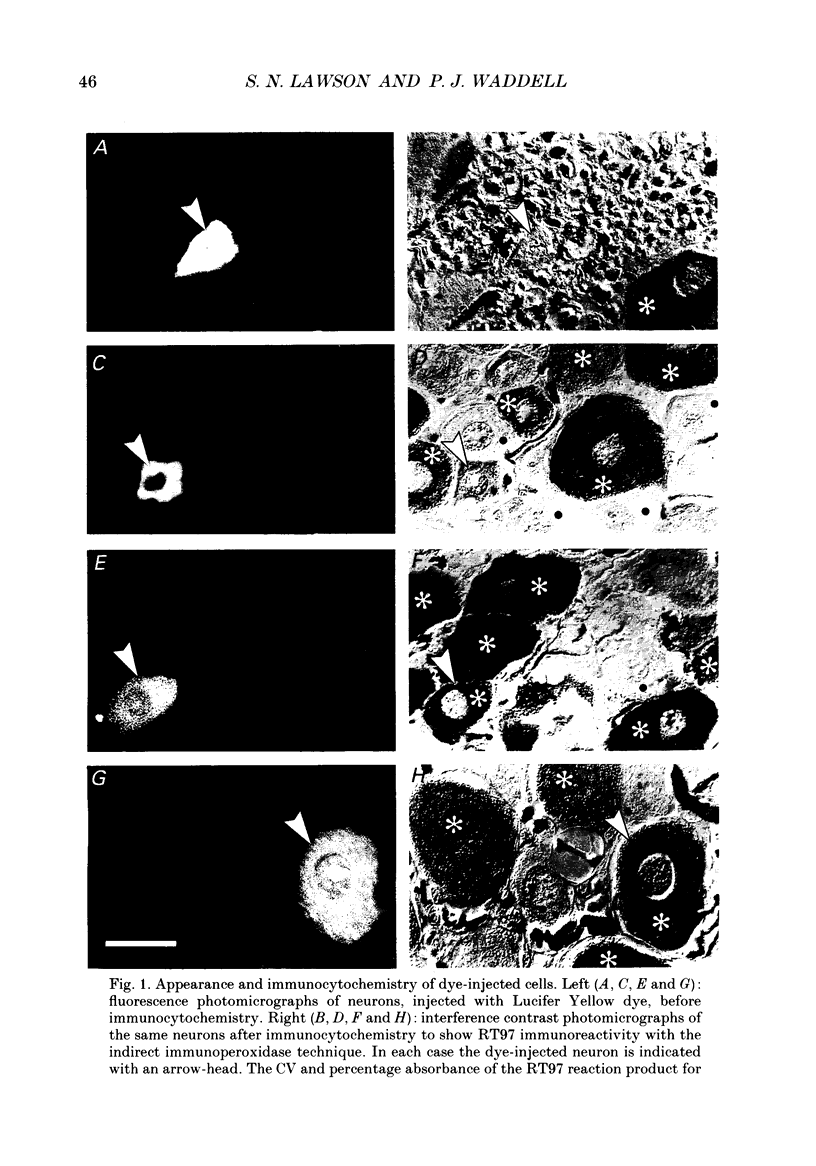
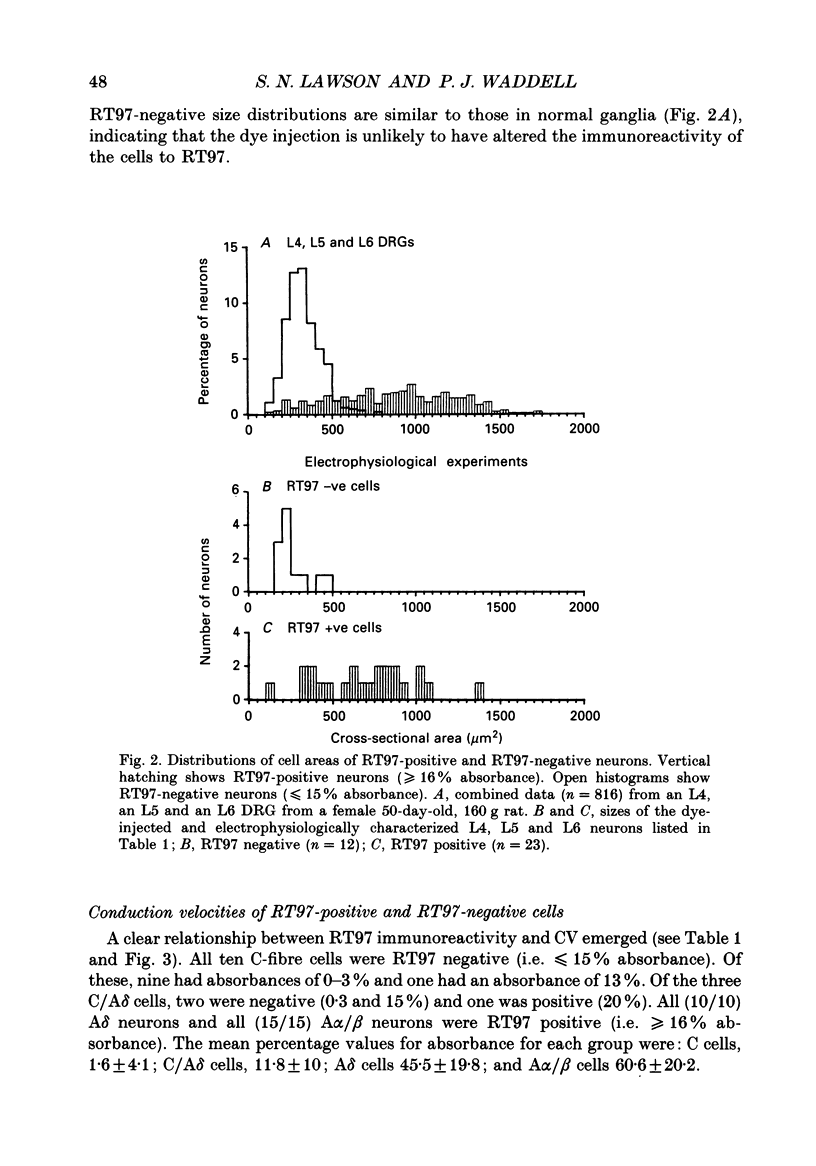
Abstract
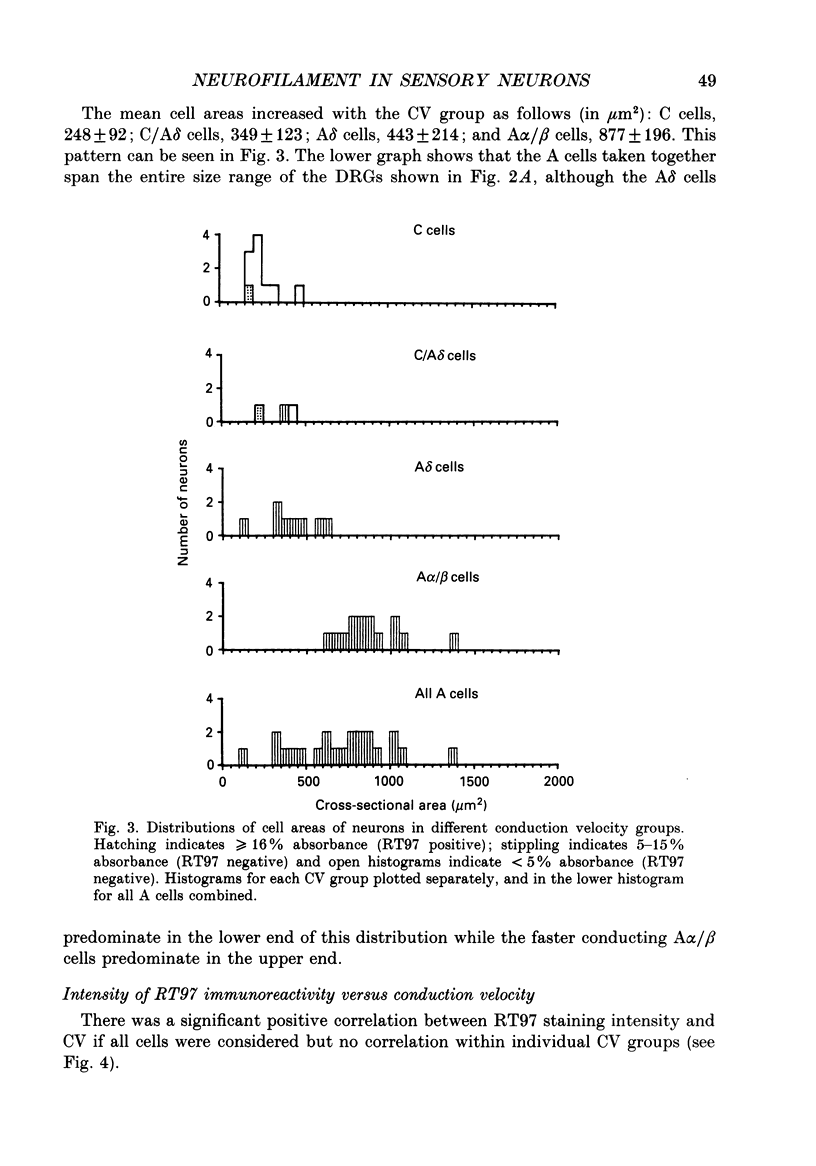
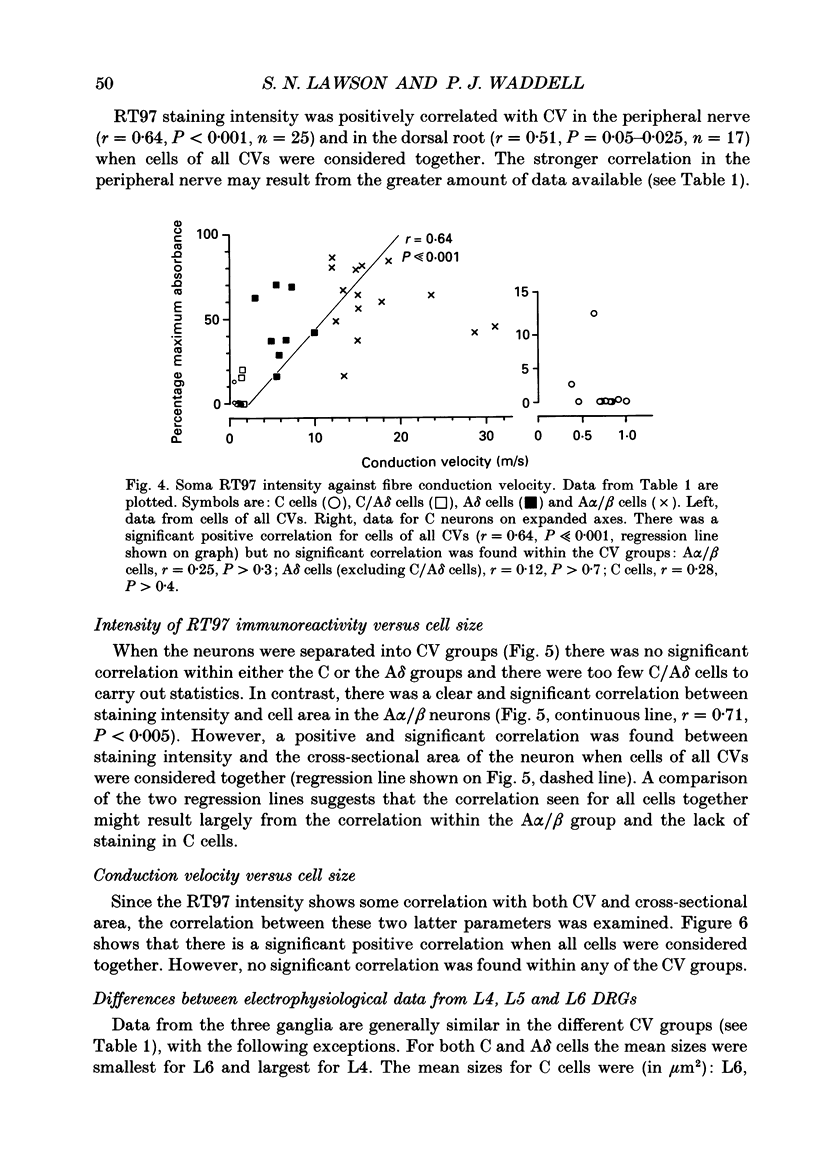
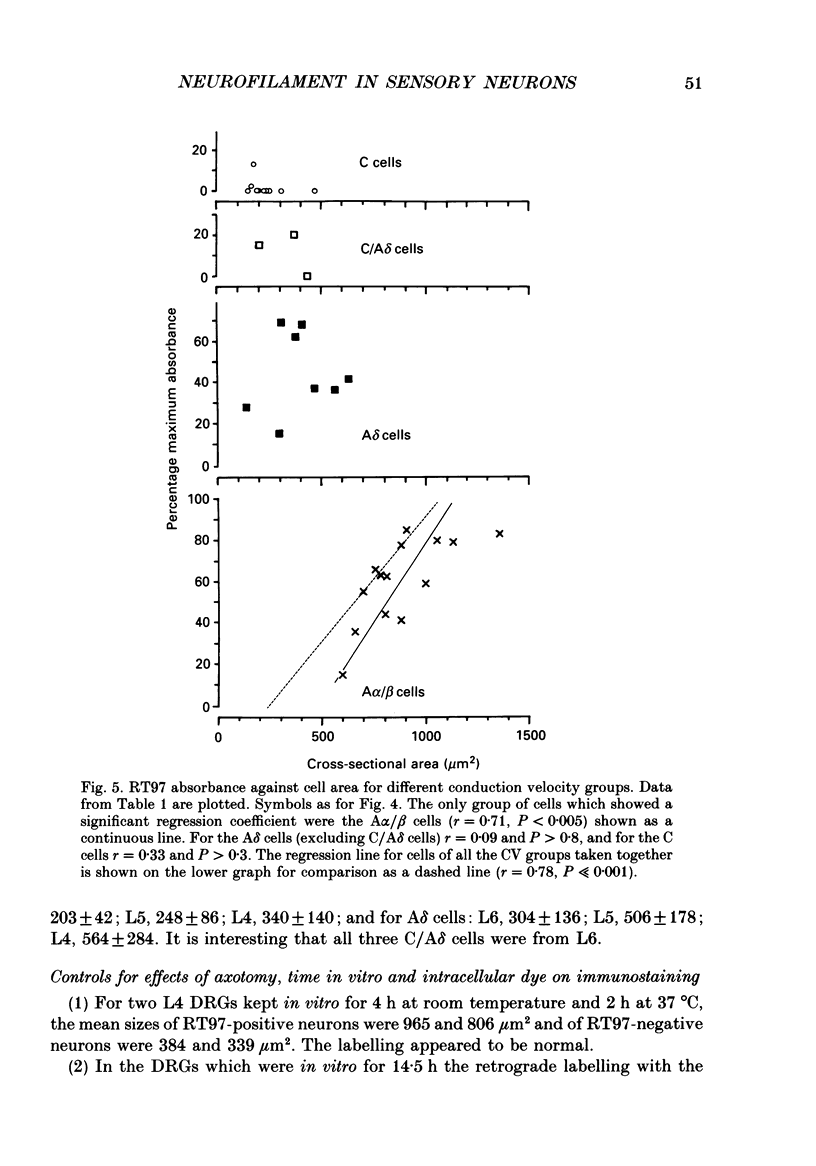
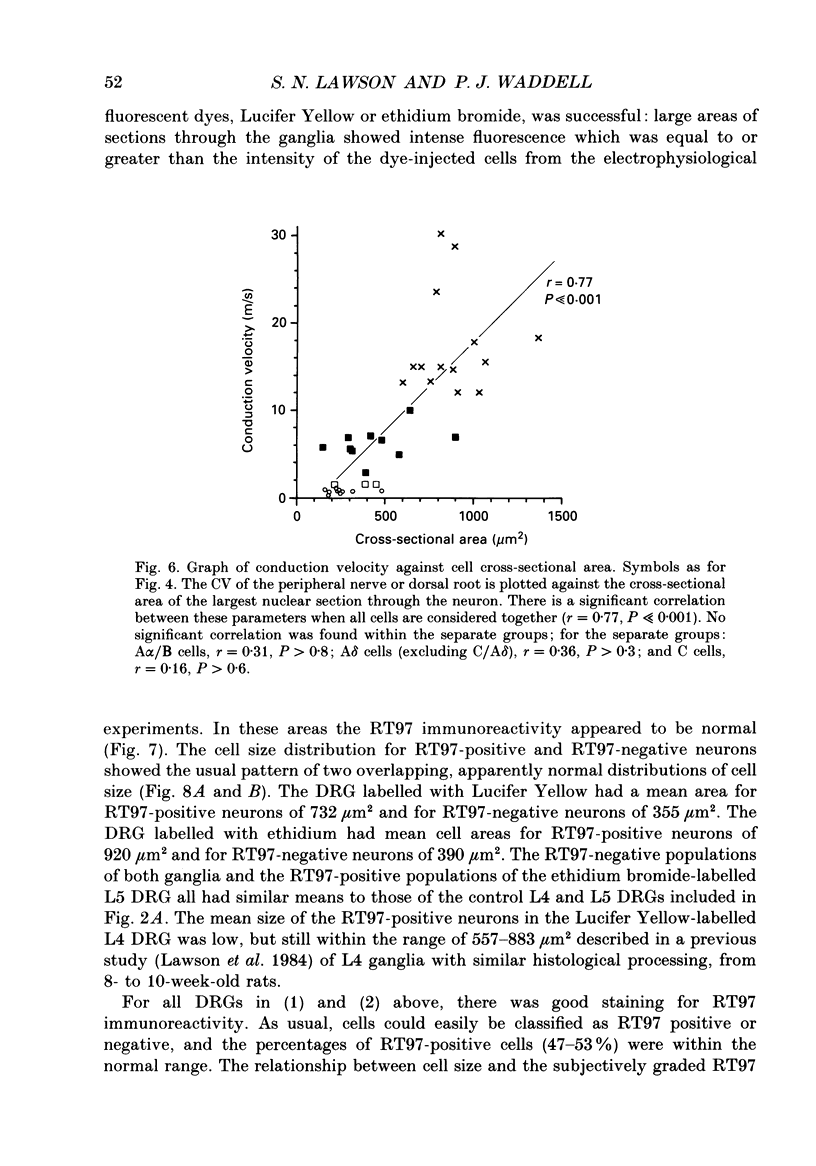
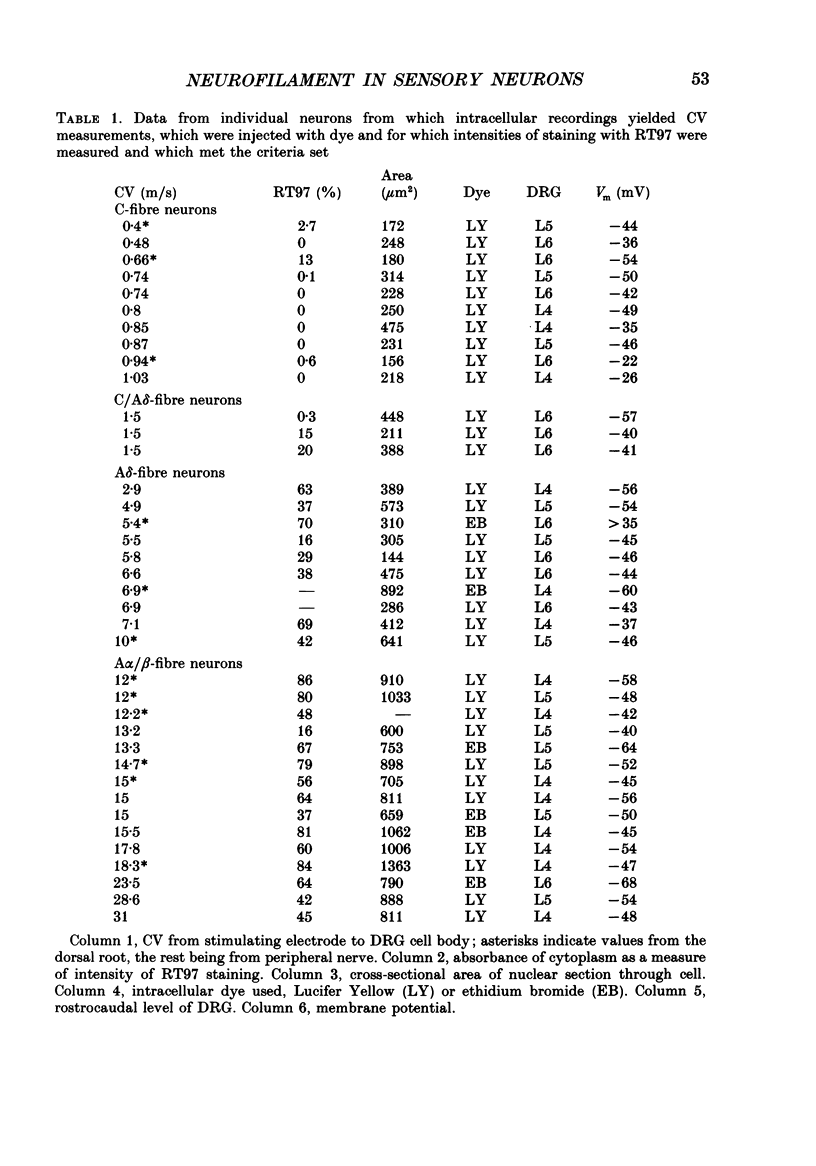
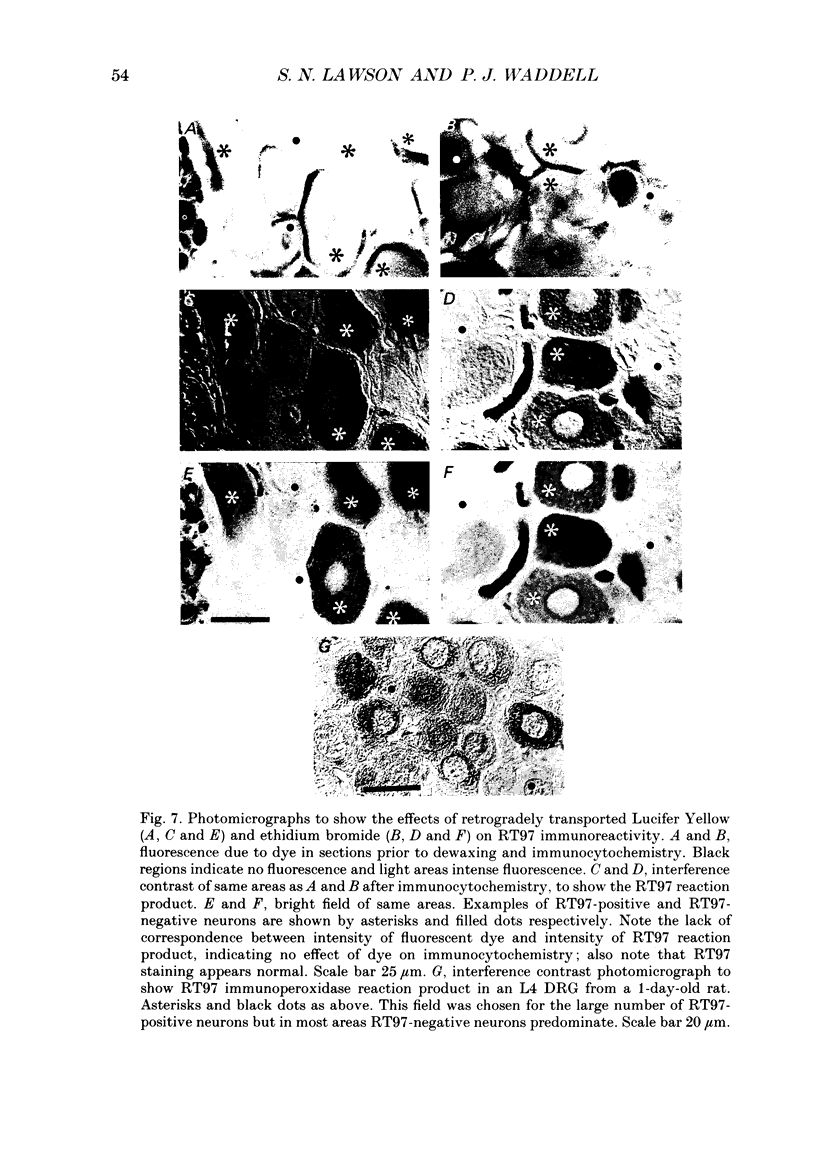
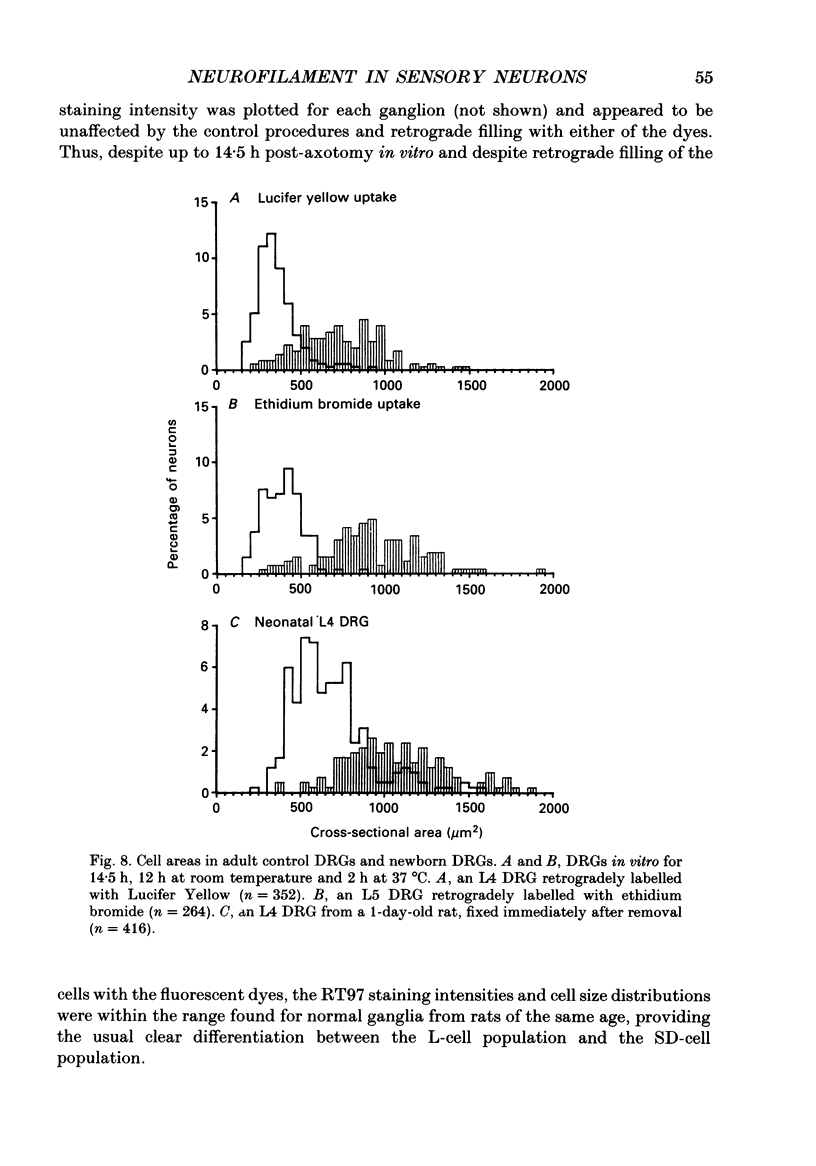
1. Intracellular recordings were made in dorsal root ganglia in vitro at 37 degrees C. The L4, L5 and L6 ganglia from 46- to 51-day-old female Wistar rats were used. In each neuron conduction velocity (CV) was measured and fluorescent dye was injected. Later the intensity of the immunoreactivity to RT97 (a monoclonal antibody to the phosphorylated 200 kDa neurofilament subunit) as well as the cell size (cross-sectional area at the nuclear level) were measured in the dye-injected neurons. RT97 was used to distinguish between the L (light, neurofilament-rich) and the SD (small dark, neurofilament-poor) neuronal somata. 2. Neurons were classified as C neurons (CV less than 1.3 m/s), C/A delta neurons (1.3-2 m/s), A delta neurons (2-12 m/s) or A alpha/beta neurons (greater than 12 m/s). 