Abstract
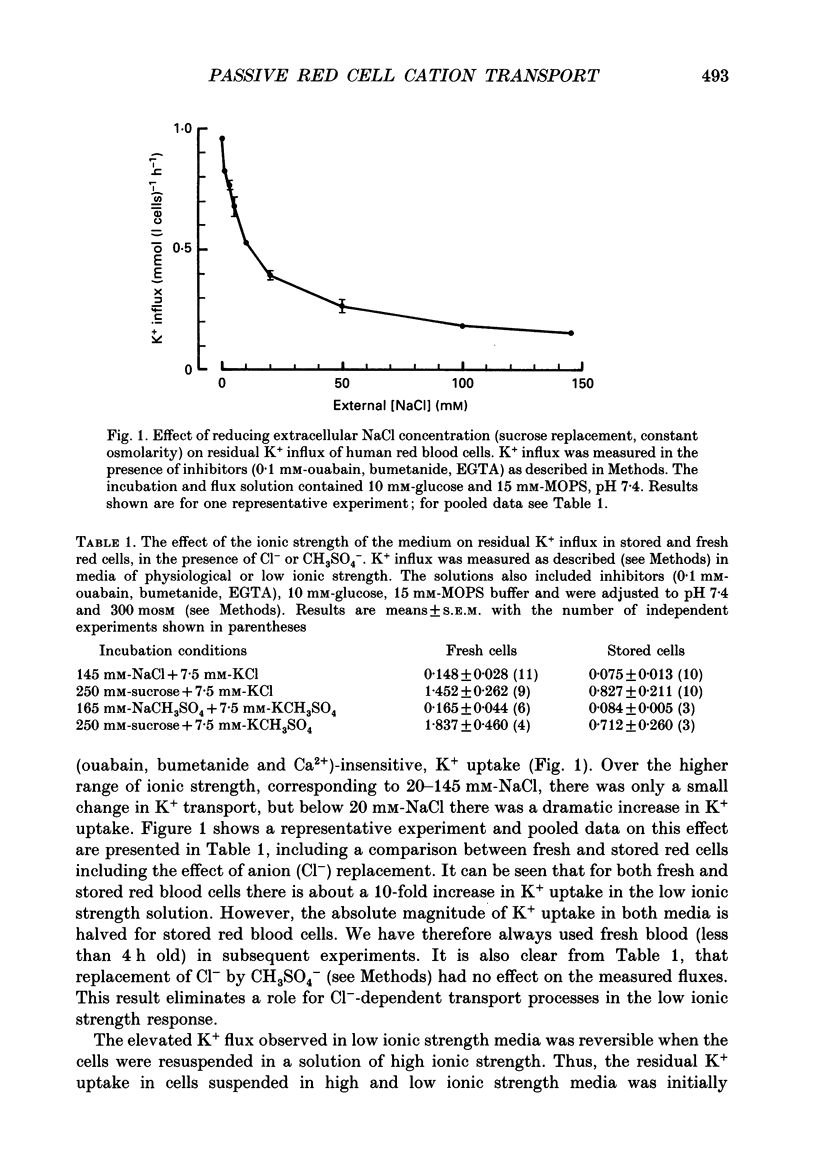
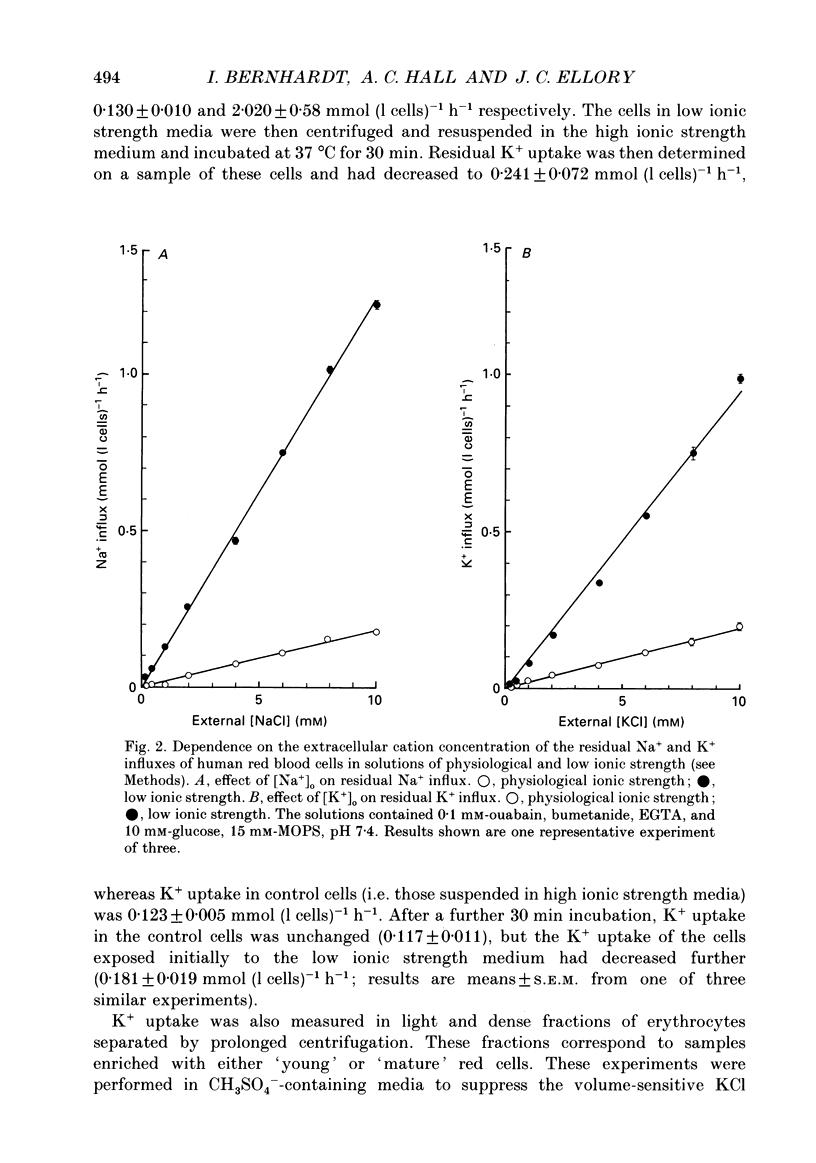
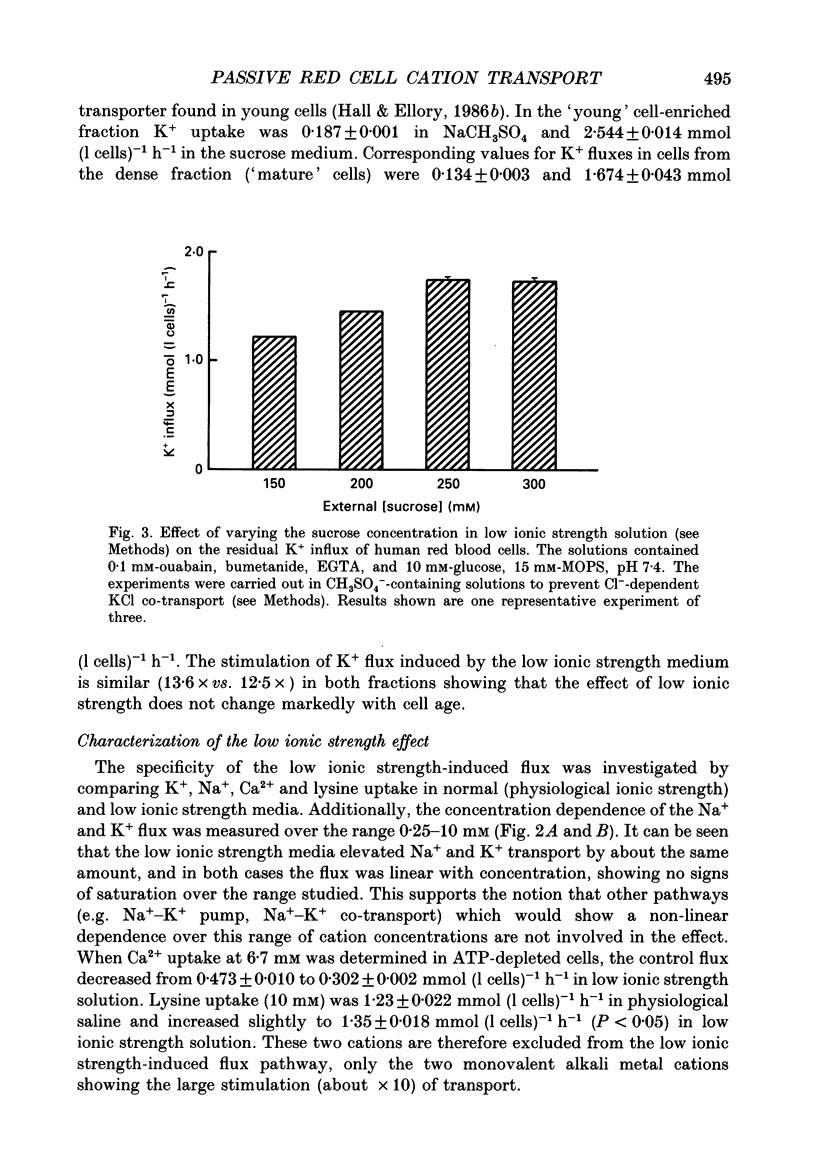
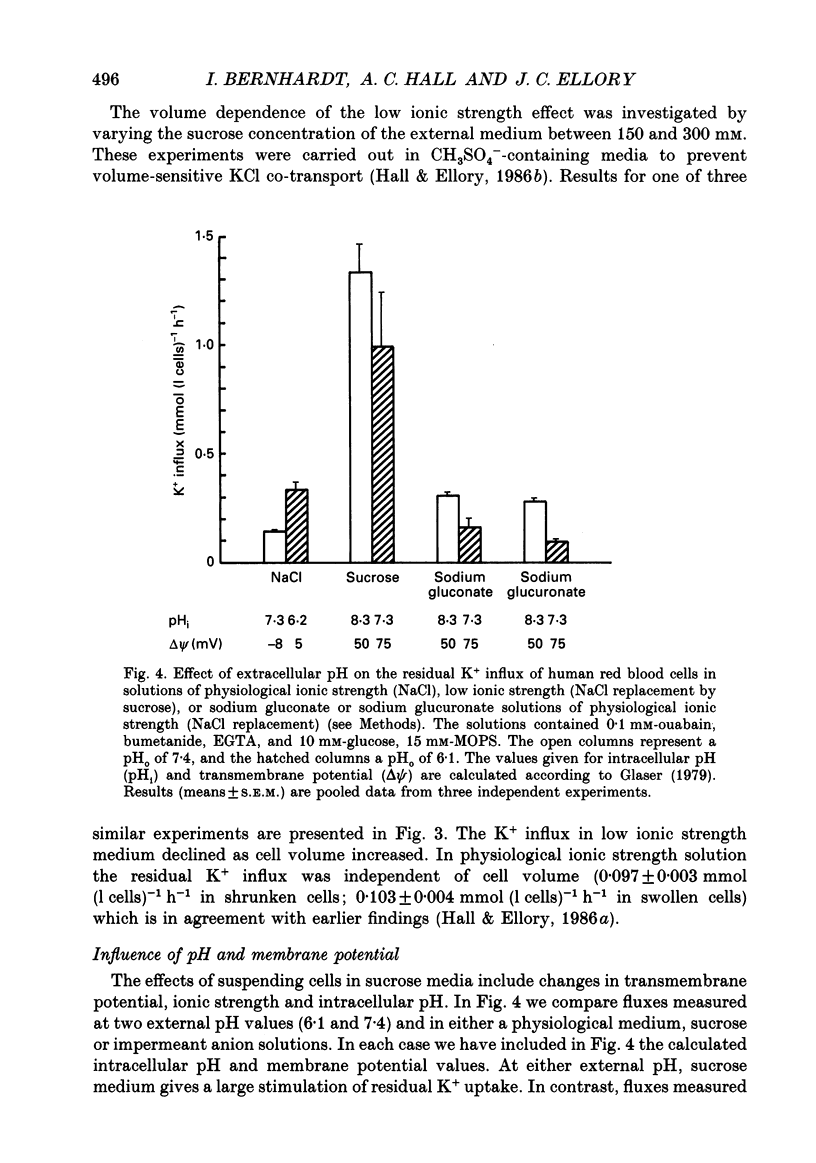
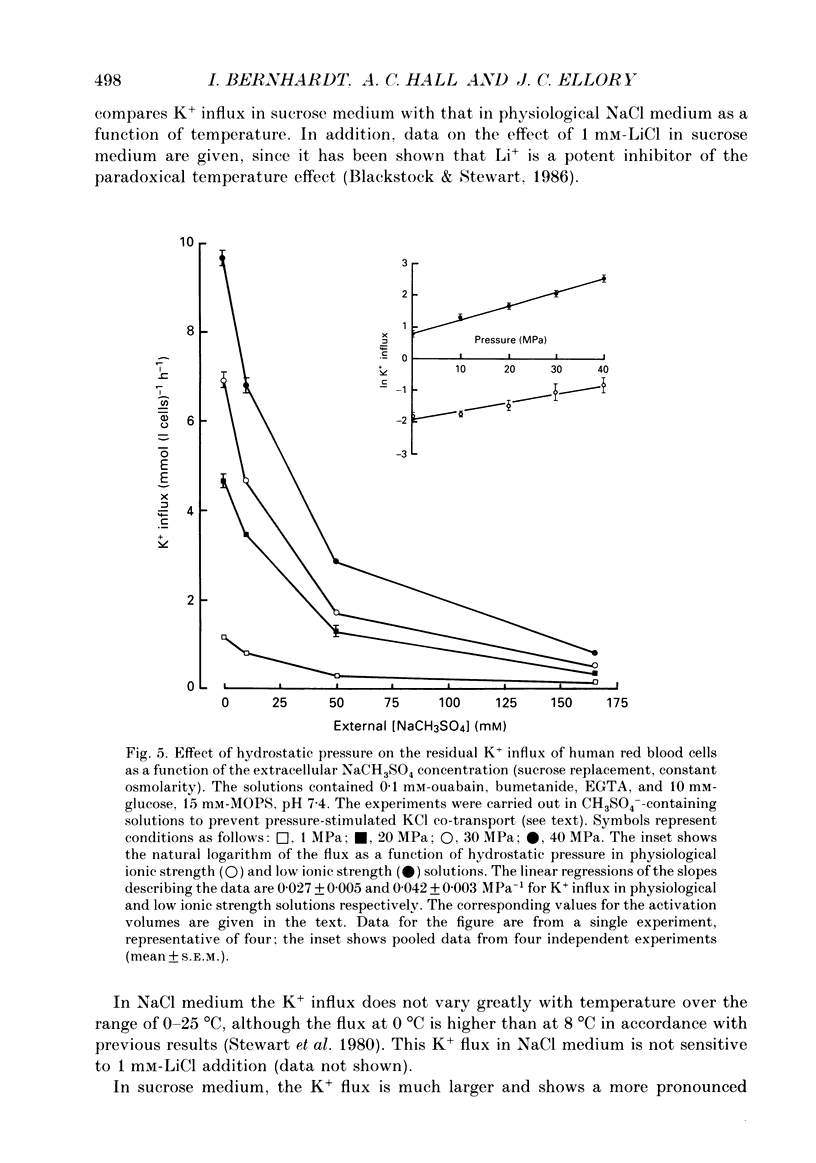
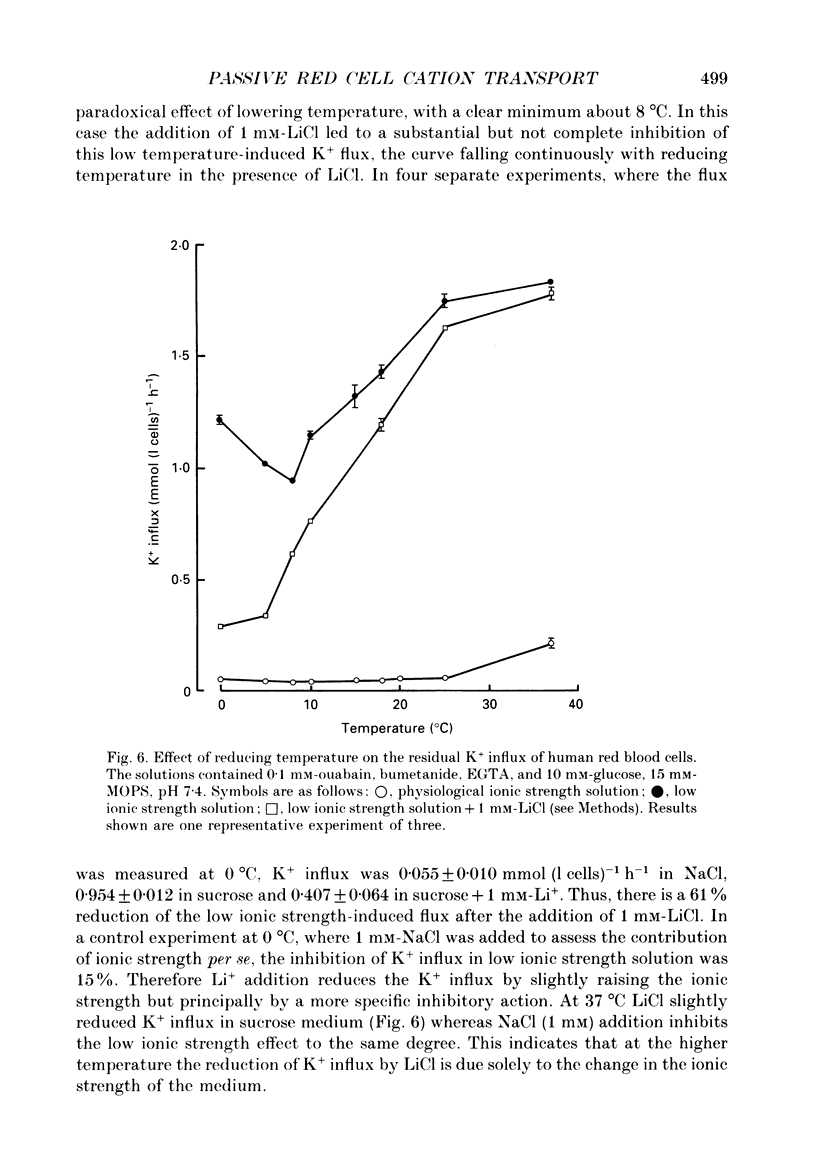
1. The effect of low ionic strength media on the residual, i.e. (ouabain + bumetanide + Ca2+)-insensitive, K+ influx was characterized in human red blood cells. 2. This K+ flux was enhanced significantly in isotonic solutions of low ionic strength using sucrose to maintain constant osmolarity. This effect was found for fresh red blood cells as well as for stored (bank) red blood cells. However, the absolute magnitude of K+ influx in solutions of low ionic strength was halved for stored red blood cells. 3. Anion replacement of Cl- by CH3SO4- did not affect residual K+ fluxes, showing that Cl- -dependent transport pathways (e.g. the KCl co-transporter) are not involved in the low ionic strength effect. 4. The enhanced K+ influx in low ionic strength media was reversible when the cells were resuspended in a solution of physiological ionic strength. 5. K+ influx measured in light and dense fractions of erythrocytes (separated by centrifugation and corresponding to samples enriched with either 'young' or 'mature' red cells) showed that the low ionic strength effect does not change markedly with cell age. 6. Low ionic strength media elevated residual, i.e. (ouabain + bumetanide + Ca2+)-insensitive, influx of both K+ and