Abstract
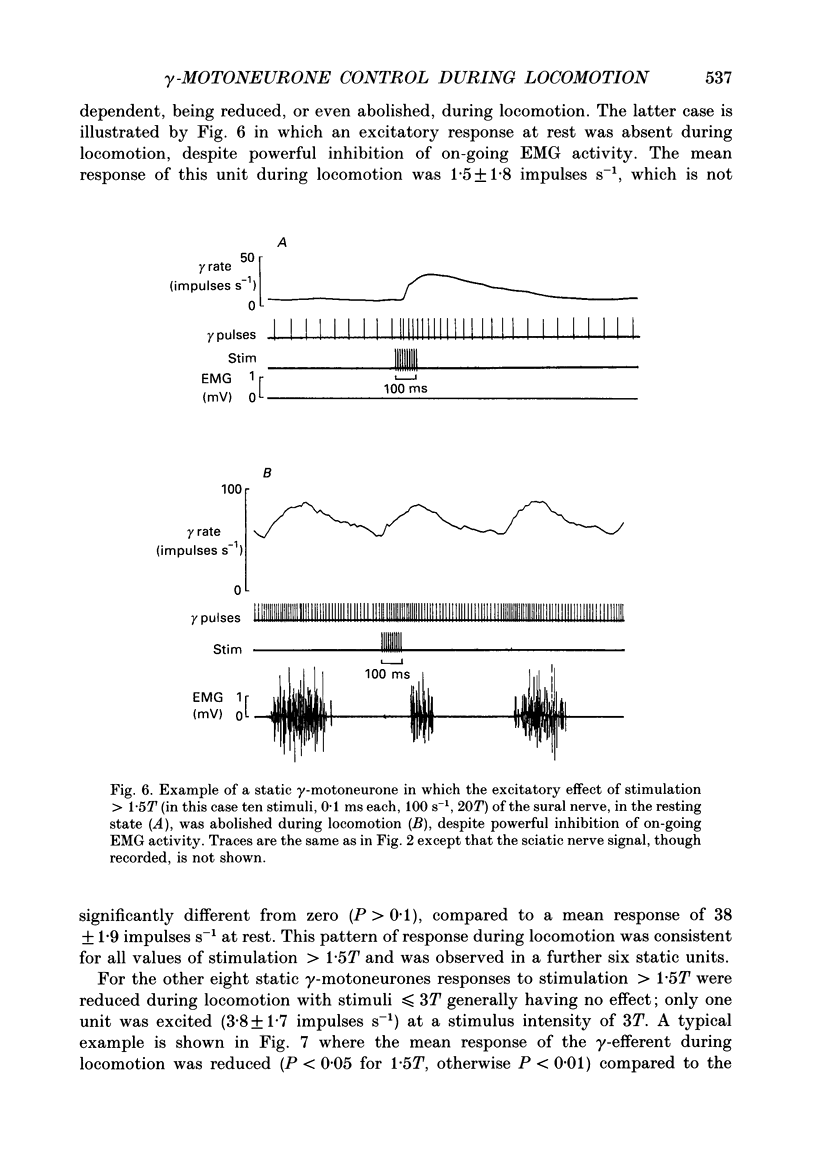
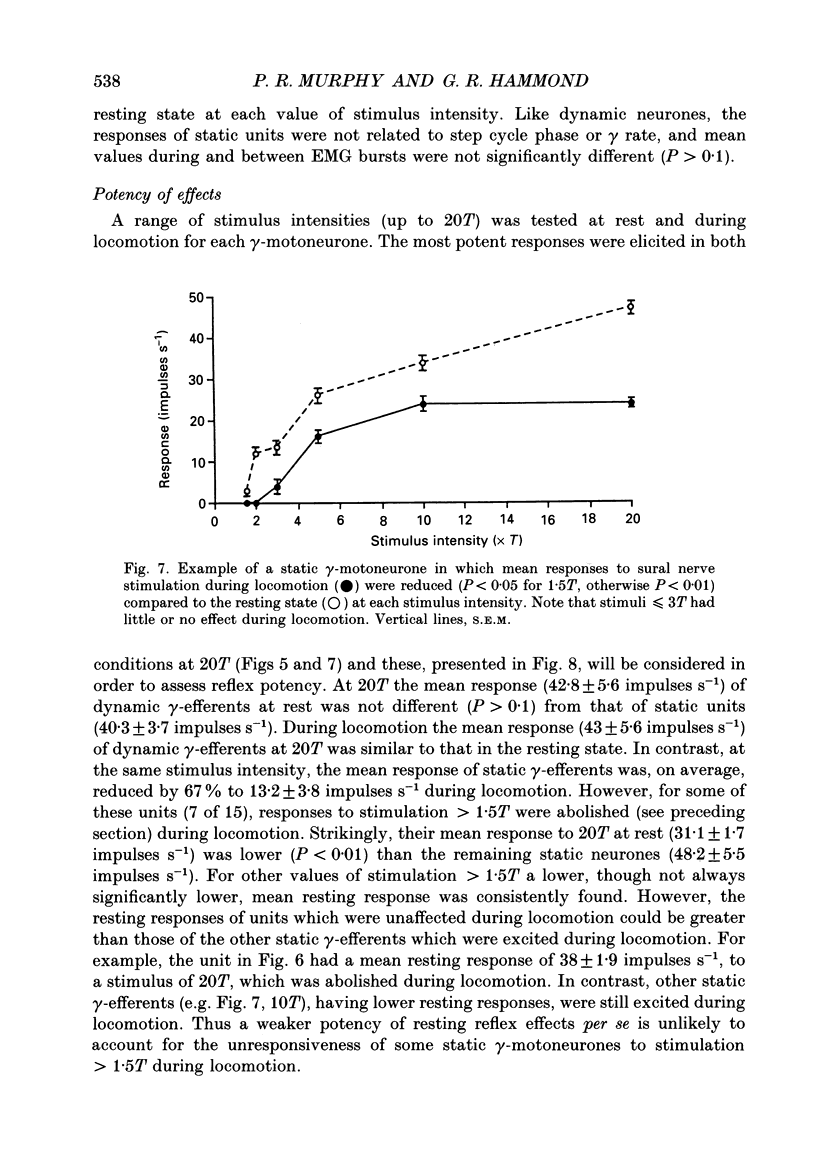
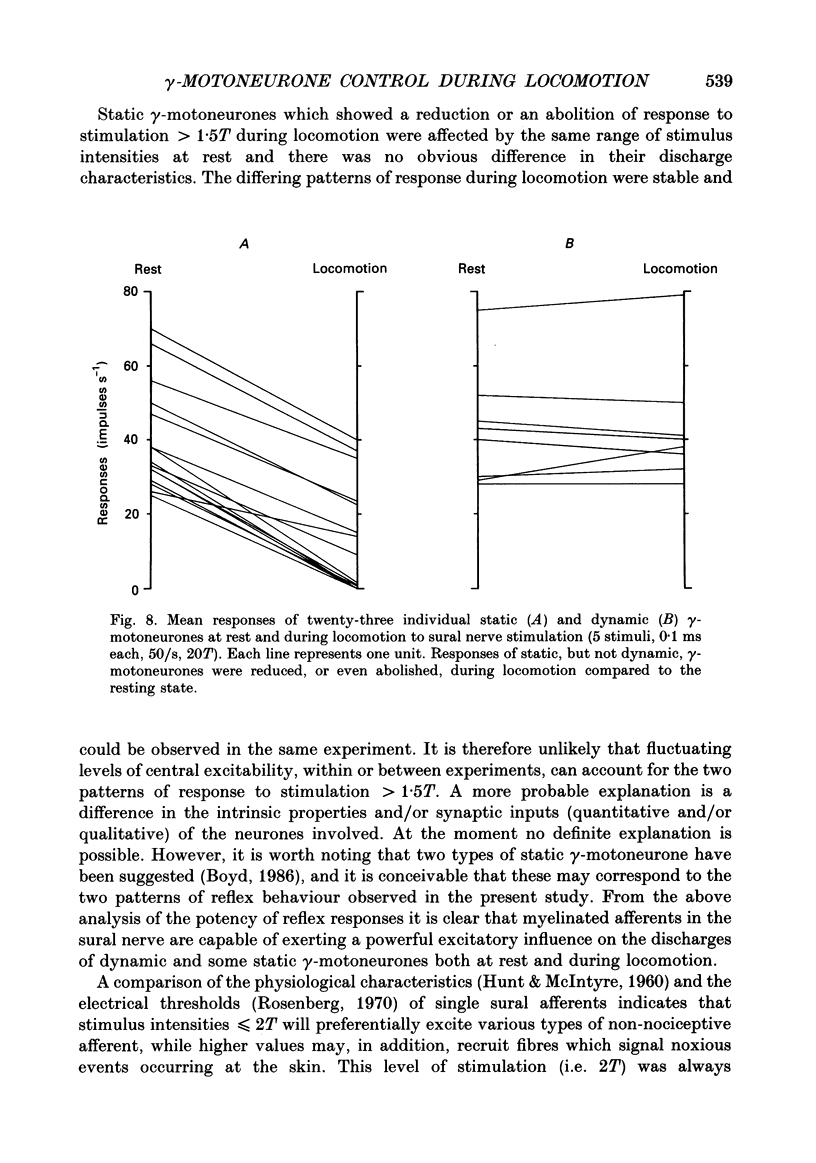
1. The effect of electrical stimulation, up to 20x threshold (T), of the sural nerve on the discharges of single medial gastrocnemius static and dynamic gamma-motoneurones has been investigated at rest and during locomotion in the decerebrate cat. 2. A total of twenty-three gamma-motoneurones were recorded. The neurones were identified as static (15) or dynamic (8) on the basis of their discharge characteristics (Murphy, Stein & Taylor, 1984). 3. Low intensity stimulation (less than or equal to 1.5T) had no effect on the discharges of most (22 of 23) gamma-efferents at rest or during locomotion. Hence the largest afferents in the sural nerve had little influence on the discharges of static or dynamic gamma-motoneurones in either condition. 