Abstract
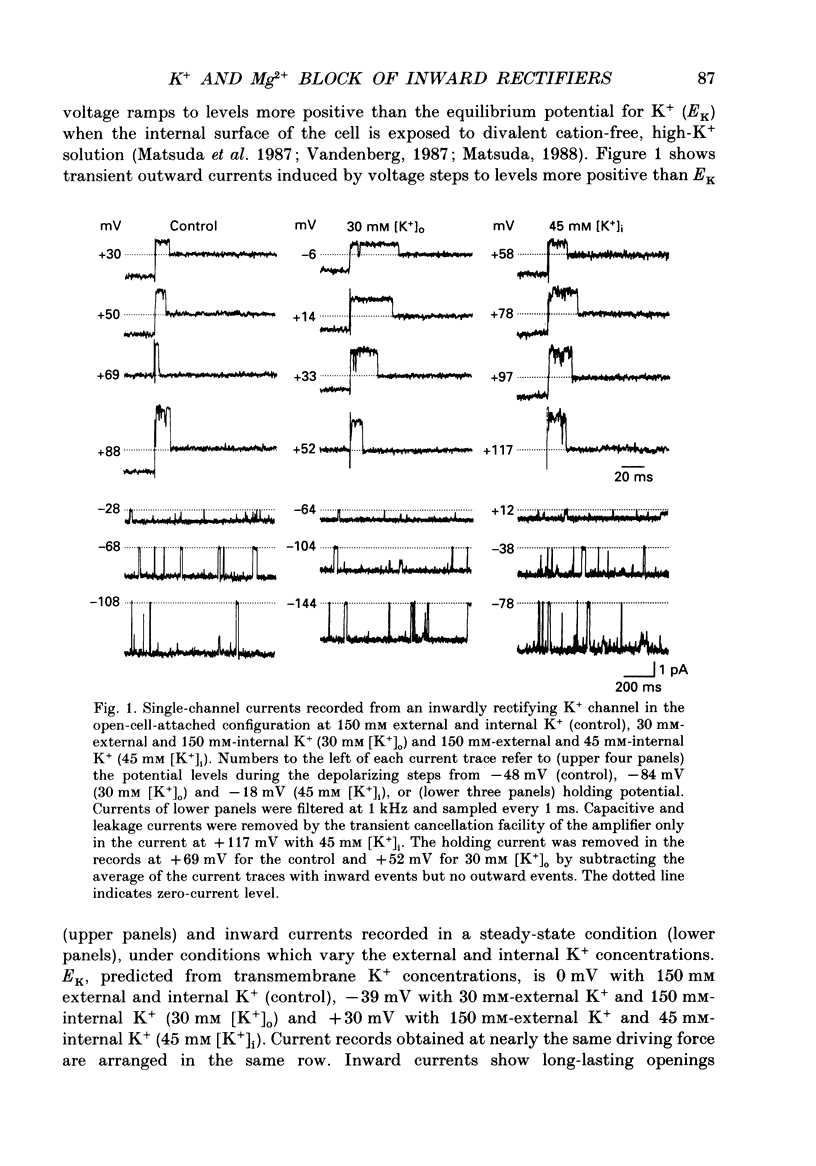
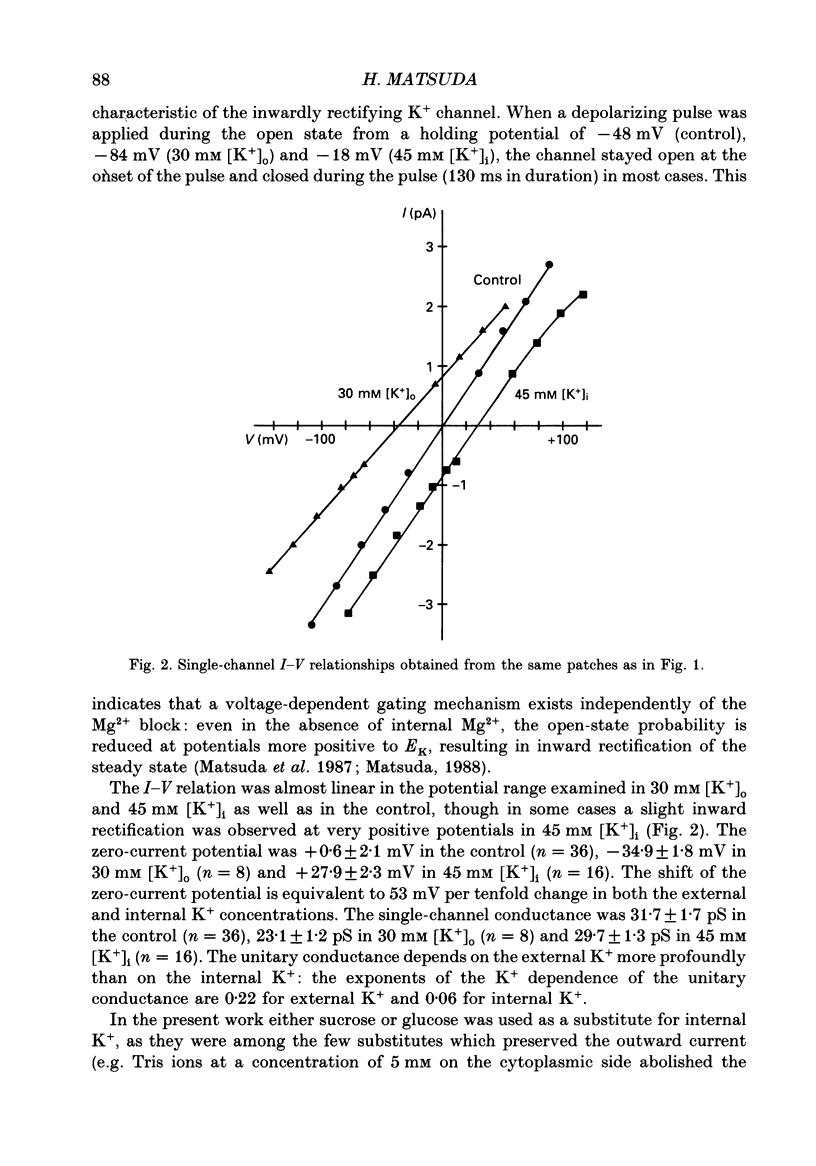
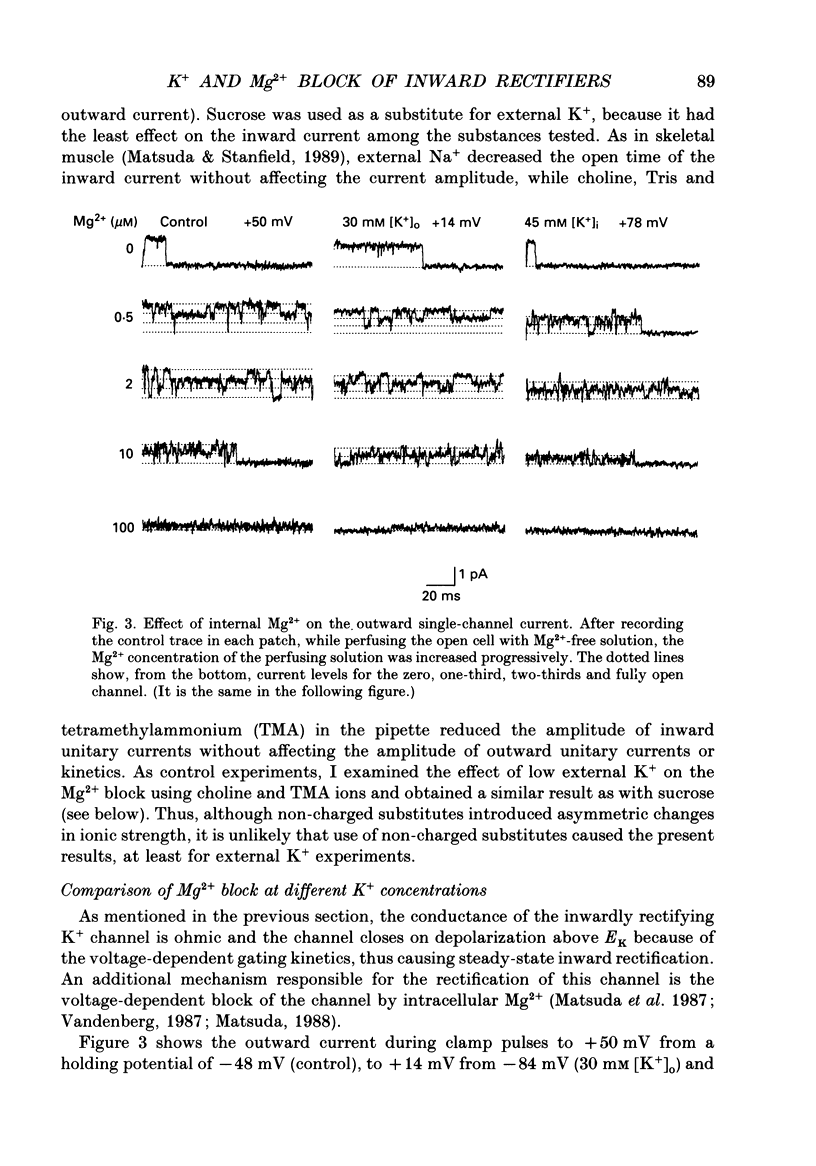
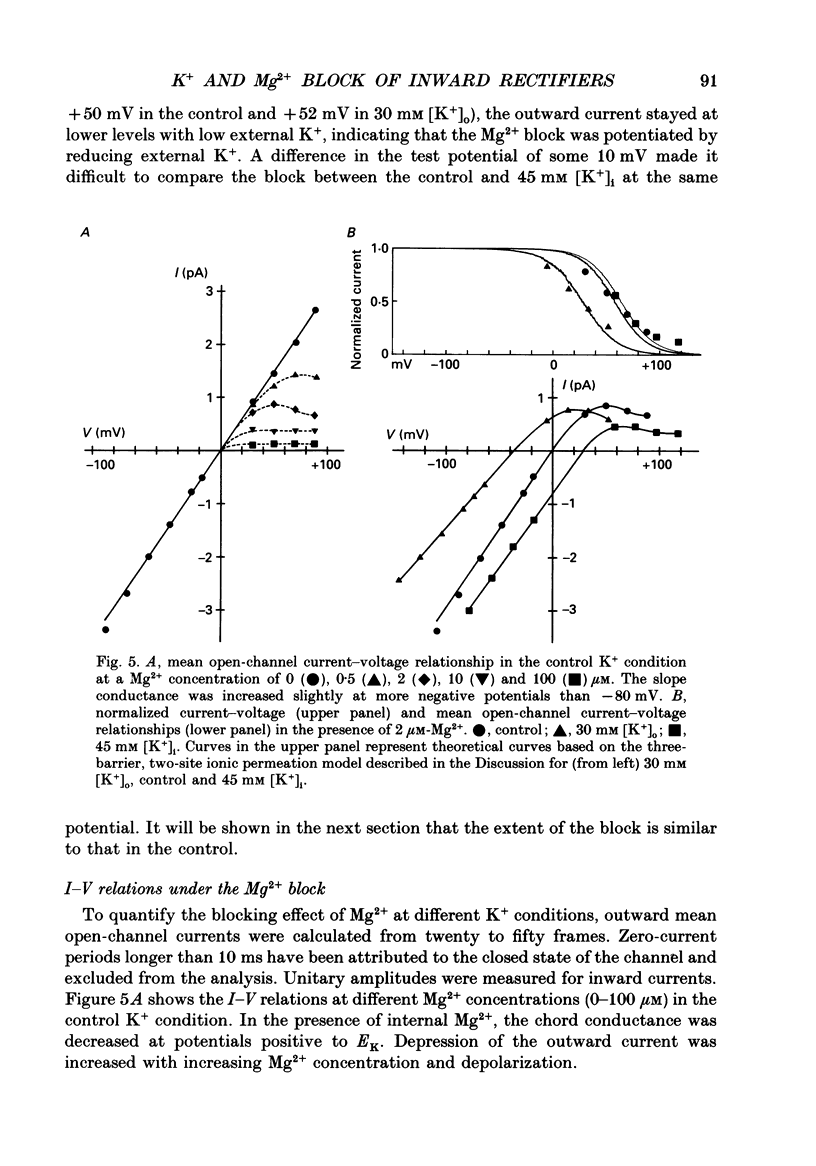
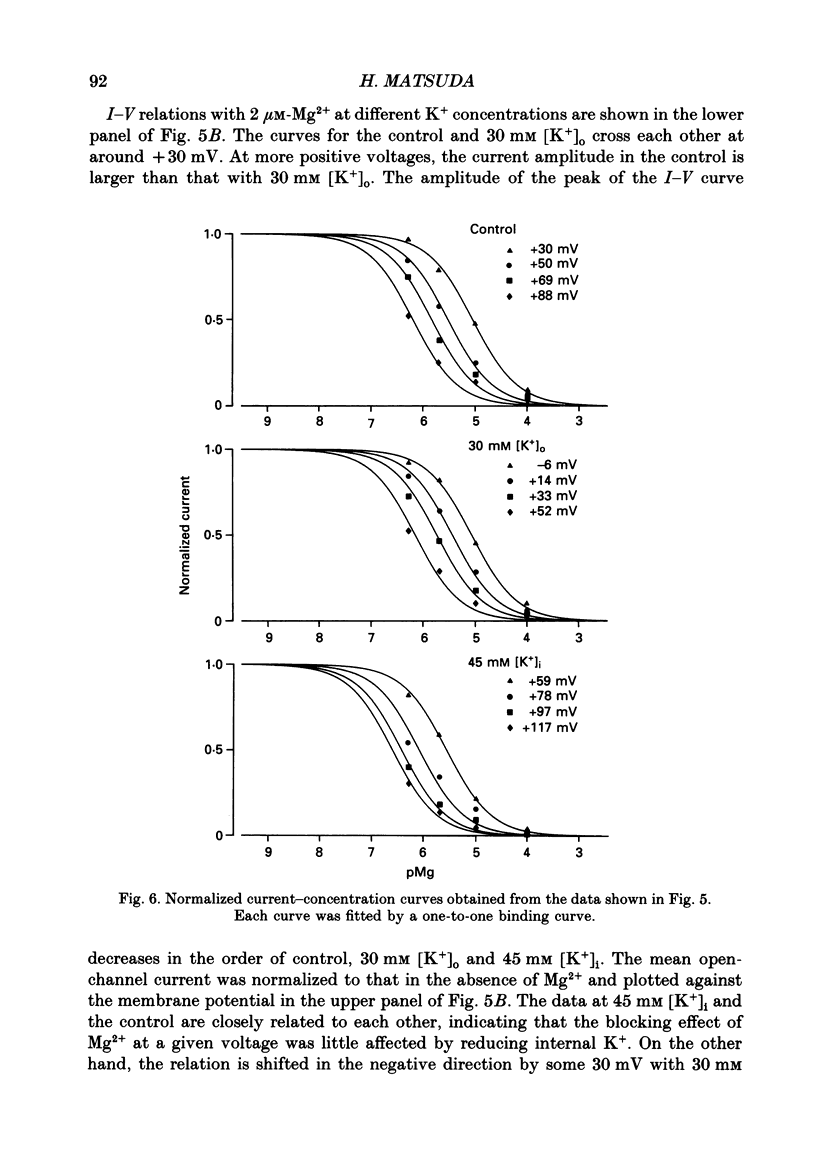
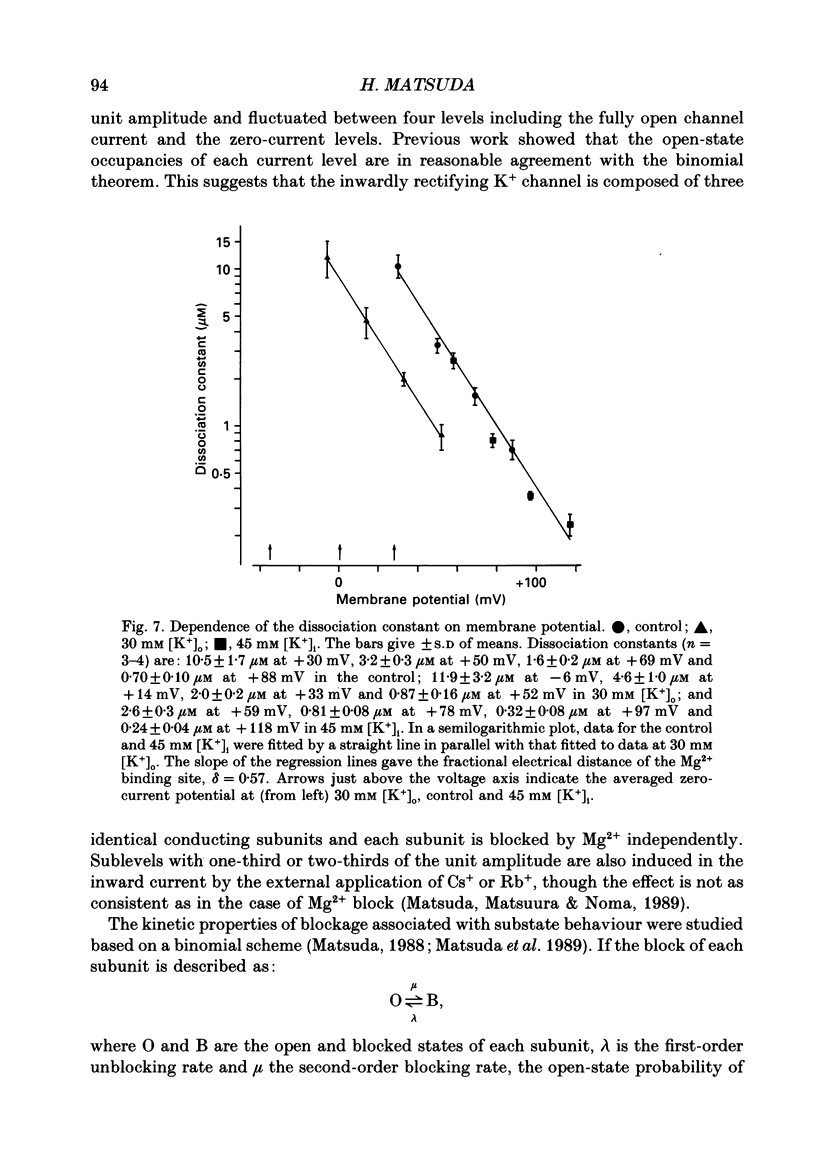
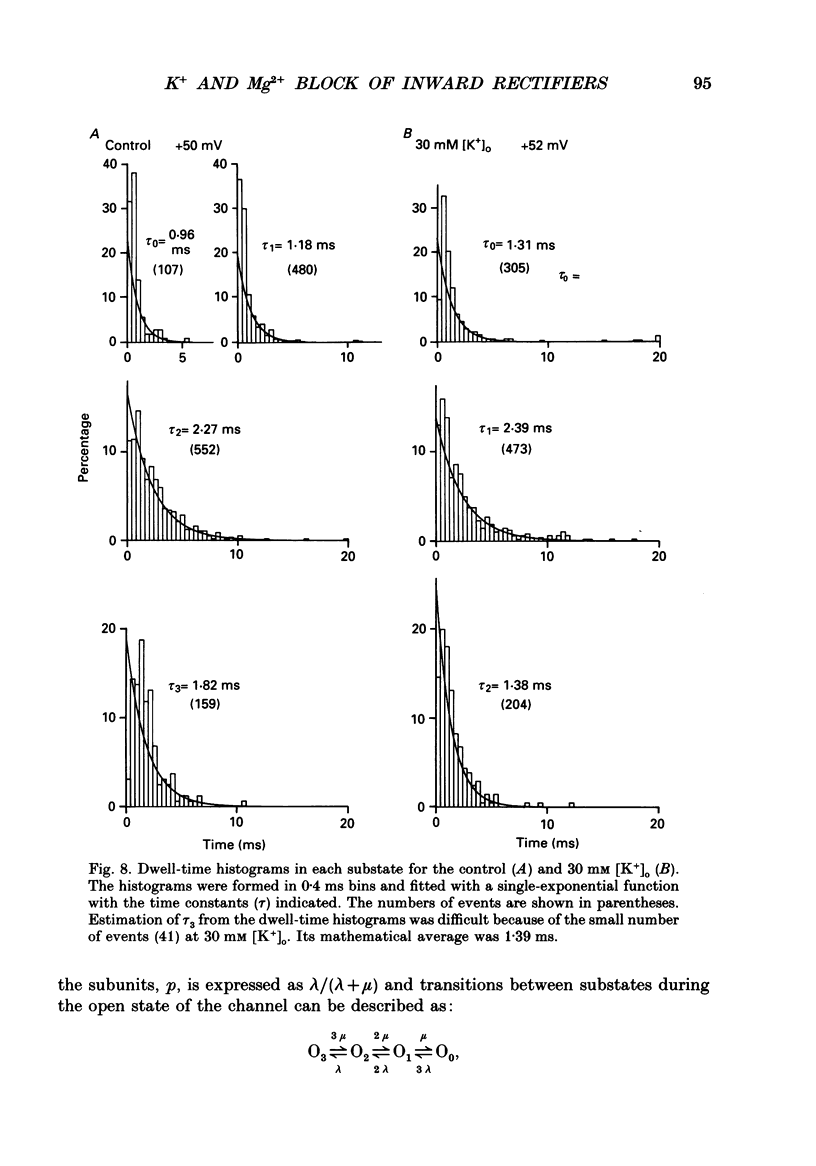
1. Block of the inwardly rectifying K+ channel by intracellular Mg2+ was studied in guinea-pig ventricular cells at varying external or internal K+ concentrations. Sucrose or glucose was mainly used as a substitute for K+. 2. The current-voltage (I-V) relation for the single channel, in the absence of internal Mg2+, was almost linear in 30 mM-external K+ and 150 mM-internal K+ (30 mM [K+]o) and in 45 mM-internal K+ and 150 mM-external K+ (45 mM [K+]i) as well as in 150 mM-external and internal K+ (the control condition). The channel conductance was 31.7 +/- 1.7 pS (mean +/- S.D., n = 36) in the control, 23.1 +/- 1.2 pS (n = 8) in 30 mM [K+]o and 29.7 +/- 1.3 pS (n = 16) in 45 mM [K+]i, respectively. 3. Mg2+ on the cytoplasmic side blocked the outward currents without affecting the inward currents. Outward mean open-channel currents were measured at different Mg2+ concentrations (0-100 microM) and voltages. The current-voltage relation rectified inwardly in the presence of internal Mg2+ in a voltage- and concentration-dependent manner. 