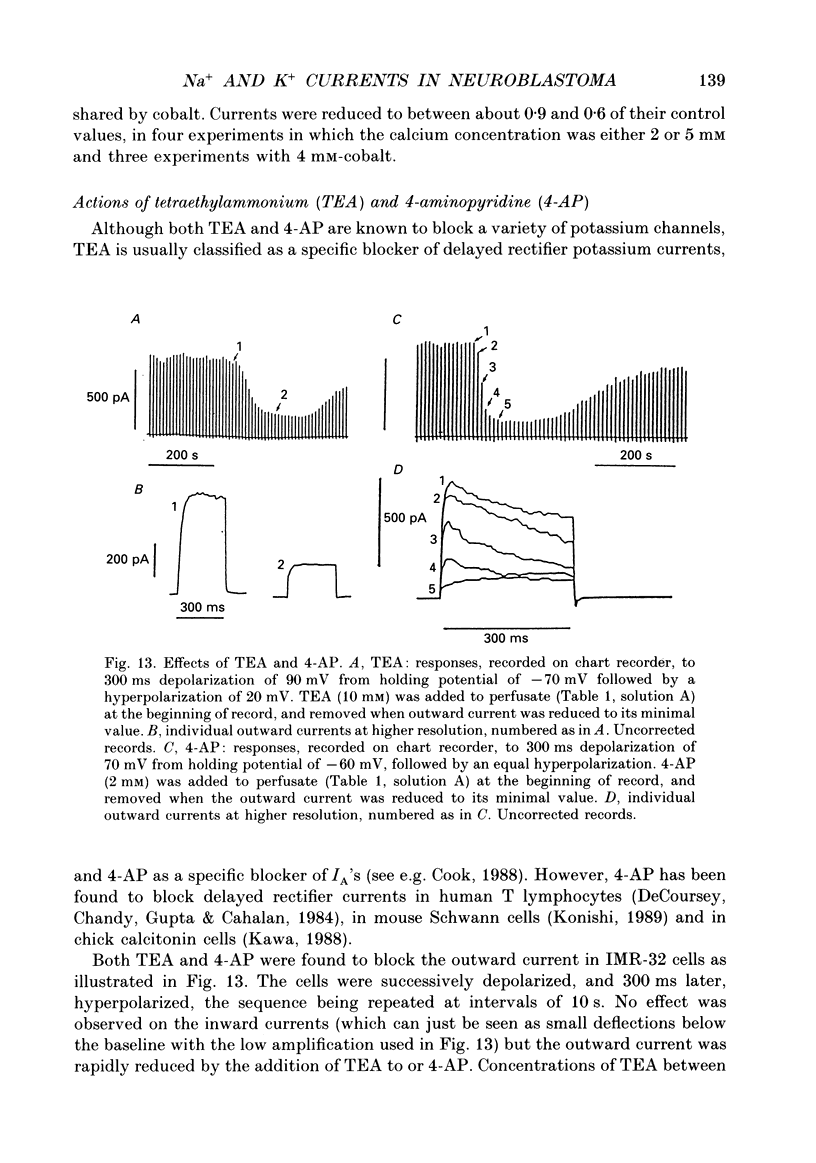
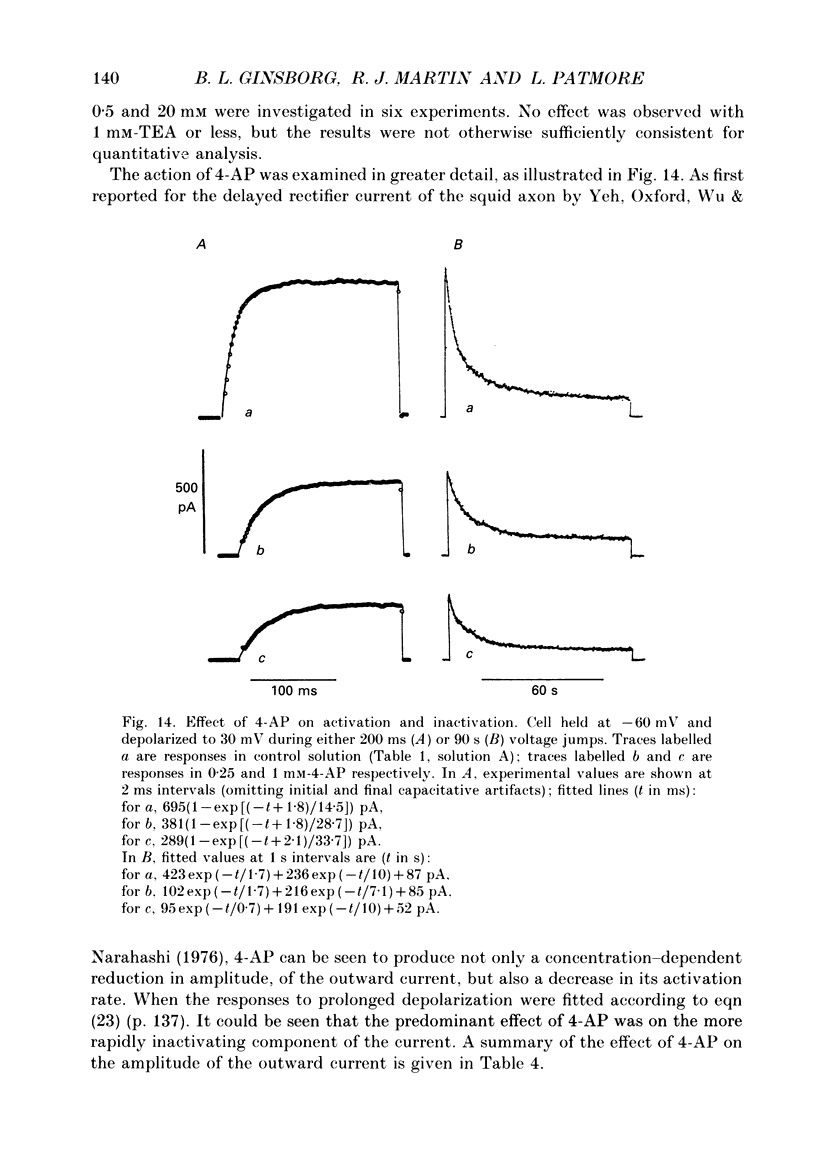
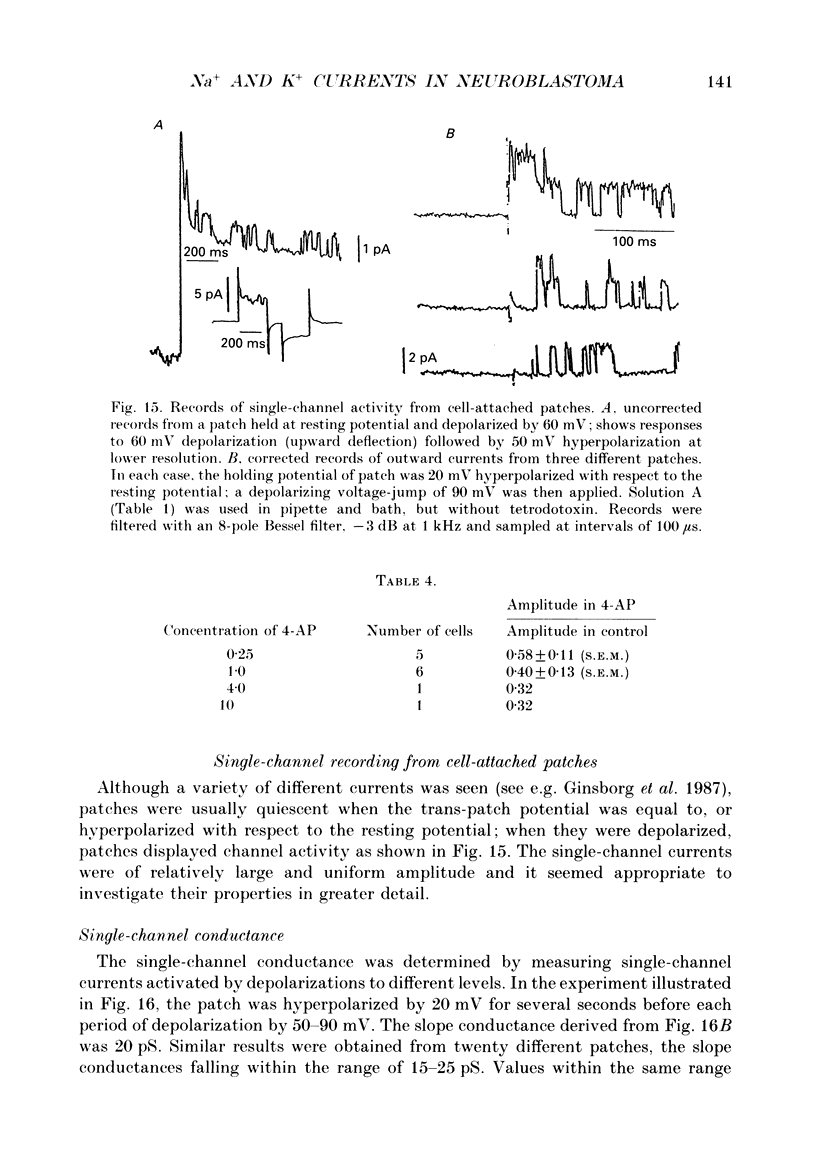
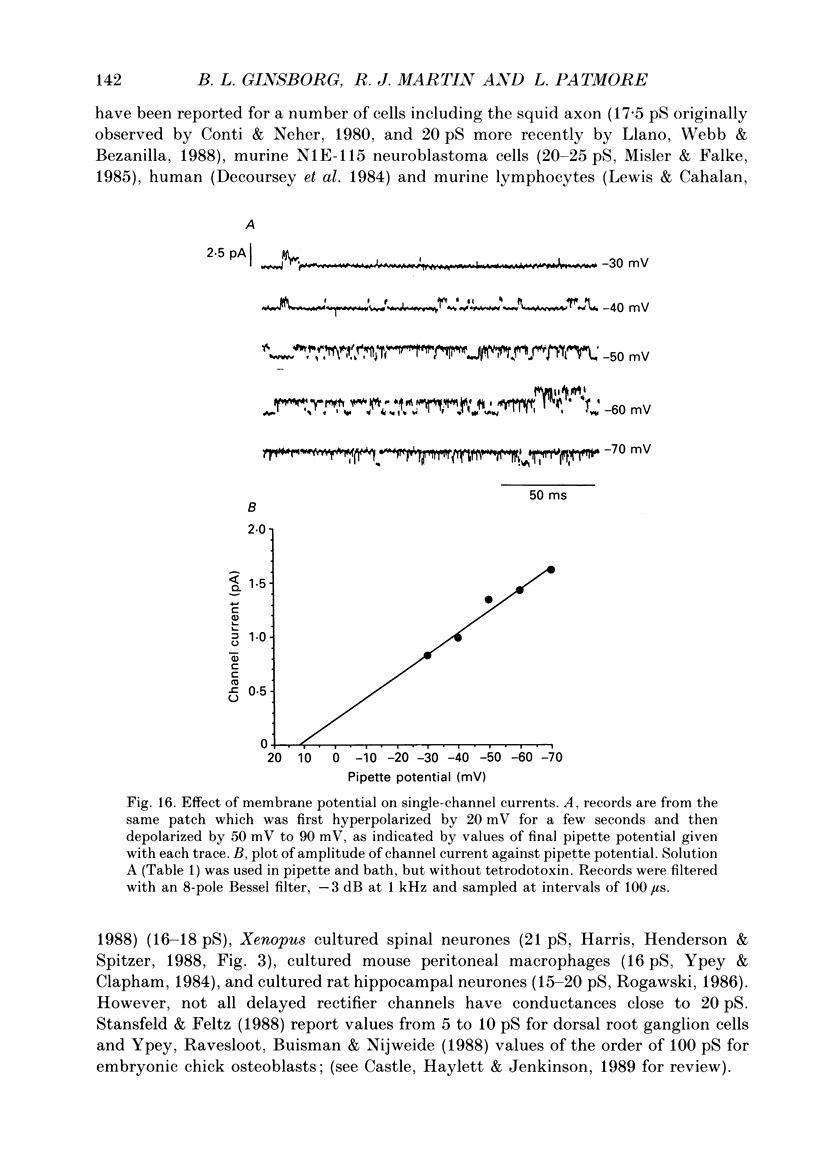
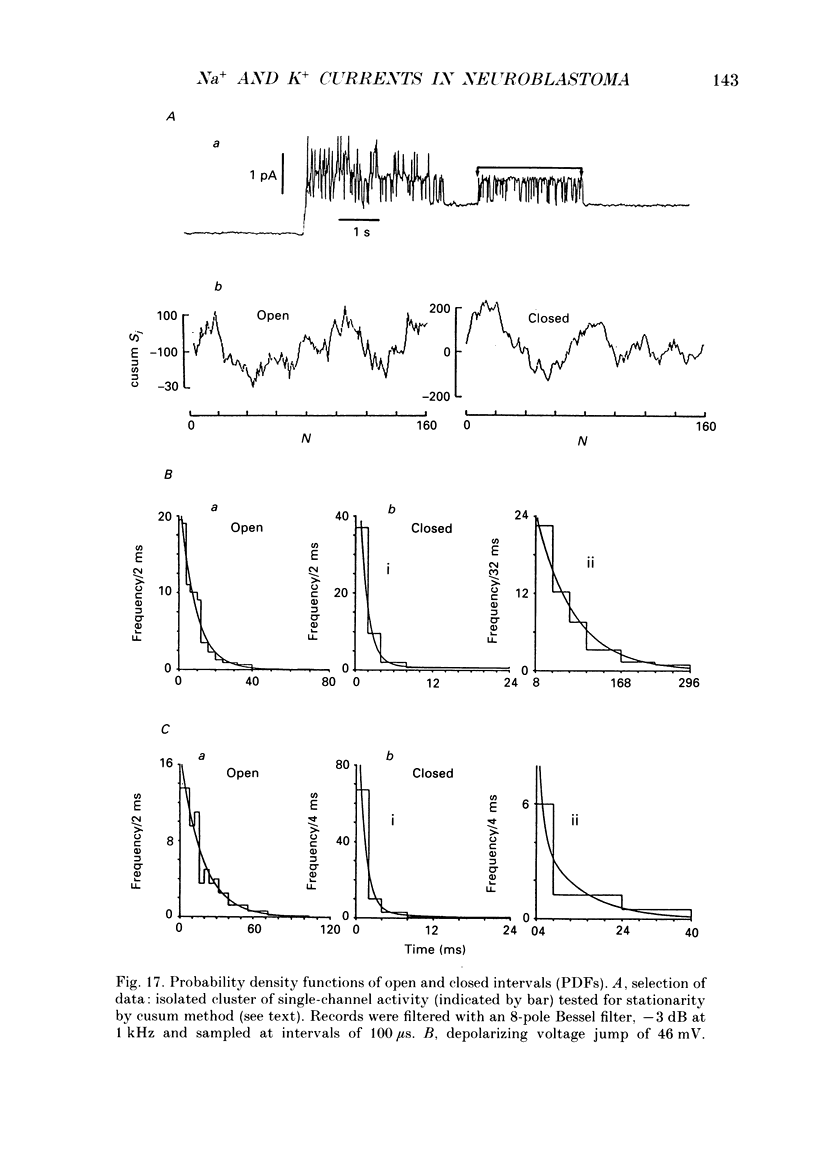


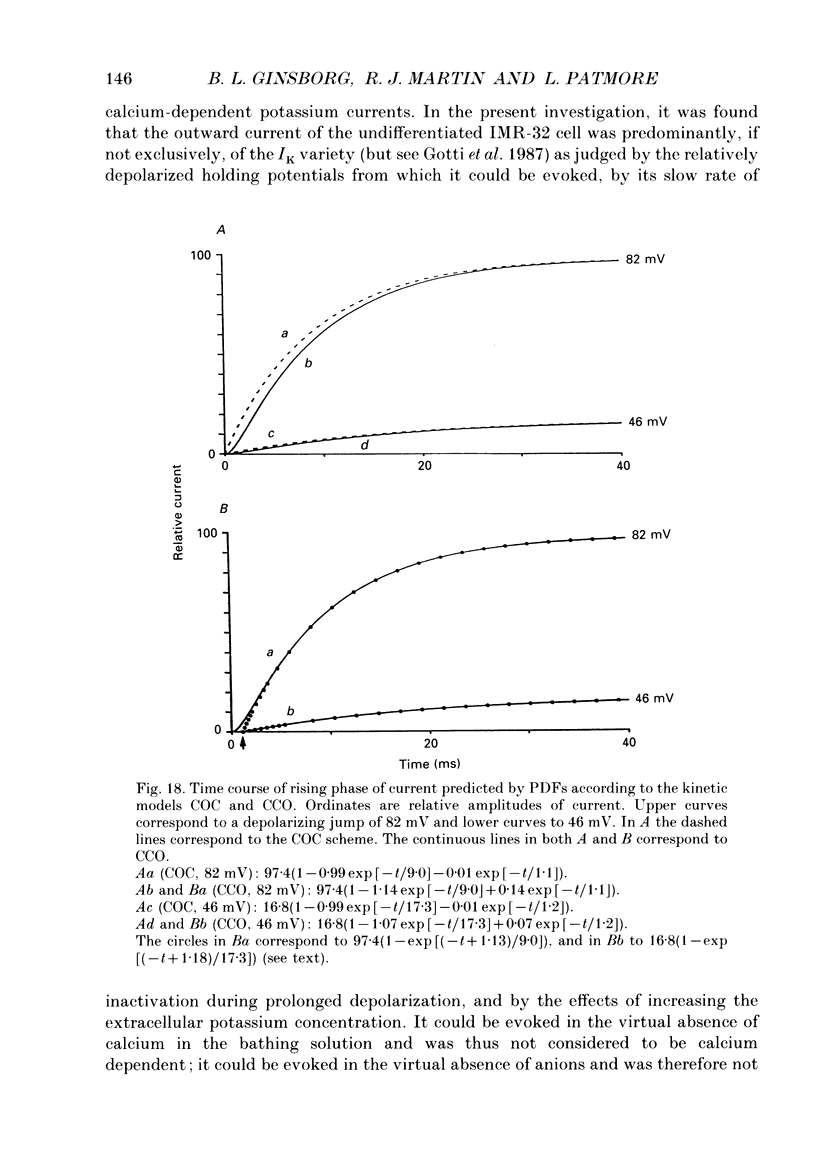



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982 Sep;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
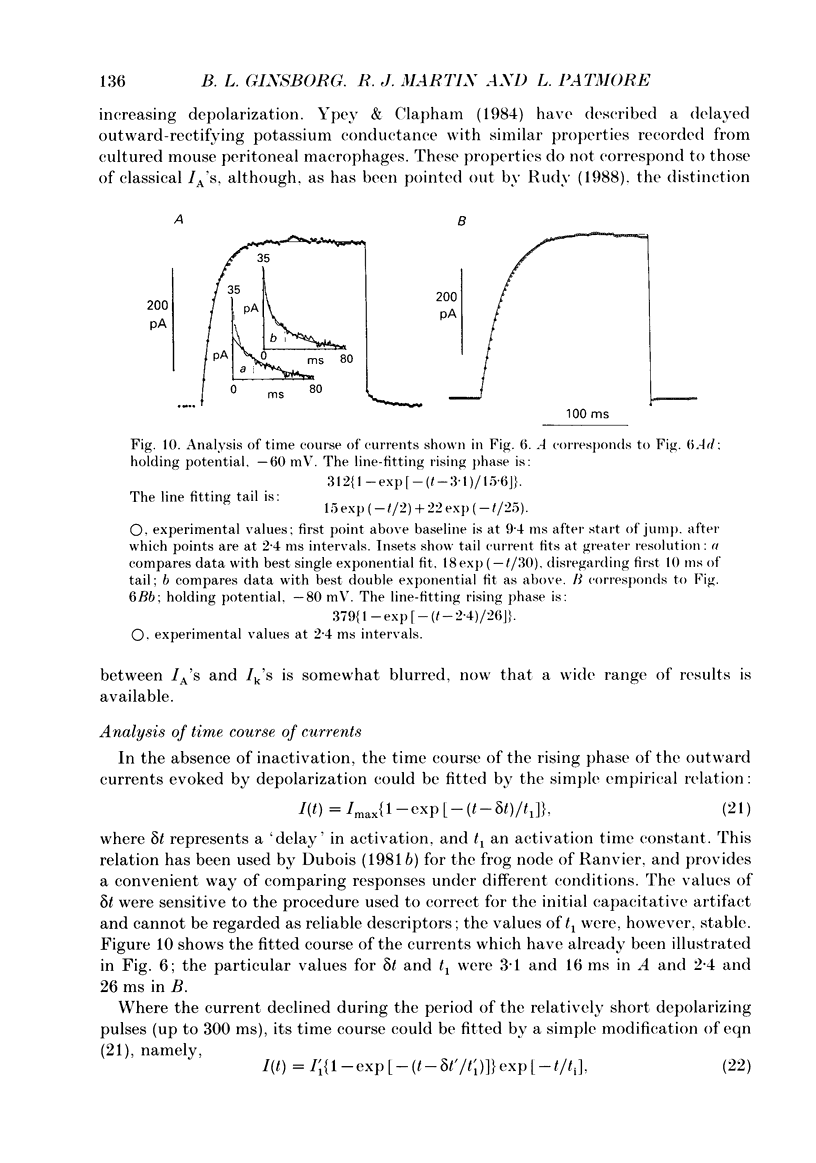
- Belluzzi O., Sacchi O. A quantitative description of the sodium current in the rat sympathetic neurone. J Physiol. 1986 Nov;380:275–291. doi: 10.1113/jphysiol.1986.sp016285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi O., Sacchi O., Wanke E. A fast transient outward current in the rat sympathetic neurone studied under voltage-clamp conditions. J Physiol. 1985 Jan;358:91–108. doi: 10.1113/jphysiol.1985.sp015542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi O., Sacchi O., Wanke E. Identification of delayed potassium and calcium currents in the rat sympathetic neurone under voltage clamp. J Physiol. 1985 Jan;358:109–129. doi: 10.1113/jphysiol.1985.sp015543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Inactivation of the sodium channel. I. Sodium current experiments. J Gen Physiol. 