Abstract
1. A cross-sectional study has been performed on 457 normal subjects to determine changes in conduction delays with age in central and peripheral motor and somatosensory pathways to the upper limb. 2. Electromagnetic stimulation was used to investigate central and peripheral conduction in motor pathways from the cortex to biceps brachii and hypothenar muscles in 308 normal human subjects aged from 32 weeks gestation to 55 years. The responses were recorded in the surface electromyogram. 3. Somatosensory potentials evoked by electrical stimulation of the median nerve have been recorded at Erb's point and over the somatosensory cortex in 149 normal subjects aged from 34 weeks gestation to 52 years to determine central and peripheral somatosensory conduction delays. 4. The conduction delays in the central components of both motor and somatosensory pathways rapidly decrease over the first 2 years after birth and thereafter remain constant at adult values. 5. The conduction delays in the peripheral components of both motor and somatosensory pathways also decrease initially but then from the age of 5 years progressively increase in proportion to arm length. 6. The threshold stimulus intensity for evoking muscle responses following electromagnetic stimulation of the cortex is high initially and falls progressively until the age of 16 years. A linear relationship exists between the threshold intensity and height for the height range 70-180 cm. 7. The threshold stimulus intensities for exciting peripheral motor and somatosensory nerves decrease up to the age of 5 years and then reach a plateau. 