Abstract
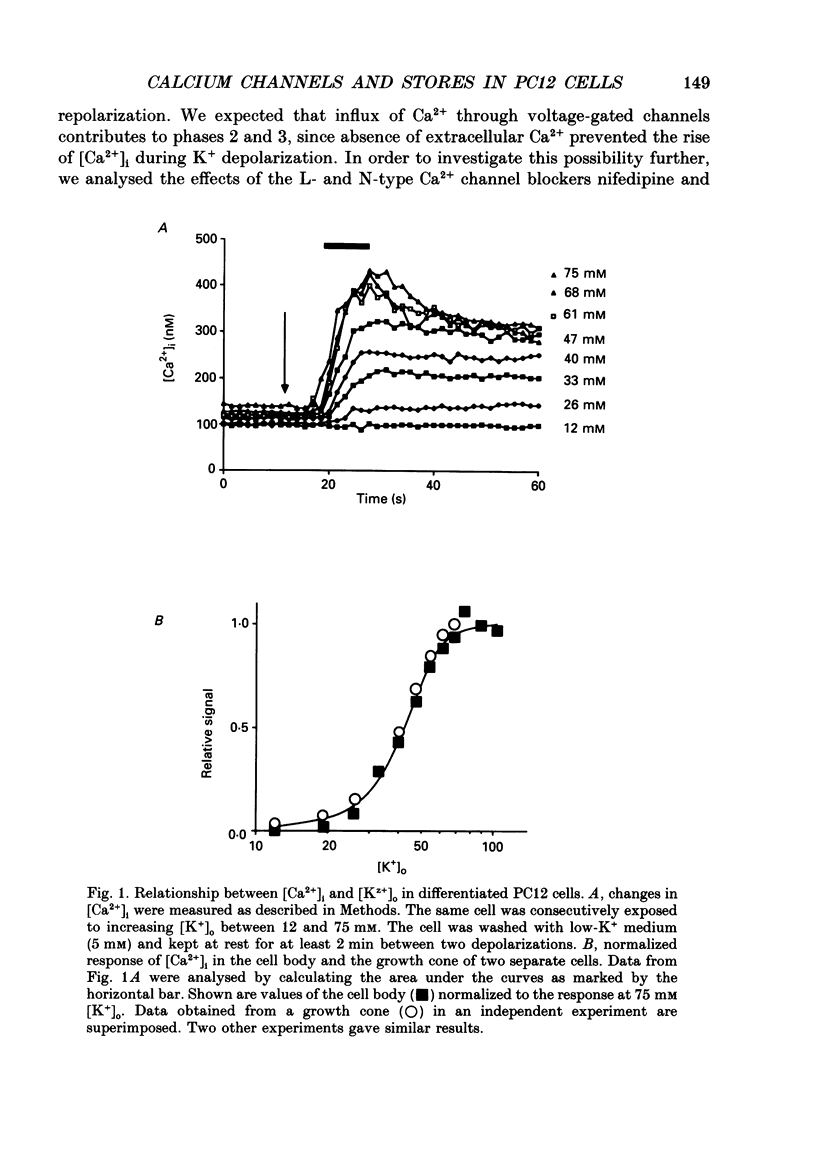