Na+ by about the same amount. In both cases the flux was linear with concentration in the range investigated (0.25-10 mM). No significant increase in the uptake of the cations Ca2+ and lysine in low ionic strength solutions could be found. 7. In CH3SO4- -containing solutions of physiological ionic strength the residual K+ influx was almost independent of cell volume, whereas this flux in CH3SO4- -containing solutions of low ionic strength declined as cell volume was increased. 8. K+ flux measurements in solutions of different external pH, where NaCl was replaced by sodium gluconate or sodium glucuronate, showed that the reduced ionic strength is of more importance for the enhanced residual K+ influx than the changed transmembrane potential or the changed intracellular pH. However, a small pH dependence could be found, the K+ flux passing through a minimum around pHi 7.3. 9. Hydrostatic pressure enhanced the residual K+ flux in media of low ionic strength synergistically, so that very large fluxes (greater than 10 mmol (1 cells)-1 h-1) were obtained at 40 MPa. The apparent activation volumes (delta V*) for the pressure-sensitive K+ flux were -108 and -69 ml mol-1 in low ionic strength or physiological ionic strength solutions respectively.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOLINGBROKE V., MAIZELS M. Calcium ions and the permeability of human erythrocytes. J Physiol. 1959 Dec;149:563–585. doi: 10.1113/jphysiol.1959.sp006361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt I., Borning M., Glaser R. Investigations on the control of ion transport in human erythrocytes. I. Passive 86Rb efflux and possibilities of its influence. Acta Biol Med Ger. 1982;41(6):531–539. [PubMed] [Google Scholar]
- Bernhardt I., Donath E., Glaser R. Influence of surface charge and transmembrane potential on rubidium-86 efflux of human red blood cells. J Membr Biol. 1984;78(3):249–255. doi: 10.1007/BF01925972. [DOI] [PubMed] [Google Scholar]
- Bernhardt I., Glaser R. Investigations on the control of ion transport in human erythrocytes. II. Influence of transmembrane potential, exterior surface potential and intracellular pH on the 22Na efflux. Acta Biol Med Ger. 1982;41(6):541–547. [PubMed] [Google Scholar]
- Blackstock E. J., Stewart G. W. The dependence on external cation of sodium and potassium fluxes across the human red cell membrane at low temperatures. J Physiol. 1986 Jun;375:403–420. doi: 10.1113/jphysiol.1986.sp016124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs J. M. Intermolecular hydrogen bonding between lipids: influence on organization and function of lipids in membranes. Can J Biochem. 1980 Oct;58(10):755–770. doi: 10.1139/o80-107. [DOI] [PubMed] [Google Scholar]
- Canessa M., Brugnara C., Cusi D., Tosteson D. C. Modes of operation and variable stoichiometry of the furosemide- sensitive Na and K fluxes in human red cells. J Gen Physiol. 1986 Jan;87(1):113–142. doi: 10.1085/jgp.87.