8. The results support the conclusion, already reported in the literature that peripheral nerves attain maximum value for fibre diameter and conduction velocity at approximately 5 years of age. 9. In contrast, it is concluded that the maximum fibre diameters in both motor and somatosensory central pathways increase in proportion to height, leading to constant central conduction delays with growth.
Full text
PDF

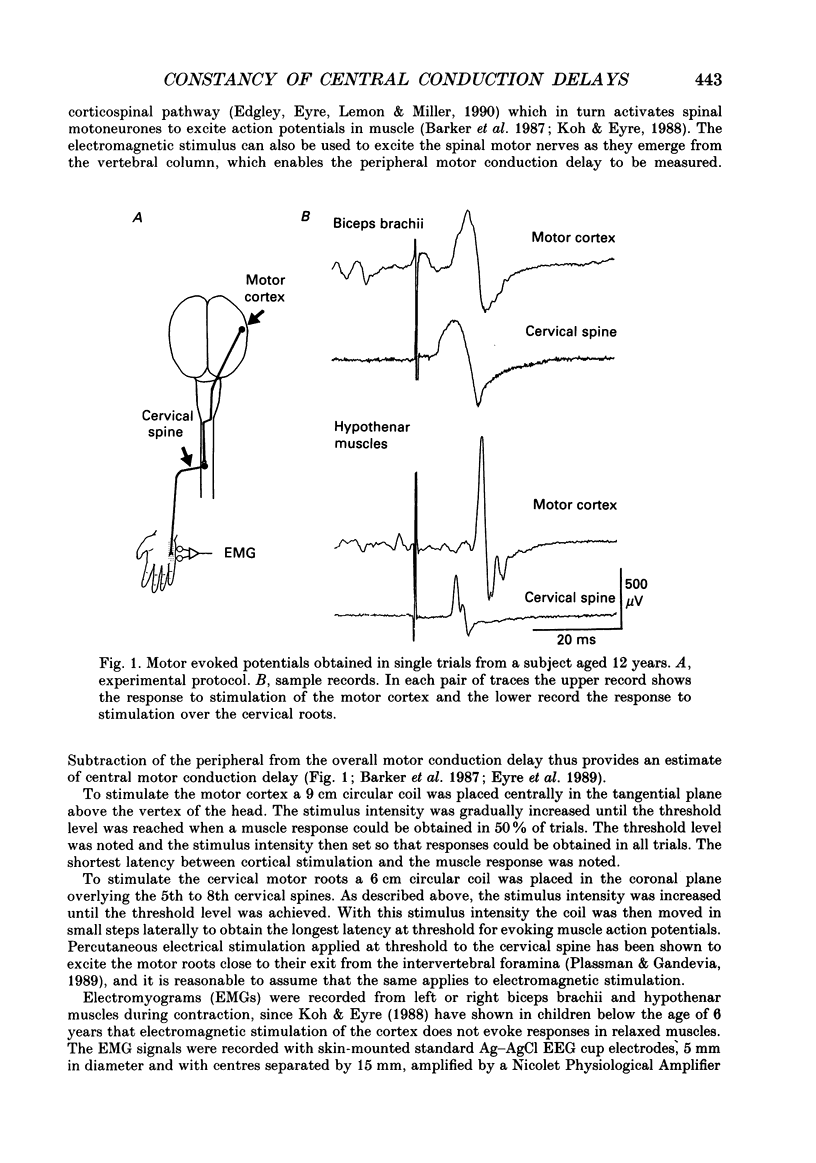
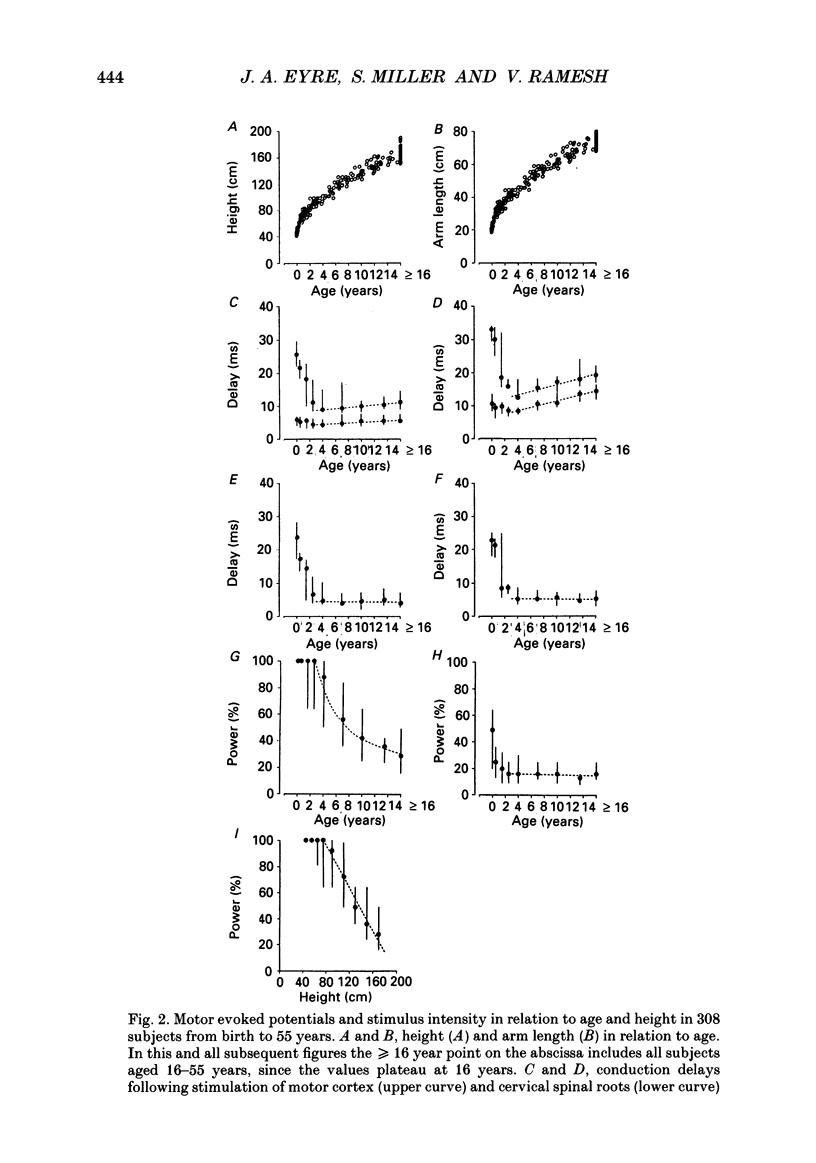

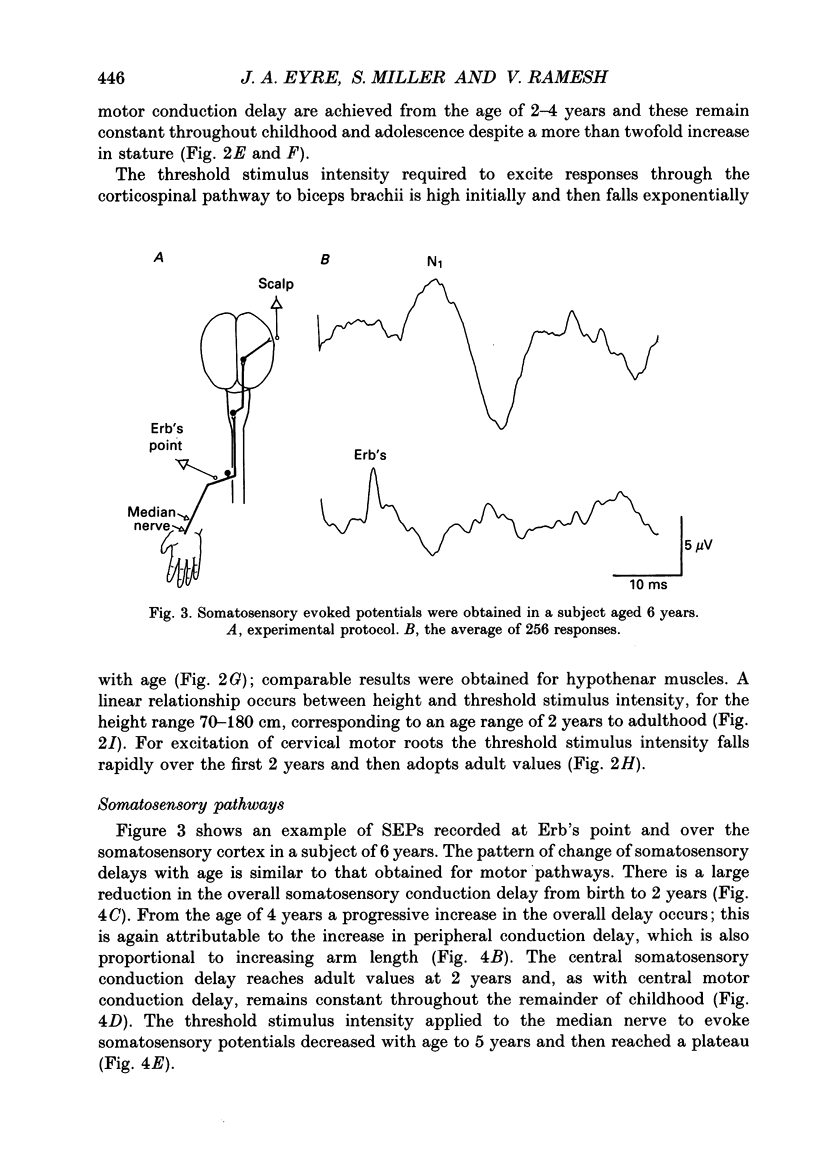
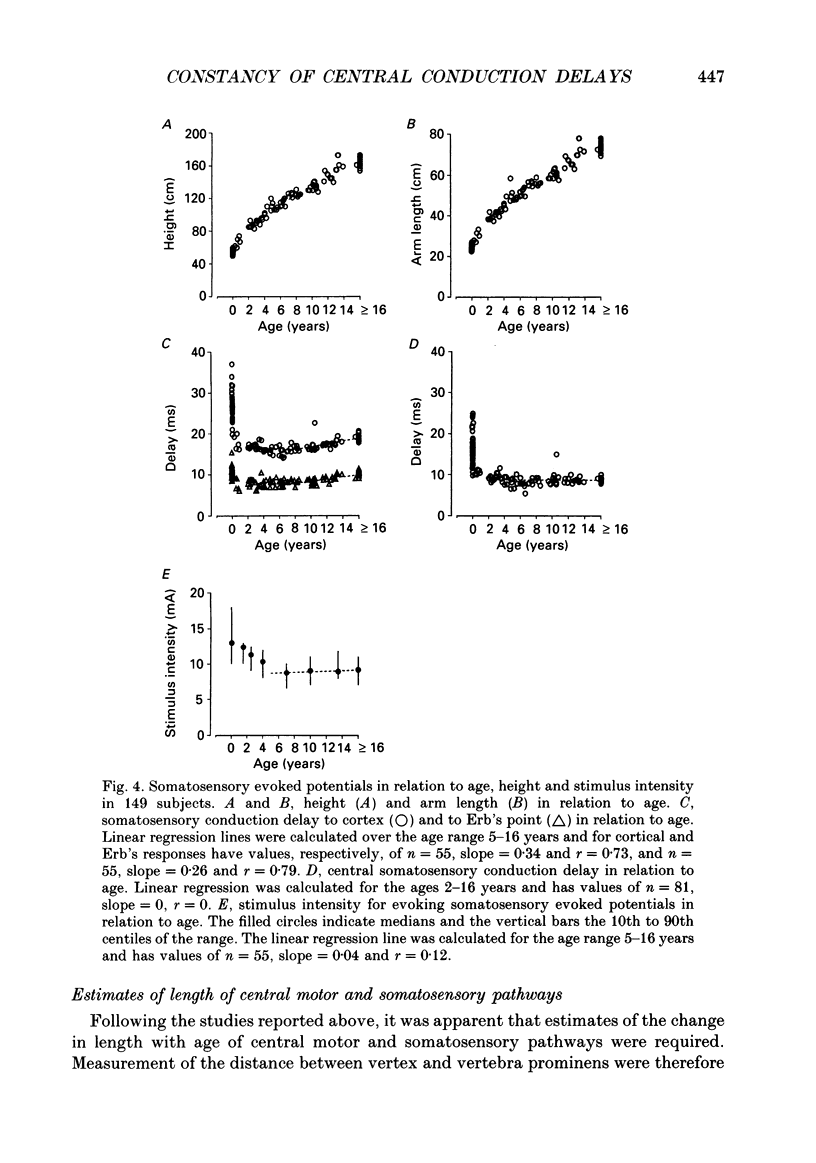



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker A. T., Freeston I. L., Jalinous R., Jarratt J. A. Magnetic stimulation of the human brain and peripheral nervous system: an introduction and the results of an initial clinical evaluation. Neurosurgery. 1987 Jan;20(1):100–109. doi: 10.1097/00006123-198701000-00024. [DOI] [PubMed] [Google Scholar]
- Barnet A. B., Friedman S. L., Weiss I. P., Ohlrich E. S., Shanks B., Lodge A. VEP development in infancy and early childhood. A longitudinal study. Electroencephalogr Clin Neurophysiol. 1980 Sep;49(5-6):476–489. doi: 10.1016/0013-4694(80)90390-9. [DOI] [PubMed] [Google Scholar]
- Cracco J. B., Cracco R. Q., Stolove R. Spinal evoked potential in man: a maturational study. Electroencephalogr Clin Neurophysiol. 1979 Jan;46(1):58–64. doi: 10.1016/0013-4694(79)90049-x. [DOI] [PubMed] [Google Scholar]