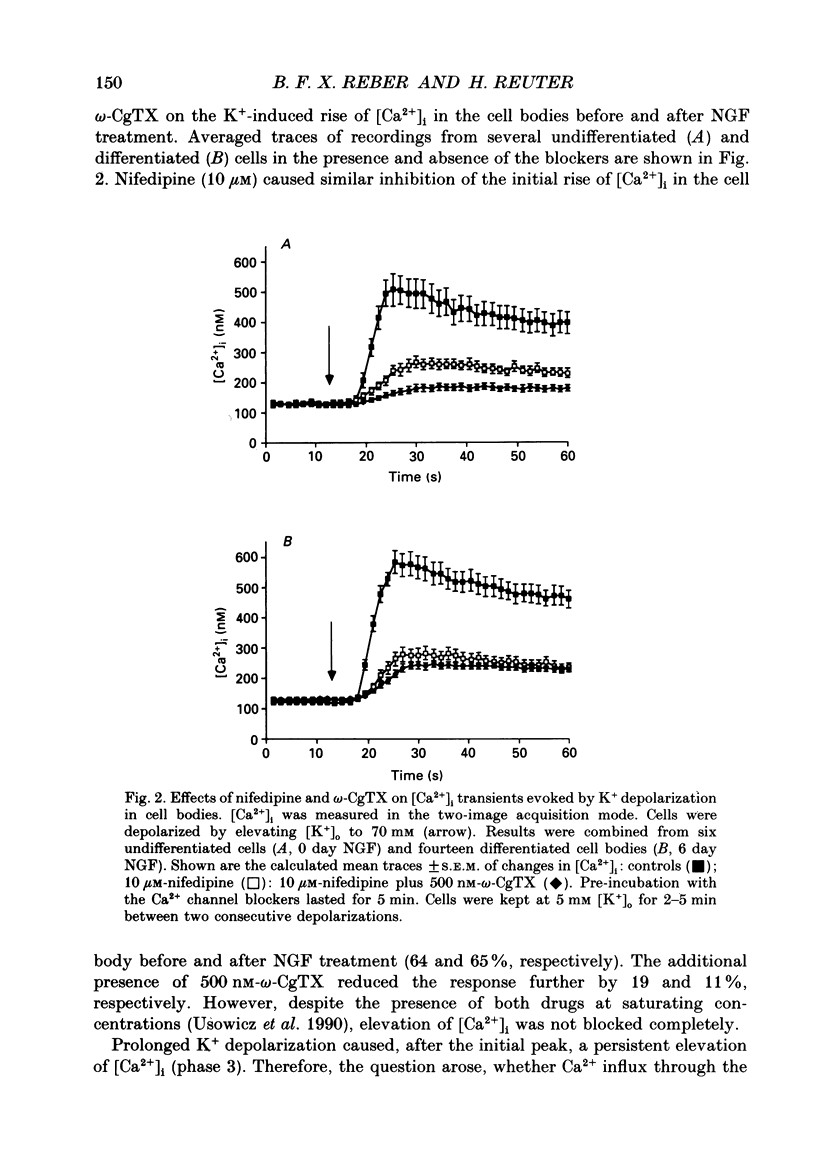
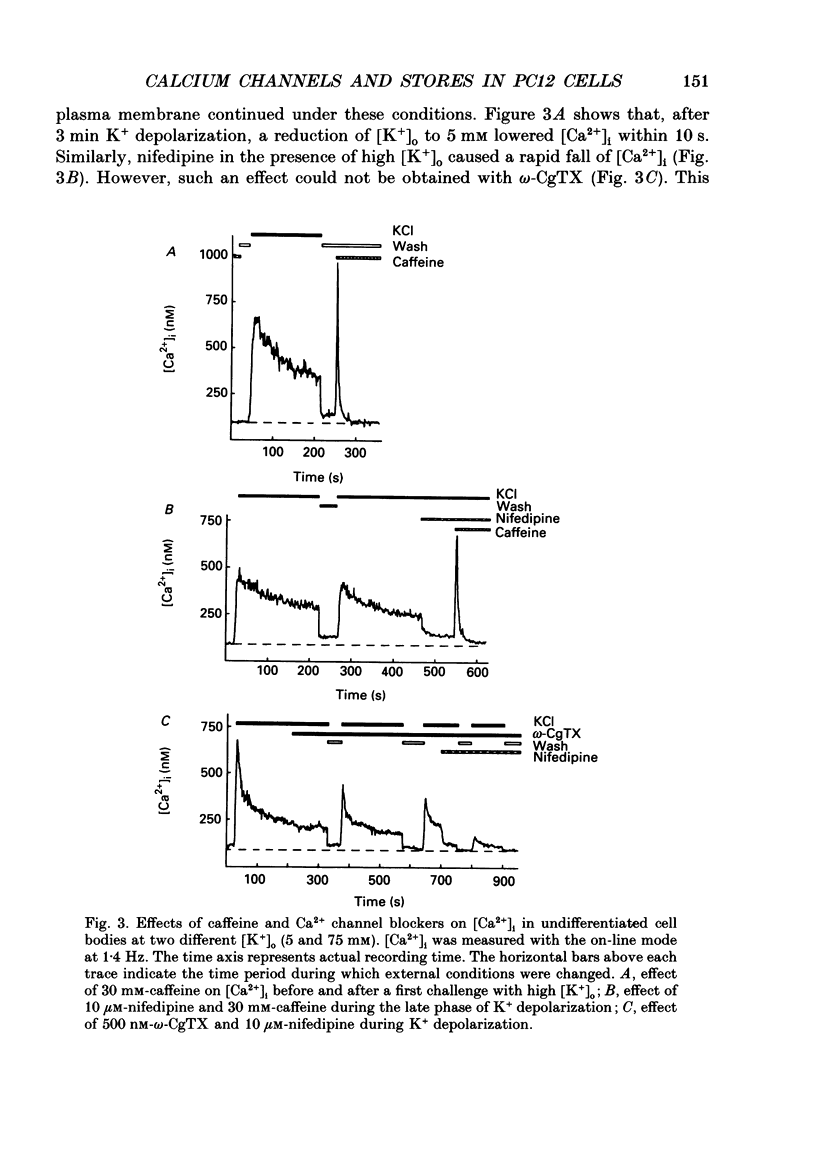