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield V. A., Macey R. I. Anion exchange in human erythrocytes has a large activation volume. Biochim Biophys Acta. 1984 Dec 5;778(2):379–384. doi: 10.1016/0005-2736(84)90382-1. [DOI] [PubMed] [Google Scholar]
- Carolin D. A., Maizels M. Effect of the duration of loading lactose-treated red cells with cations on the rate of subsequent cation efflux. J Physiol. 1965 Jul;179(1):54–94. doi: 10.1113/jphysiol.1965.sp007649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipperfield A. R., Shennan D. B. The influence of pH and membrane potential on passive Na+ and K+ fluxes in human red blood cells. Biochim Biophys Acta. 1986 May 29;886(3):373–382. doi: 10.1016/0167-4889(86)90172-2. [DOI] [PubMed] [Google Scholar]
- Chipperfield A. R. The (Na+-K+-Cl-) co-transport system. Clin Sci (Lond) 1986 Nov;71(5):465–476. doi: 10.1042/cs0710465. [DOI] [PubMed] [Google Scholar]
- Davson H. Studies on the permeability of erythrocytes: The effect of reducing the salt content of the medium surrounding the cell. Biochem J. 1939 Mar;33(3):389–401. doi: 10.1042/bj0330389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuticke B., Poser B., Lütkemeier P., Haest C. W. Formation of aqueous pores in the human erythrocyte membrane after oxidative cross-linking of spectrin by diamide. Biochim Biophys Acta. 1983 Jun 10;731(2):196–210. doi: 10.1016/0005-2736(83)90009-3. [DOI] [PubMed] [Google Scholar]