3. All A-fibre somata were RT97 positive (L) and all C-fibre somata were RT97 negative (SD), although in the C/A delta group both positive and negative neurons were seen. Thus, RT97-negative somata had C (unmyelinated) or C/A delta fibres, while RT97-positive somata had A (myelinated) or C/A delta fibres. 4. The size distributions of A neurons and C neurons were consistent with their classification as L- and SD-cell neurons respectively. The size distribution of A delta cells was skewed with a peak of small cells and a tail of medium-sized cells. 5. There was a loose positive correlation between cell size and fibre CV. 6. RT97 intensity was positively correlated with CV if all neurons were considered together, but no correlation was seen within the C, A delta or A alpha/beta CV groups. 7. RT97 intensity was positively correlated with cell size when all neurons were considered together. Although no correlation was seen within the C or the A delta CV groups, a clear positive correlation was seen for A alpha/beta neurons. 8. The relationship of RT97 intensity to cell size was not demonstrably altered by axotomy, time in vitro or the presence of intracellular dye in control experiments. 9. RT97-negative and -positive neurons could be seen in neonatal rat ganglia. Their size distributions resembled, respectively, the SD- and L-neuron populations at this age. RT97 immunoreactivity may therefore be a useful predictor of the cell type and myelinated state which a sensory cell is destined to reach in the adult rat.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calvert R., Anderton B. H. In vivo metabolism of mammalian neurofilament polypeptides in developing and adult rat brain. FEBS Lett. 1982 Aug 23;145(2):171–175. doi: 10.1016/0014-5793(82)80160-9. [DOI] [PubMed] [Google Scholar]
- Cameron A. A., Leah J. D., Snow P. J. The electrophysiological and morphological characteristics of feline dorsal root ganglion cells. Brain Res. 1986 Jan 1;362(1):1–6. doi: 10.1016/0006-8993(86)91391-0. [DOI] [PubMed] [Google Scholar]