4. Higher intensity stimulation (greater than 1.5T) excited both types of gamma-efferent in the resting state and response size was graded with stimulus intensity. For most neurones (20 of 23) excitatory effects appeared in the range 1.5-2T. 5. Stimulation at intensities greater than 1.5T also excited dynamic and some static gamma-motoneurones during locomotion. The responses of dynamic gamma-motoneurones were unchanged during locomotion compared to the resting state. In contrast, the responses of static neurones were significantly reduced, or even abolished, during locomotion and stimuli less than or equal to 3T generally (12 of 13) had no effect. Thus the responses of static, but not dynamic, gamma-efferents were task dependent. Further, the thresholds of responses indicate that activation of low threshold mechanoreceptors in the sural receptive field excites both types of gamma-efferent at rest, and dynamic neurones during locomotion. In contrast, it is proposed that the same peripheral input does not affect static gamma-efferents during locomotion. 6. The responses of static and dynamic gamma-motoneurones during locomotion were not obviously related to step cycle phase, or gamma rate, and responses occurring during or between homonymous electromyogram (EMG) bursts were not significantly different. Thus gamma responses during locomotion were not phase dependent. 7. Stimulation at intensities greater than 3T excited dynamic and some static gamma-motoneurones during locomotion but simultaneously inhibited on-going EMG activity. Peripheral inputs are therefore capable of influencing alpha- and gamma-motoneurones independently during locomotion. 8. The significance of the results is discussed in relation to the control and function of gamma-motoneurones.