- Dunham P. B., Stewart G. W., Ellory J. C. Chloride-activated passive potassium transport in human erythrocytes. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1711–1715. doi: 10.1073/pnas.77.3.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellory J. C., Dunham P. B., Logue P. J., Stewart G. W. Anion-dependent cation transport in erythrocytes. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 1;299(1097):483–495. doi: 10.1098/rstb.1982.0146. [DOI] [PubMed] [Google Scholar]
- Ellory J. C., Hall A. C. Human red cell volume regulation in hypotonic media. Comp Biochem Physiol A Comp Physiol. 1988;90(4):533–537. doi: 10.1016/0300-9629(88)90663-9. [DOI] [PubMed] [Google Scholar]
- Escobales N., Canessa M. Amiloride-sensitive Na+ transport in human red cells: evidence for a Na/H exchange system. J Membr Biol. 1986;90(1):21–28. doi: 10.1007/BF01869682. [DOI] [PubMed] [Google Scholar]
- Glaser R. The shape of red blood cells as a function of membrane potential and temperature. J Membr Biol. 1979 Dec 31;51(3-4):217–228. doi: 10.1007/BF01869085. [DOI] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M., Schmidt W. F., 3rd p-Chloromercuribenzenesulfonic acid stimulation of chloride-dependent sodium and potassium transport in human red blood cells. Biochim Biophys Acta. 1985 Mar 28;814(1):43–49. doi: 10.1016/0005-2736(85)90417-1. [DOI] [PubMed] [Google Scholar]
- Hall A. C., Ellory J. C. Effects of high hydrostatic pressure on 'passive' monovalent cation transport in human red cells. J Membr Biol. 1986;94(1):1–17. doi: 10.1007/BF01901009. [DOI] [PubMed] [Google Scholar]