- Dahl D., Labkovsky B., Bignami A. Neurofilament phosphorylation in axons and perikarya: immunofluorescence study of the rat spinal cord and dorsal root ganglia with monoclonal antibodies. J Comp Neurol. 1988 May 15;271(3):445–450. doi: 10.1002/cne.902710311. [DOI] [PubMed] [Google Scholar]
- Duce I. R., Keen P. An ultrastructural classification of the neuronal cell bodies of the rat dorsal root ganglion using zinc iodide-osmium impregnation. Cell Tissue Res. 1977 Dec 13;185(2):263–277. doi: 10.1007/BF00220670. [DOI] [PubMed] [Google Scholar]
- Friede R. L., Samorajski T. Axon caliber related to neurofilaments and microtubules in sciatic nerve fibers of rats and mice. Anat Rec. 1970 Aug;167(4):379–387. doi: 10.1002/ar.1091670402. [DOI] [PubMed] [Google Scholar]
- Harper A. A., Lawson S. N. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J Physiol. 1985 Feb;359:31–46. doi: 10.1113/jphysiol.1985.sp015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugh M. C., Probst A., Ulrich J., Kahn J., Anderton B. H. Alzheimer neurofibrillary tangles contain phosphorylated and hidden neurofilament epitopes. J Neurol Neurosurg Psychiatry. 1986 Nov;49(11):1213–1220. doi: 10.1136/jnnp.49.11.