- Edgley S. A., Eyre J. A., Lemon R. N., Miller S. Excitation of the corticospinal tract by electromagnetic and electrical stimulation of the scalp in the macaque monkey. J Physiol. 1990 Jun;425:301–320. doi: 10.1113/jphysiol.1990.sp018104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisengart M. A. Reflex-arc latency measurements in newborn infants and children. Pediatrics. 1970 Jul;46(1):28–35. [PubMed] [Google Scholar]
- Gutrecht J. A., Dyck P. J. Quantitative teased-fiber and histologic studies of human sural nerve during postnatal development. J Comp Neurol. 1970 Jan;138(1):117–129. doi: 10.1002/cne.901380109. [DOI] [PubMed] [Google Scholar]
- Hume A. L., Cant B. R., Shaw N. A., Cowan J. C. Central somatosensory conduction time from 10 to 79 years. Electroencephalogr Clin Neurophysiol. 1982 Jul;54(1):49–54. doi: 10.1016/0013-4694(82)90230-9. [DOI] [PubMed] [Google Scholar]
- Koh T. H., Eyre J. A. Maturation of corticospinal tracts assessed by electromagnetic stimulation of the motor cortex. Arch Dis Child. 1988 Nov;63(11):1347–1352. doi: 10.1136/adc.63.11.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg A. Multisensory control of spinal reflex pathways. Prog Brain Res. 1979;50:11–28. doi: 10.1016/S0079-6123(08)60803-1. [DOI] [PubMed] [Google Scholar]