- Hall A. C., Ellory J. C. Evidence for the presence of volume-sensitive KCl transport in 'young' human red cells. Biochim Biophys Acta. 1986 Jun 26;858(2):317–320. doi: 10.1016/0005-2736(86)90338-x. [DOI] [PubMed] [Google Scholar]
- Hall A. C., Ellory J. C. Measurement and stoichiometry of bumetanide-sensitive (2Na:1K:3Cl) cotransport in ferret red cells. J Membr Biol. 1985;85(3):205–213. doi: 10.1007/BF01871515. [DOI] [PubMed] [Google Scholar]
- Hall A. C., Willis J. S. The temperature dependence of passive potassium permeability in mammalian erythrocytes. Cryobiology. 1986 Oct;23(5):395–405. doi: 10.1016/0011-2240(86)90024-6. [DOI] [PubMed] [Google Scholar]
- Halperin J. A., Brugnara C., Tosteson M. T., Van Ha T., Tosteson D. C. Voltage-activated cation transport in human erythrocytes. Am J Physiol. 1989 Nov;257(5 Pt 1):C986–C996. doi: 10.1152/ajpcell.1989.257.5.C986. [DOI] [PubMed] [Google Scholar]
- Heller K. B., Poser B., Haest C. W., Deuticke B. Oxidative stress of human erythrocytes by iodate and periodate. Reversible formation of aqueous membrane pores due to SH-group oxidation. Biochim Biophys Acta. 1984 Oct 17;777(1):107–116. doi: 10.1016/0005-2736(84)90502-9. [DOI] [PubMed] [Google Scholar]
- Jarvis S. M., Hammond J. R., Paterson A. R., Clanachan A. S. Species differences in nucleoside transport. A study of uridine transport and nitrobenzylthioinosine binding by mammalian erythrocytes. Biochem J. 1982 Oct 