4. Outward mean open-channel currents were normalized to that measured in the absence of Mg2+. The normalized current-voltage relation in 45 mM [K+]i was almost superimposable on that obtained in the control at the same Mg2+ concentration, while that in 30 mM [K+]o was shifted in the negative direction by some 30 mV. 5. The normalized current-Mg2+ concentration curve was fitted by a one-to-one binding curve at each K+ condition and voltage. In a semilogarithmic plot of dissociation constant versus membrane potential, data points for 45 mM [K+]i were located on the same line as the control, whereas data points for 30 mM [K+]o were shifted in the negative direction by about 30 mV. The dissociation constant at 0 mV is 37 microM in the control and 45 mM [K+]i and 8.8 microM in 30 mM [K+]o. The voltage dependence of dissociation constants gives a value for the fractional electrical distance of the Mg2+ binding site of 0.57. 6. Subconductance levels with one-third and two-thirds of the unitary amplitude were seen with low internal Mg2+ at 45 mM [K+]i or 30 mM [K+]o as well as in the control condition. Blocking and unblocking rates were calculated on the assumption that the channel is composed of three identical conducting units and each unit is blocked by Mg2+ independently.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., McCleskey E. W. Non-selective conductance in calcium channels of frog muscle: calcium selectivity in a single-file pore. J Physiol. 1984 Aug;353:585–608. doi: 10.1113/jphysiol.1984.sp015352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begenisich T. B., Cahalan M. D. Sodium channel permeation in squid axons. I: Reversal potential experiments. J Physiol. 1980 Oct;307:217–242. doi: 10.1113/jphysiol.1980.sp013432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciani S., Krasne S., Hagiwara S. A model for the effects of potential and external K+ concentration on the Cs+ blocking of inward rectification. Biophys J. 1980 Apr;30(1):199–204. doi: 10.1016/S0006-3495(80)85089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Findlay I. ATP-sensitive K+ channels in rat ventricular myocytes are blocked and inactivated by internal divalent cations. Pflugers Arch. 1987 Oct;410(3):313–320. doi: 10.1007/BF00580282. [DOI] [PubMed] [Google Scholar]