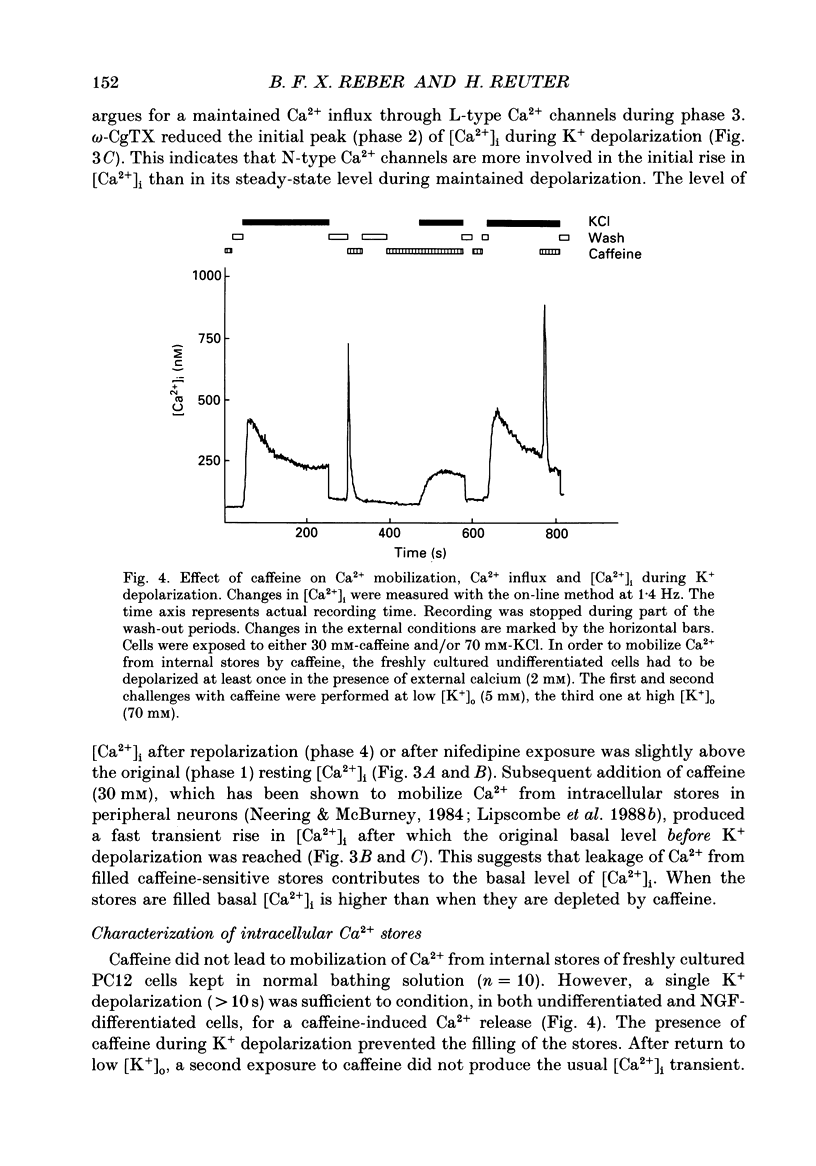