1977 Nov;70(5):549–566. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. M-currents: an update. Trends Neurosci. 1988 Jul;11(7):294–299. doi: 10.1016/0166-2236(88)90089-6. [DOI] [PubMed] [Google Scholar]
- Cahalan M. D., Chandy K. G., DeCoursey T. E., Gupta S. A voltage-gated potassium channel in human T lymphocytes. J Physiol. 1985 Jan;358:197–237. doi: 10.1113/jphysiol.1985.sp015548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E., Sher E., Clementi F. Ca currents in human neuroblastoma IMR32 cells: kinetics, permeability and pharmacology. Pflugers Arch. 1990 Apr;416(1-2):170–179. doi: 10.1007/BF00370239. [DOI] [PubMed] [Google Scholar]
- Castle N. A., Haylett D. G., Jenkinson D. H. Toxins in the characterization of potassium channels. Trends Neurosci. 1989 Feb;12(2):59–65. doi: 10.1016/0166-2236(89)90137-9. [DOI] [PubMed] [Google Scholar]
- Clementi F., Cabrini D., Gotti C., Sher E. Pharmacological characterization of cholinergic receptors in a human neuroblastoma cell line. J Neurochem. 1986 Jul;47(1):291–297. doi: 10.1111/j.1471-4159.1986.tb02861.x. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of single ion channels. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):205–235. doi: 10.1098/rspb.1981.0003. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F., Neher E. Single channel recordings of K+ currents in squid axons. Nature. 1980 May 15;285(5761):140–143. doi: 10.1038/285140a0. [DOI] [PubMed] [Google Scholar]
- Cook N. S. The pharmacology of potassium channels and their therapeutic potential. Trends Pharmacol Sci. 1988 Jan;9(1):21–28. doi: 10.1016/0165-6147(88)90238-6. [DOI] [PubMed] [Google Scholar]
- DeCoursey T. E., Chandy K. G., Gupta S., Cahalan M. D. Voltage-gated K+ channels in human T lymphocytes: a role in mitogenesis? Nature. 1984 Feb 2;307(5950):465–468. doi: 10.1038/307465a0. [DOI] [PubMed] [Google Scholar]
- Dubois J. M. Evidence for the existence of three types of potassium channels in the frog Ranvier node membrane. J Physiol. 1981 Sep;318:297–316. doi: 10.1113/jphysiol.1981.sp013865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J. M. Potassium currents in the frog node of Ranvier. Prog Biophys Mol Biol. 1983;42(1):1–20. doi: 10.1016/0079-6107(83)90002-0. [DOI] [PubMed] [Google Scholar]