Full text
PDF



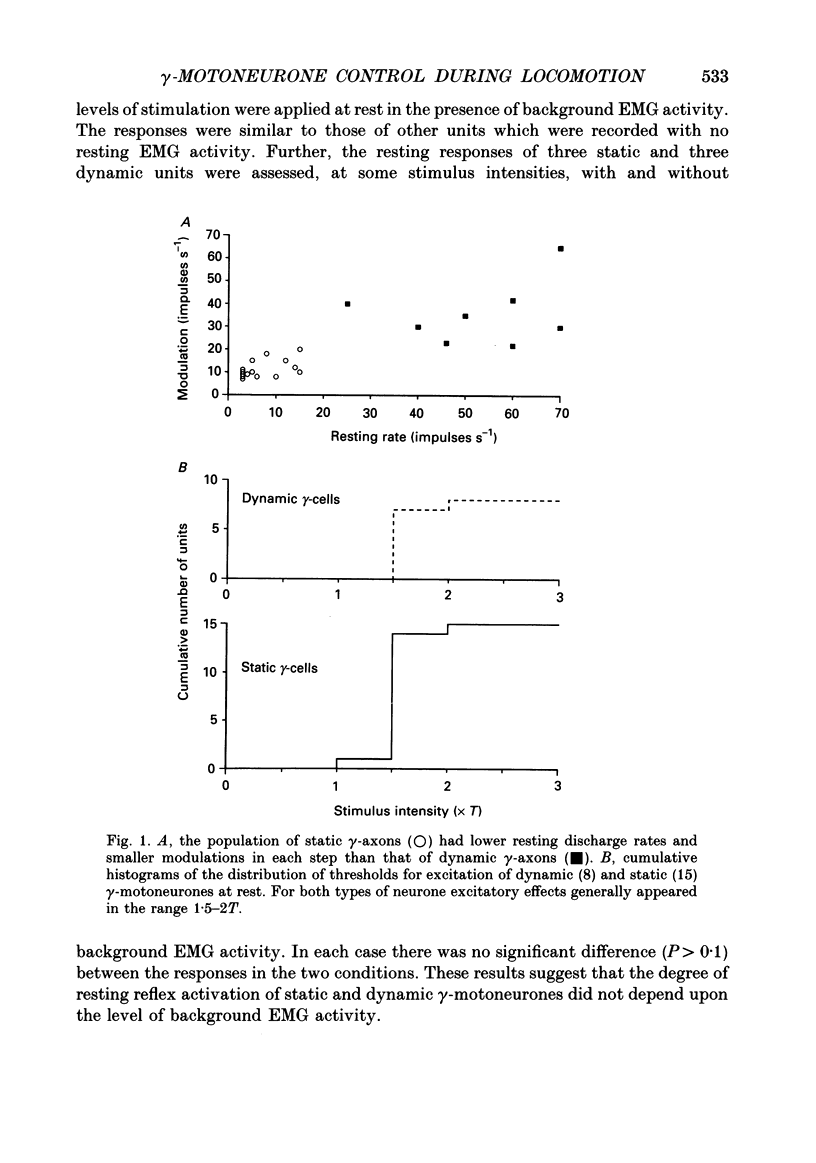








Selected References