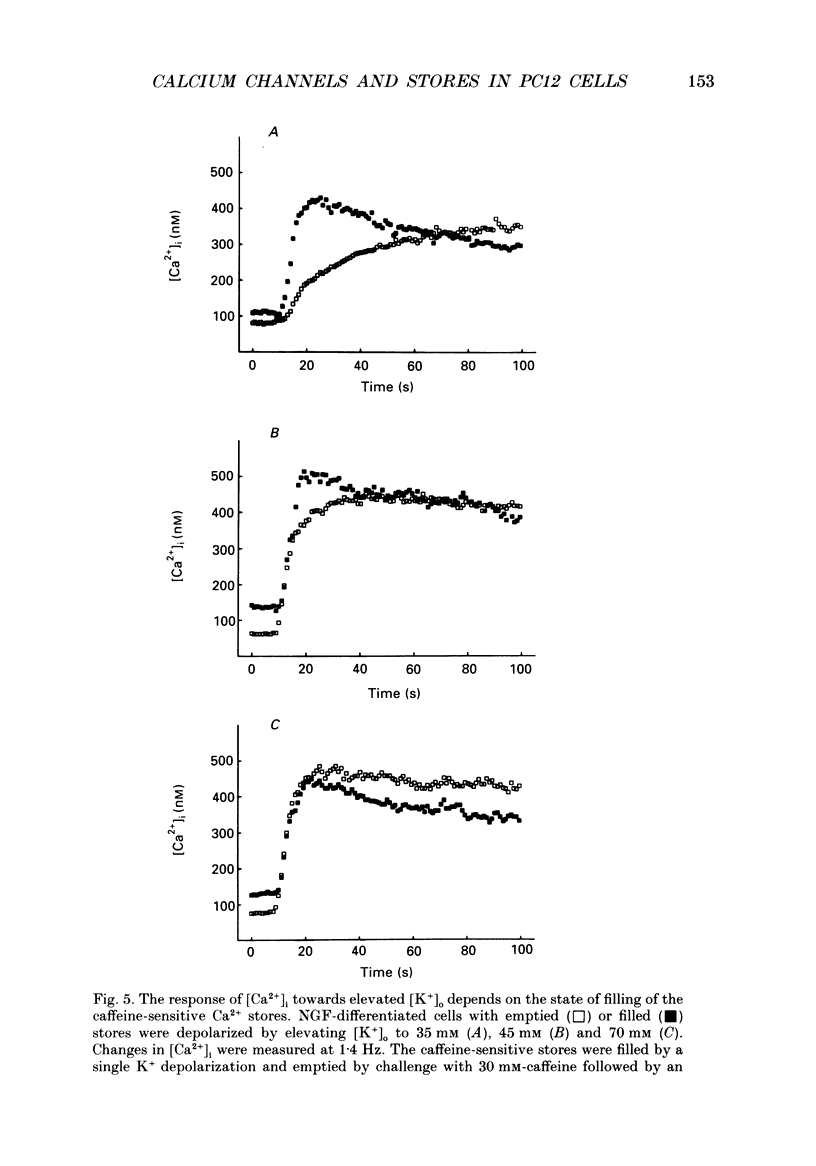