15;208(1):83–88. doi: 10.1042/bj2080083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. S., Knauf P. A. Mechanism of the increase in cation permeability of human erythrocytes in low-chloride media. Involvement of the anion transport protein capnophorin. J Gen Physiol. 1985 Nov;86(5):721–738. doi: 10.1085/jgp.86.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf P. A., Rothstein A. Chemical modification of membranes. II. Permeation paths for sulfhydryl agents. J Gen Physiol. 1971 Aug;58(2):211–223. doi: 10.1085/jgp.58.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kracke G. R., Dunham P. B. Effect of membrane potential on furosemide-inhibitable sodium influxes in human red blood cells. J Membr Biol. 1987;98(2):117–124. doi: 10.1007/BF01872124. [DOI] [PubMed] [Google Scholar]
- LaCelle P. L., Rothsteto A. The passive permeability of the red blood cell in cations. J Gen Physiol. 1966 Sep;50(1):171–188. doi: 10.1085/jgp.50.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew V. L. On the ATP dependence of the Ca 2+ -induced increase in K + permeability observed in human red cells. Biochim Biophys Acta. 1971 Jun 1;233(3):827–830. doi: 10.1016/0005-2736(71)90185-4. [DOI] [PubMed] [Google Scholar]
- Macdonald A. G. The effects of pressure on the molecular structure and physiological functions of cell membranes. Philos Trans R Soc Lond B Biol Sci. 1984 Jan 7;304(1118):47–68. doi: 10.1098/rstb.1984.0008. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Wohlhueter R. M. Kinetics of nucleoside transport in human erythrocytes. Alterations during blood preservation. Biochim Biophys Acta. 1984 Nov 21;778(1):176–184. doi: 10.1016/0005-2736(84)90460-7. [DOI] [PubMed] [Google Scholar]