- Moskowitz A., Sokol S. Developmental changes in the human visual system as reflected by the latency of the pattern reversal VEP. Electroencephalogr Clin Neurophysiol. 1983 Jul;56(1):1–15. doi: 10.1016/0013-4694(83)90002-0. [DOI] [PubMed] [Google Scholar]
- NATHAN P. W., SMITH M. C. Long descending tracts in man. I. Review of present knowledge. Brain. 1955;78(2):248–303. doi: 10.1093/brain/78.2.248. [DOI] [PubMed] [Google Scholar]
- Neilson P. D., Neilson M. D., O'Dwyer N. J. Internal models and intermittency: a theoretical account of human tracking behavior. Biol Cybern. 1988;58(2):101–112. doi: 10.1007/BF00364156. [DOI] [PubMed] [Google Scholar]
- Plassman B. L., Gandevia S. C. High-voltage stimulation over the human spinal cord: sources of latency variation. J Neurol Neurosurg Psychiatry. 1989 Feb;52(2):213–217. doi: 10.1136/jnnp.52.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSHTON W. A. H. A theory of the effects of fibre size in medullated nerve. J Physiol. 1951 Sep;115(1):101–122. doi: 10.1113/jphysiol.1951.sp004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlandson P. H., Stephens J. A. Maturation of cutaneous reflex responses recorded in the lower limb in man. Dev Med Child Neurol. 1985 Aug;27(4):425–433. doi: 10.1111/j.1469-8749.1985.tb04565.x. [DOI] [PubMed] [Google Scholar]
- Sakamoto T., Arissian K., Asanuma H. Functional role of the sensory cortex in learning motor skills in cats. Brain Res. 1989 Dec 4;503(2):258–264. doi: 10.1016/0006-8993(89)91672-7. [DOI] [PubMed] [Google Scholar]