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson S. N., Biscoe T. J. Development of mouse dorsal root ganglia: an autoradiographic and quantitative study. J Neurocytol. 1979 Jun;8(3):265–274. doi: 10.1007/BF01236122. [DOI] [PubMed] [Google Scholar]
- Lawson S. N., Caddy K. W., Biscoe T. J. Development of rat dorsal root ganglion neurones. Studies of cell birthdays and changes in mean cell diameter. Cell Tissue Res. 1974;153(3):399–413. doi: 10.1007/BF00229167. [DOI] [PubMed] [Google Scholar]
- Lawson S. N., Harper A. A., Harper E. I., Garson J. A., Anderton B. H. A monoclonal antibody against neurofilament protein specifically labels a subpopulation of rat sensory neurones. J Comp Neurol. 1984 Sep 10;228(2):263–272. doi: 10.1002/cne.902280211. [DOI] [PubMed] [Google Scholar]
- Lawson S. N. The postnatal development of large light and small dark neurons in mouse dorsal root ganglia: a statistical analysis of cell numbers and size. J Neurocytol. 1979 Jun;8(3):275–294. doi: 10.1007/BF01236123. [DOI] [PubMed] [Google Scholar]
- Lee K. H., Chung K., Chung J. M., Coggeshall R. E. Correlation of cell body size, axon size, and signal conduction velocity for individually labelled dorsal root ganglion cells in the cat. J Comp Neurol. 1986 Jan 15;243(3):335–346. doi: 10.1002/cne.902430305. [DOI] [PubMed] [Google Scholar]
- Mansour H., Bignami A., Labkovsky B., Dahl D. Neurofilament phosphorylation in neuronal perikarya following axotomy: a study of rat spinal cord with ventral and dorsal root transection. J Comp Neurol. 1989 May 22;283(4):481–485. doi: 10.1002/cne.902830404. [DOI] [PubMed] [Google Scholar]
- McCarthy P. W., Lawson S. N. Cell type and conduction velocity of rat primary sensory neurons with calcitonin gene-related peptide-like immunoreactivity. Neuroscience. 1990;34(3):623–632. doi: 10.1016/0306-4522(90)90169-5. [DOI] [PubMed] [Google Scholar]
- McCarthy P. W., Lawson S. N. Differential intracellular labelling of identified neurones with two fluorescent dyes. Brain Res Bull. 1988 Feb;20(2):261–265. doi: 10.1016/0361-9230(88)90188-8. [DOI] [PubMed] [Google Scholar]