- Dubois J. M. Simultaneous changes in the equilibrium potential and potassium conductance in voltage clamped Ranvier node in the frog. J Physiol. 1981 Sep;318:279–295. doi: 10.1113/jphysiol.1981.sp013864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasbey C. A., Martin R. J. Exploratory and confirmatory plots of single-channel records. J Neurosci Methods. 1986 May;16(3):239–249. doi: 10.1016/0165-0270(86)90041-5. [DOI] [PubMed] [Google Scholar]
- Gotti C., Sher E., Cabrini D., Bondiolotti G., Wanke E., Mancinelli E., Clementi F. Cholinergic receptors, ion channels, neurotransmitter synthesis, and neurite outgrowth are independently regulated during the in vitro differentiation of a human neuroblastoma cell line. Differentiation. 1987;34(2):144–155. doi: 10.1111/j.1432-0436.1987.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Gupta M., Notter M. F., Felten S., Gash D. M. Differentiation characteristics of human neuroblastoma cells in the presence of growth modulators and antimitotic drugs. Brain Res. 1985 Mar;351(1):21–29. doi: 10.1016/0165-3806(85)90227-5. [DOI] [PubMed] [Google Scholar]
- Gustafsson B., Galvan M., Grafe P., Wigström H. A transient outward current in a mammalian central neurone blocked by 4-aminopyridine. Nature. 1982 Sep 16;299(5880):252–254. doi: 10.1038/299252a0. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harris G. L., Henderson L. P., Spitzer N. C. Changes in densities and kinetics of delayed rectifier potassium channels during neuronal differentiation. Neuron. 1988 Oct;1(8):739–750. doi: 10.1016/0896-6273(88)90172-9. [DOI] [PubMed] [Google Scholar]
- Kawa K. Voltage-gated sodium and potassium currents and their variation in calcitonin-secreting cells of the chick. J Physiol. 1988 May;399:93–113. doi: 10.1113/jphysiol.1988.sp017070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi T. Voltage-dependent potassium channels in mouse Schwann cells. J Physiol. 1989 Apr;411:115–130. doi: 10.1113/jphysiol.1989.sp017564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G., Veselovsky N. S., Fedulova S. A., Tsyndrenko A. Y. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons-III. Potassium currents. Neuroscience. 1981;6(12):2439–2444. doi: 10.1016/0306-4522(81)90090-7. [DOI] [PubMed] [Google Scholar]