- Shucard D. W., Shucard J. L., Thomas D. G. Auditory event-related potentials in waking infants and adults: a developmental perspective. Electroencephalogr Clin Neurophysiol. 1987 Jul;68(4):303–310. doi: 10.1016/0168-5597(87)90051-7. [DOI] [PubMed] [Google Scholar]
- THOMAS J. E., LAMBERT E. H. Ulnar nerve conduction velocity and H-reflex in infants and children. J Appl Physiol. 1960 Jan;15:1–9. doi: 10.1152/jappl.1960.15.1.1. [DOI] [PubMed] [Google Scholar]
- Tanner J. M., Whitehouse R. H., Takaishi M. Standards from birth to maturity for height, weight, height velocity, and weight velocity: British children, 1965. II. Arch Dis Child. 1966 Dec;41(220):613–635. doi: 10.1136/adc.41.220.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. J., Fagan E. R. SEPs to median nerve stimulation: normative data for paediatrics. Electroencephalogr Clin Neurophysiol. 1988 Sep-Oct;71(5):323–330. doi: 10.1016/0168-5597(88)90034-2. [DOI] [PubMed] [Google Scholar]
- Vecchierini-Blineau M. F., Guiheneuc P. Electrophysiological study of the peripheral nervous system in children. Changes in proximal and distal conduction velocities from birth to age 5 years. J Neurol Neurosurg Psychiatry. 1979 Aug;42(8):753–759. doi: 10.1136/jnnp.42.8.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAGMAN I. H., LESSE H. Maximum conduction velocities of motor fibers of ulnar nerve in human subjects of various ages and sizes. J Neurophysiol. 1952 May;15(3):235–244. doi: 10.1152/jn.1952.15.3.235. [DOI] [PubMed] [Google Scholar]