- Solomon A. K., Chasan B., Dix J. A., Lukacovic M. F., Toon M. R., Verkman A. S. The aqueous pore in the red cell membrane: band 3 as a channel for anions, cations, nonelectrolytes, and water. Ann N Y Acad Sci. 1983;414:97–124. doi: 10.1111/j.1749-6632.1983.tb31678.x. [DOI] [PubMed] [Google Scholar]
- Stewart G. W., Ellory J. C., Klein R. A. Increased human red cell cation passive permeability below 12 degrees C. Nature. 1980 Jul 24;286(5771):403–404. doi: 10.1038/286403a0. [DOI] [PubMed] [Google Scholar]
- TOSTESON D. C., HOFFMAN J. F. Regulation of cell volume by active cation transport in high and low potassium sheep red cells. J Gen Physiol. 1960 Sep;44:169–194. doi: 10.1085/jgp.44.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser M. B., Razin M., Stein W. D. Kinetic tests of models for sugar transport in human erythrocytes and a comparison of fresh and cold-stored cells. Biochim Biophys Acta. 1983 Jan 19;727(2):379–388. doi: 10.1016/0005-2736(83)90423-6. [DOI] [PubMed] [Google Scholar]
- Zade-Oppen A. M., Adragna N. C., Tosteson D. C. Effects of pH, potential, chloride and furosemide on passive Na+ and K+ effluxes from human red blood cells. J Membr Biol. 1988 Aug;103(3):217–225. doi: 10.1007/BF01993981. [DOI] [PubMed] [Google Scholar]