- Lewis R. S., Cahalan M. D. Subset-specific expression of potassium channels in developing murine T lymphocytes. Science. 1988 Feb 12;239(4841 Pt 1):771–775. doi: 10.1126/science.2448877. [DOI] [PubMed] [Google Scholar]
- Llano I., Webb C. K., Bezanilla F. Potassium conductance of the squid giant axon. Single-channel studies. J Gen Physiol. 1988 Aug;92(2):179–196. doi: 10.1085/jgp.92.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A., Neher E. Potassium channels in cultured bovine adrenal chromaffin cells. J Physiol. 1985 Oct;367:117–141. doi: 10.1113/jphysiol.1985.sp015817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. J., Smith G. L. EGTA purity and the buffering of calcium ions in physiological solutions. Am J Physiol. 1984 Jan;246(1 Pt 1):C160–C166. doi: 10.1152/ajpcell.1984.246.1.C160. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Spector I. Ionic currents in cultured mouse neuroblastoma cells under voltage-clamp conditions. J Physiol. 1978 May;278:265–286. doi: 10.1113/jphysiol.1978.sp012303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Two fast transient current components during voltage clamp on snail neurons. J Gen Physiol. 1971 Jul;58(1):36–53. doi: 10.1085/jgp.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski M. A. Single voltage-dependent potassium channels in cultured rat hippocampal neurons. J Neurophysiol. 1986 Aug;56(2):481–493. doi: 10.1152/jn.1986.56.2.481. [DOI] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Sah P., Gibb A. J., Gage P. W. Potassium current activated by depolarization of dissociated neurons from adult guinea pig hippocampus. J Gen Physiol. 1988 Aug;92(2):263–278. doi: 10.1085/jgp.92.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea-pig heart. J Physiol. 1984 Feb;347:641–657. doi: 10.1113/jphysiol.1984.sp015088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher E., Gotti C., Pandiella A., Madeddu L., Clementi F. Intracellular calcium homeostasis in a human neuroblastoma cell line: modulation by depolarization, cholinergic receptors, and alpha-latrotoxin. J Neurochem. 1988 Jun;50(6):1708–1713. doi: 10.1111/j.1471-4159.1988.tb02467.x. [DOI] [PubMed] [Google Scholar]