- Fukushima Y. Blocking kinetics of the anomalous potassium rectifier of tunicate egg studied by single channel recording. J Physiol. 1982 Oct;331:311–331. doi: 10.1113/jphysiol.1982.sp014374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Moody W., Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. J Physiol. 1978 Jun;279:167–185. doi: 10.1113/jphysiol.1978.sp012338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Rosenthal N. P. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J Gen Physiol. 1976 Jun;67(6):621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Yoshii M. Effects of internal potassium and sodium on the anomalous rectification of the starfish egg as examined by internal perfusion. J Physiol. 1979 Jul;292:251–265. doi: 10.1113/jphysiol.1979.sp012849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hess P., Tsien R. W. Mechanism of ion permeation through calcium channels. 1984 May 31-Jun 6Nature. 309(5967):453–456. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- Hestrin S. The interaction of potassium with the activation of anomalous rectification in frog muscle membrane. J Physiol. 1981 Aug;317:497–508. doi: 10.1113/jphysiol.1981.sp013839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Schwarz W. Potassium channels as multi-ion single-file pores. J Gen Physiol. 1978 Oct;72(4):409–442. doi: 10.1085/jgp.72.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie M., Irisawa H. Dual effects of intracellular magnesium on muscarinic potassium channel current in single guinea-pig atrial cells. J Physiol. 1989 Jan;408:313–332. doi: 10.1113/jphysiol.1989.sp017461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie M., Irisawa H., Noma A. Voltage-dependent magnesium block of adenosine-triphosphate-sensitive potassium channel in guinea-pig ventricular cells. J Physiol. 1987 Jun;387:251–272. doi: 10.1113/jphysiol.1987.sp016572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie M., Irisawa H. Rectification of muscarinic K+ current by magnesium ion in guinea pig atrial cells. Am J Physiol. 1987 Jul;253(1 Pt 2):H210–H214. doi: 10.1152/ajpheart.1987.253.1.H210. [DOI] [PubMed] [Google Scholar]
- Leech C. A., Stanfield P. R. Inward rectification in frog skeletal muscle fibres and its dependence on membrane potential and external potassium. J Physiol. 1981;319:295–309. doi: 10.1113/jphysiol.1981.sp013909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A. Blocking of large unitary calcium-dependent potassium currents by internal sodium ions. Pflugers Arch. 1983 Feb;396(2):179–181. doi: 10.1007/BF00615524. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Matsuura H., Noma A. Triple-barrel structure of inwardly rectifying K+ channels revealed by Cs+ and Rb+ block in guinea-pig heart cells. J Physiol. 1989 Jun;413:139–157. doi: 10.1113/jphysiol.1989.sp017646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H. Open-state substructure of inwardly rectifying potassium channels revealed by magnesium block in guinea-pig heart cells. J Physiol. 1988 Mar;397:237–258. doi: 10.1113/jphysiol.1988.sp016998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H., Saigusa A., Irisawa H. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+. Nature. 1987 Jan 8;325(7000):156–159. doi: 10.1038/325156a0. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Stanfield P. R. Single inwardly rectifying potassium channels in cultured muscle cells from rat and mouse. J Physiol. 1989 Jul;414:111–124. doi: 10.1113/jphysiol.1989.sp017679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea-pig heart. J Physiol. 1984 Feb;347:641–657. doi: 10.1113/jphysiol.1984.sp015088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senyk O. External [K+] and the block of the K+ inward rectifier by external Cs+ in frog skeletal muscle. Biophys J. 1986 Oct;50(4):677–683. doi: 10.1016/S0006-3495(86)83508-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. A potential- and time-dependent blockade of inward rectification in frog skeletal muscle fibres by barium and strontium ions. J Physiol. 1978 Jul;280:169–191. doi: 10.1113/jphysiol.1978.sp012379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J. Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium. Biochim Biophys Acta. 1980 Jul;599(2):623–638. doi: 10.1016/0005-2736(80)90205-9. [DOI] [PubMed] [Google Scholar]
- Vandenberg C. A. Inward rectification of a potassium channel in cardiac ventricular cells depends on internal magnesium ions. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2560–2564. doi: 10.1073/pnas.84.8.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Kimitsuki T., Noma A. Conductance properties of the Na(+)-activated K+ channel in guinea-pig ventricular cells. J Physiol. 1991 Feb;433:241–257. doi: 10.1113/jphysiol.1991.sp018424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. Relief of Na+ block of Ca2+-activated K+ channels by external cations. J Gen Physiol. 1984 Aug;84(2):187–199. doi: 10.1085/jgp.84.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]