- RUSHTON W. A. H. A theory of the effects of fibre size in medullated nerve. J Physiol. 1951 Sep;115(1):101–122. doi: 10.1113/jphysiol.1951.sp004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambourg A., Clermont Y., Beaudet A. Ultrastructural features of six types of neurons in rat dorsal root ganglia. J Neurocytol. 1983 Feb;12(1):47–66. doi: 10.1007/BF01148087. [DOI] [PubMed] [Google Scholar]
- Sharp G. A., Shaw G., Weber K. Immunoelectronmicroscopical localization of the three neurofilament triplet proteins along neurofilaments of cultured dorsal root ganglion neurones. Exp Cell Res. 1982 Feb;137(2):403–413. doi: 10.1016/0014-4827(82)90042-8. [DOI] [PubMed] [Google Scholar]
- Shaw G., Osborn M., Weber K. Reactivity of a panel of neurofilament antibodies on phosphorylated and dephosphorylated neurofilaments. Eur J Cell Biol. 1986 Oct;42(1):1–9. [PubMed] [Google Scholar]
- Sternberger L. A., Sternberger N. H. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh Y. S., Chung K., Coggeshall R. E. A study of axonal diameters and areas in lumbosacral roots and nerves in the rat. J Comp Neurol. 1984 Feb 1;222(4):473–481. doi: 10.1002/cne.902220402. [DOI] [PubMed] [Google Scholar]