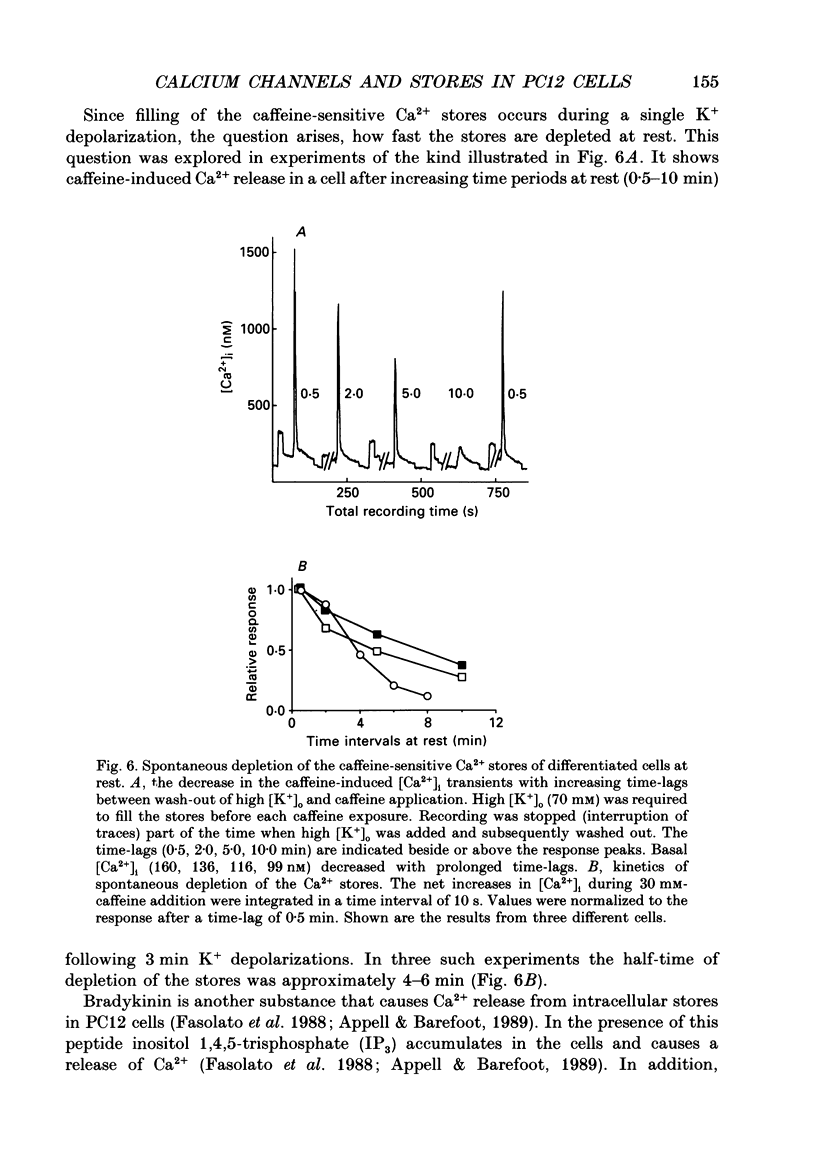
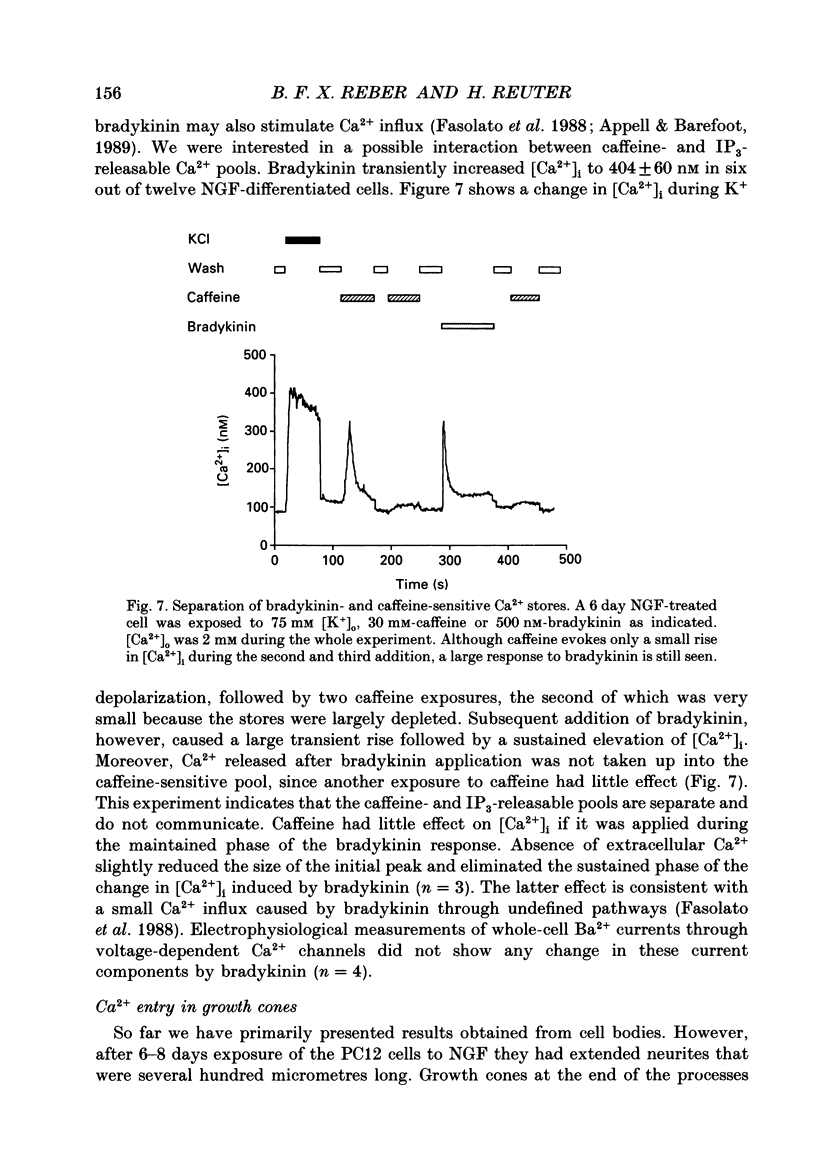