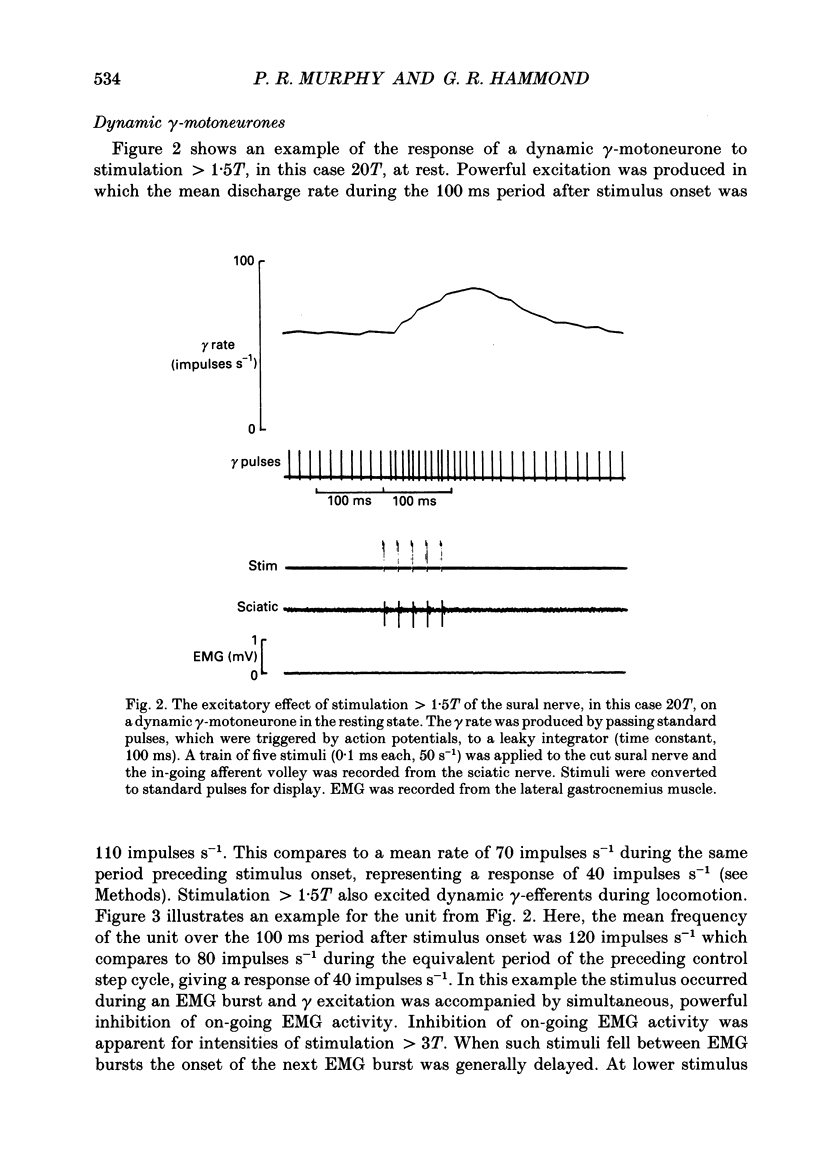
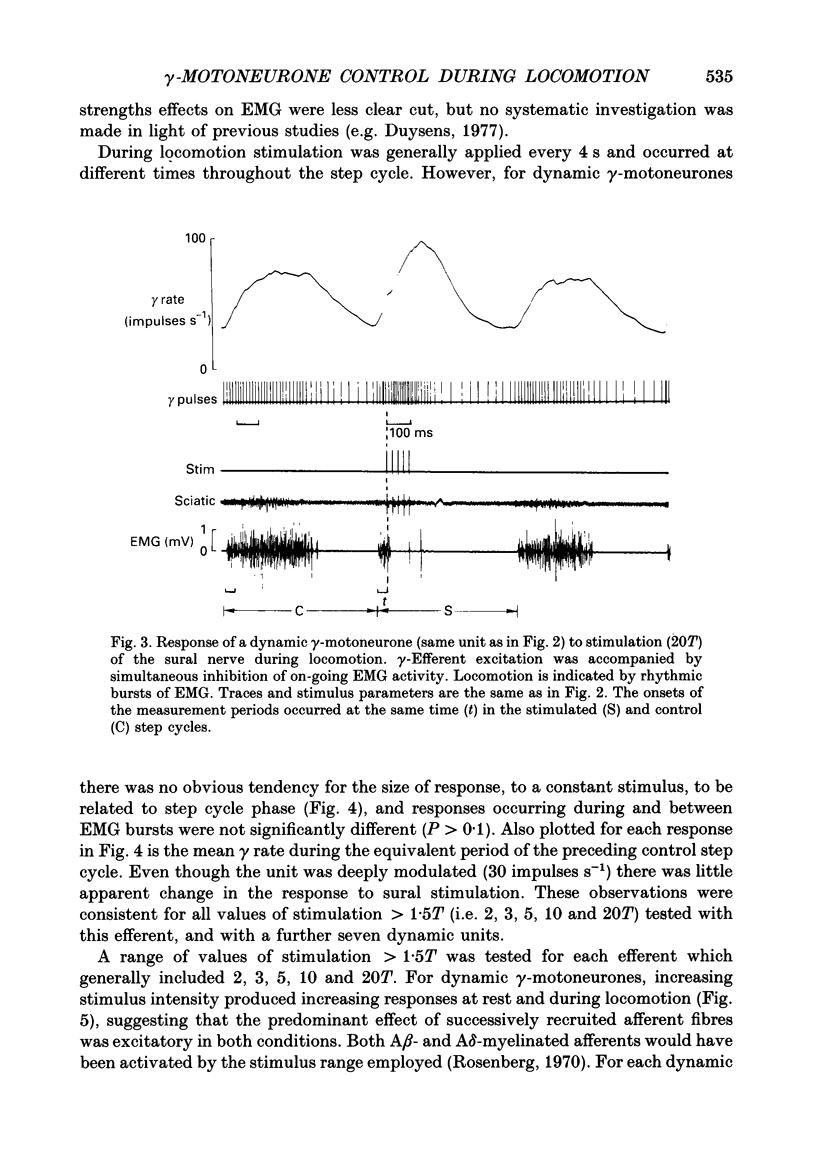
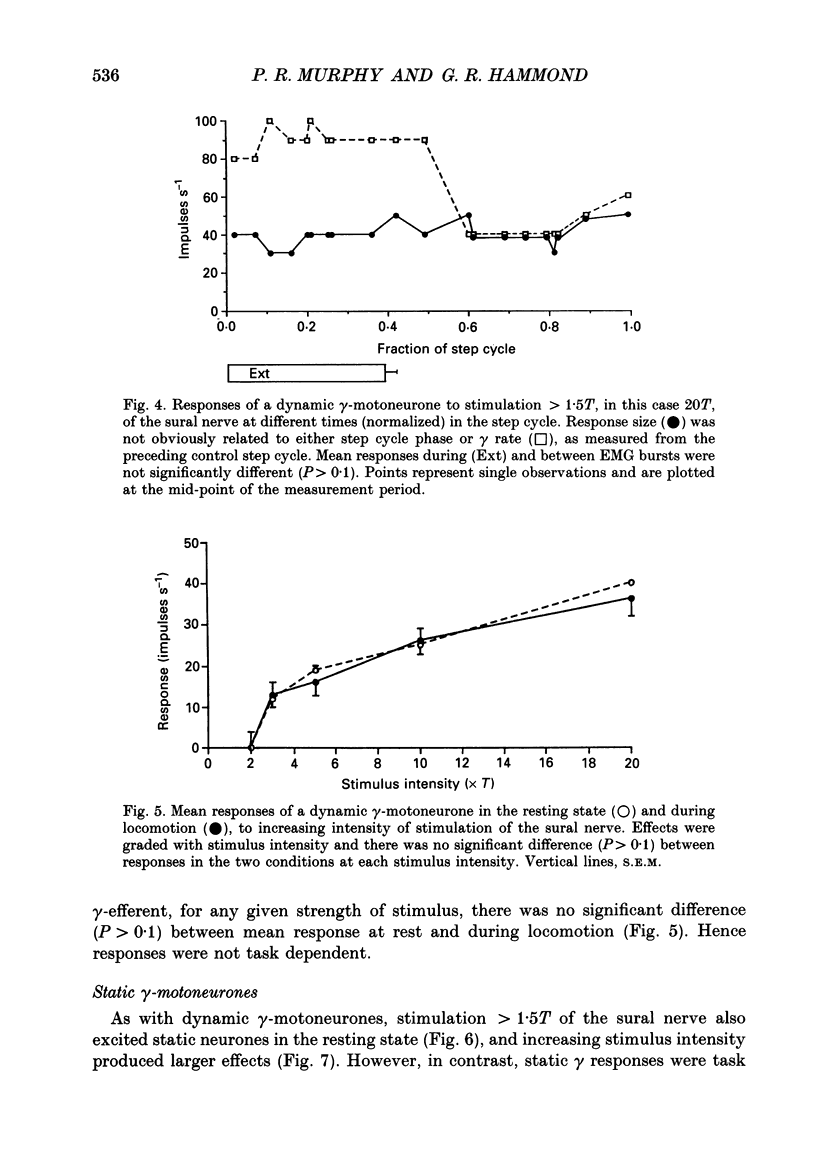
These references are in PubMed. This may not be the complete list of references from this article.