- Waddell P. J., Lawson S. N., McCarthy P. W. Conduction velocity changes along the processes of rat primary sensory neurons. Neuroscience. 1989;30(3):577–584. doi: 10.1016/0306-4522(89)90152-8. [DOI] [PubMed] [Google Scholar]
- Wong J., Oblinger M. M. Changes in neurofilament gene expression occur after axotomy of dorsal root ganglion neurons: an in situ hybridization study. Metab Brain Dis. 1987 Dec;2(4):291–303. doi: 10.1007/BF00999699. [DOI] [PubMed] [Google Scholar]
- Wood J. N., Anderton B. H. Monoclonal antibodies to mammalian neurofilaments. Biosci Rep. 1981 Mar;1(3):263–268. doi: 10.1007/BF01114913. [DOI] [PubMed] [Google Scholar]
- Yamadori T. A light and electron microscopic study on the postnatal development of spinal ganglia in rats. Kaibogaku Zasshi. 1970 Aug 1;45(4):191–205. [PubMed] [Google Scholar]
- Yoshida S., Matsuda Y. Studies on sensory neurons of the mouse with intracellular-recording and horseradish peroxidase-injection techniques. J Neurophysiol. 1979 Jul;42(4):1134–1145. doi: 10.1152/jn.1979.42.4.1134. [DOI] [PubMed] [Google Scholar]