- Stansfeld C., Feltz A. Dendrotoxin-sensitive K+ channels in dorsal root ganglion cells. Neurosci Lett. 1988 Oct 31;93(1):49–55. doi: 10.1016/0304-3940(88)90011-0. [DOI] [PubMed] [Google Scholar]
- Tumilowicz J. J., Nichols W. W., Cholon J. J., Greene A. E. Definition of a continuous human cell line derived from neuroblastoma. Cancer Res. 1970 Aug;30(8):2110–2118. [PubMed] [Google Scholar]
- Yeh J. Z., Oxford G. S., Wu C. H., Narahashi T. Dynamics of aminopyridine block of potassium channels in squid axon membrane. J Gen Physiol. 1976 Nov;68(5):519–535. doi: 10.1085/jgp.68.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ypey D. L., Clapham D. E. Development of a delayed outward-rectifying K+ conductance in cultured mouse peritoneal macrophages. Proc Natl Acad Sci U S A. 1984 May;81(10):3083–3087. doi: 10.1073/pnas.81.10.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ypey D. L., Ravesloot J. H., Buisman H. P., Nijweide P. J. Voltage-activated ionic channels and conductances in embryonic chick osteoblast cultures. J Membr Biol. 1988;101(2):141–150. doi: 10.1007/BF01872829. [DOI] [PubMed] [Google Scholar]