- Akazawa K., Aldridge J. W., Steeves J. D., Stein R. B. Modulation of stretch reflexes during locomotion in the mesencephalic cat. J Physiol. 1982 Aug;329:553–567. doi: 10.1113/jphysiol.1982.sp014319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg B., Hulliger M., Johansson H., Sojka P. Actions on gamma-motoneurones elicited by electrical stimulation of group III muscle afferent fibres in the hind limb of the cat. J Physiol. 1983 Feb;335:275–292. doi: 10.1113/jphysiol.1983.sp014533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd I. A., Gladden M. H., McWilliam P. N., Ward J. Control of dynamic and static nuclear bag fibres and nuclear chain fibres by gamma and beta axons in isolated cat muscle spindels. J Physiol. 1977 Feb;265(1):133–162. doi: 10.1113/jphysiol.1977.sp011709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd I. A. Two types of static gamma-axon in cat muscle spindles. Q J Exp Physiol. 1986 Apr;71(2):307–327. doi: 10.1113/expphysiol.1986.sp002987. [DOI] [PubMed] [Google Scholar]
- Campbell G. D., Edwards F. R., Hirst G. D., O'Shea J. E. Effects of vagal stimulation and applied acetylcholine on pacemaker potentials in the guinea-pig heart. J Physiol. 1989 Aug;415:57–68. doi: 10.1113/jphysiol.1989.sp017711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaday C., Stein R. B. Amplitude modulation of the soleus H-reflex in the human during walking and standing. J Neurosci. 1986 May;6(5):1308–1313. doi: 10.1523/JNEUROSCI.06-05-01308.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey N. J., Ellaway P. H. Facilitation of individual gamma-motoneurones by the discharge of single slowly adapting type 1 mechanoreceptors in cats. J Physiol. 1989 Apr;411:97–114. doi: 10.1113/jphysiol.1989.sp017563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dueñas S. H., Rudomin P. Excitability changes of ankle extensor group Ia and Ib fibers during fictive locomotion in the cat. Exp Brain Res. 1988;70(1):15–25. doi: 10.1007/BF00271842. [DOI] [PubMed] [Google Scholar]
- Duysens J. Reflex control of locomotion as revealed by stimulation of cutaneous afferents in spontaneously walking premammillary cats. J Neurophysiol. 1977 Jul;40(4):737–751. doi: 10.1152/jn.1977.40.4.737. [DOI] [PubMed] [Google Scholar]
- Emonet-Dénand F., Laporte Y., Matthews P. B., Petit J. On the subdivision of static and dynamic fusimotor actions on the primary ending of the cat muscle spindle. J Physiol. 1977 Jul;268(3):827–861. doi: 10.1113/jphysiol.1977.sp011884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans A. L., Harrison L. M., Stephens J. A. Task-dependent changes in cutaneous reflexes recorded from various muscles controlling finger movement in man. J Physiol. 1989 Nov;418:1–12. doi: 10.1113/jphysiol.1989.sp017825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C., McINTYRE A. K. An analysis of fibre diameter and receptor characteristics of myelinated cutaneous afferent fibres in cat. J Physiol. 1960 Aug;153:99–112. doi: 10.1113/jphysiol.1960.sp006521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson H., Sojka P. Actions on gamma-motoneurones elicited by electrical stimulation of cutaneous afferent fibres in the hind limb of the cat. J Physiol. 1985 Sep;366:343–363. doi: 10.1113/jphysiol.1985.sp015802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W., HUNT C. C., QUILLIAM J. P. Function of medullated small-nerve fibers in mammalian ventral roots; efferent muscle spindle innervation. J Neurophysiol. 1951 Jan;14(1):29–54. doi: 10.1152/jn.1951.14.1.29. [DOI] [PubMed] [Google Scholar]
- Lennerstrand G., Thoden U. Position and velocity sensitivity of muscle spindles in the cat. 3. Static fusimotor single-fibre activation of primary and secondary endings. Acta Physiol Scand. 1968 Sep-Oct;74(1):30–49. doi: 10.1111/j.1748-1716.1968.tb04212.x. [DOI] [PubMed] [Google Scholar]
- Loeb G. E., Duysens J. Activity patterns in individual hindlimb primary and secondary muscle spindle afferents during normal movements in unrestrained cats. J Neurophysiol. 1979 Mar;42(2):420–440. doi: 10.1152/jn.1979.42.2.420. [DOI] [PubMed] [Google Scholar]
- Loeb G. E., Hoffer J. A., Marks W. B. Activity of spindle afferents from cat anterior thigh muscles. III. Effects of external stimuli. J Neurophysiol. 1985 Sep;54(3):578–591. doi: 10.1152/jn.1985.54.3.578. [DOI] [PubMed] [Google Scholar]
- Loeb G. E. Somatosensory unit input to the spinal cord during normal walking. Can J Physiol Pharmacol. 1981 Jul;59(7):627–635. doi: 10.1139/y81-097. [DOI] [PubMed] [Google Scholar]
- MATTHEWS P. B. The differentiation of two types of fusimotor fibre by their effects on the dynamic response of muscle spindle primary endings. Q J Exp Physiol Cogn Med Sci. 1962 Oct;47:324–333. doi: 10.1113/expphysiol.1962.sp001616. [DOI] [PubMed] [Google Scholar]
- Murphy P. R., Hammond G. R. Gamma-motoneurone discharge patterns during fictive locomotion in the decerebrate cat. Exp Physiol. 1990 Jan;75(1):107–110. doi: 10.1113/expphysiol.1990.sp003376. [DOI] [PubMed] [Google Scholar]
- Murphy P. R., Stein R. B., Taylor J. Phasic and tonic modulation of impulse rates in gamma-motoneurons during locomotion in premammillary cats. J Neurophysiol. 1984 Aug;52(2):228–243. doi: 10.1152/jn.1984.52.2.228. [DOI] [PubMed] [Google Scholar]
- Prochazka A., Hulliger M., Trend P., Llewellyn M., Dürmüller N. Muscle afferent contribution to control of paw shakes in normal cats. J Neurophysiol. 1989 Mar;61(3):550–562. doi: 10.1152/jn.1989.61.3.550. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Sensorimotor gain control: a basic strategy of motor systems? Prog Neurobiol. 1989;33(4):281–307. doi: 10.1016/0301-0082(89)90004-x. [DOI] [PubMed] [Google Scholar]
- Prochazka A., Westerman R. A., Ziccone S. P. Ia afferent activity during a variety of voluntary movements in the cat. J Physiol. 1977 Jun;268(2):423–448. doi: 10.1113/jphysiol.1977.sp011864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M. E. Synaptic connexions of alpha extensor motoneurones with ipsilateral and contralateral cutaneous nerves. J Physiol. 1970 Mar;207(1):231–255. doi: 10.1113/jphysiol.1970.sp009058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B. J., Meyers D. E., Tokuriki M., Burke R. E. Modulation of short latency cutaneous excitation in flexor and extensor motoneurons during fictive locomotion in the cat. Exp Brain Res. 1989;77(1):57–68. doi: 10.1007/BF00250567. [DOI] [PubMed] [Google Scholar]
- Taylor J., Stein R. B., Murphy P. R. Impulse rates and sensitivity to stretch of soleus muscle spindle afferent fibers during locomotion in premammillary cats. J Neurophysiol. 1985 Feb;53(2):341–360. doi: 10.1152/jn.1985.53.2.341. [DOI] [PubMed] [Google Scholar]