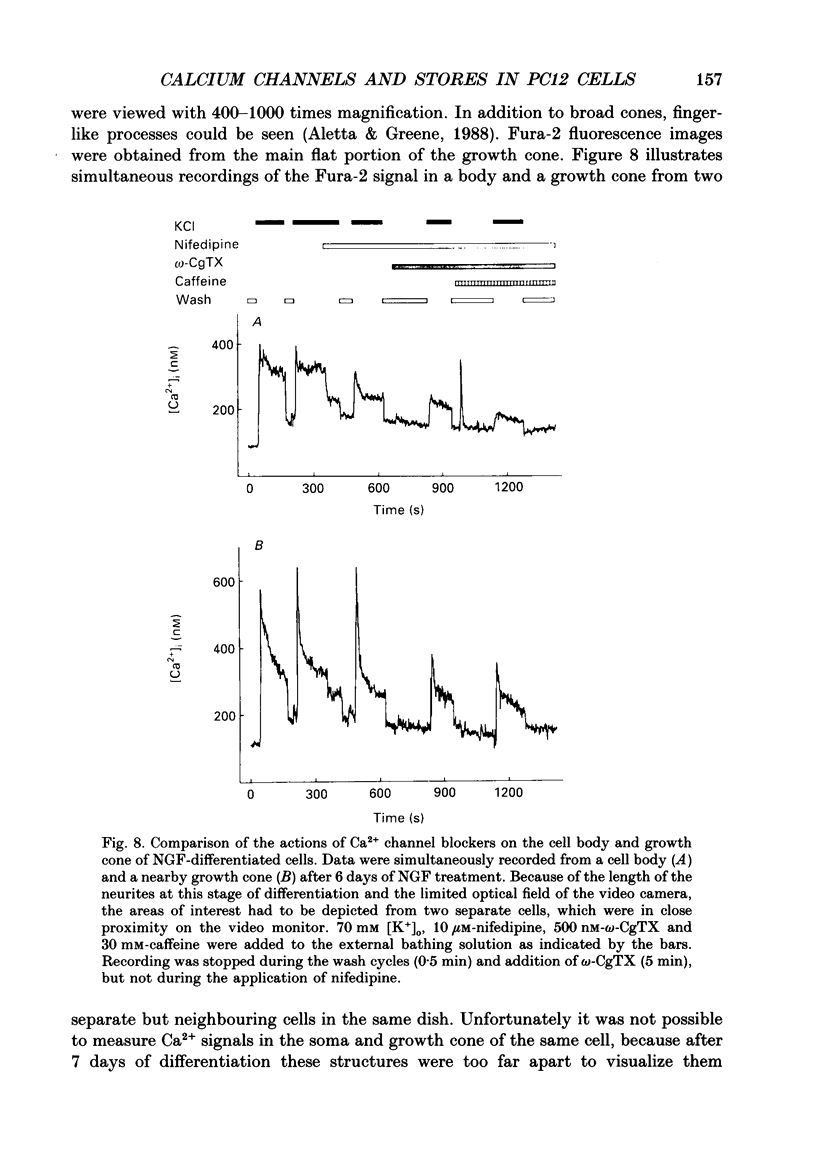
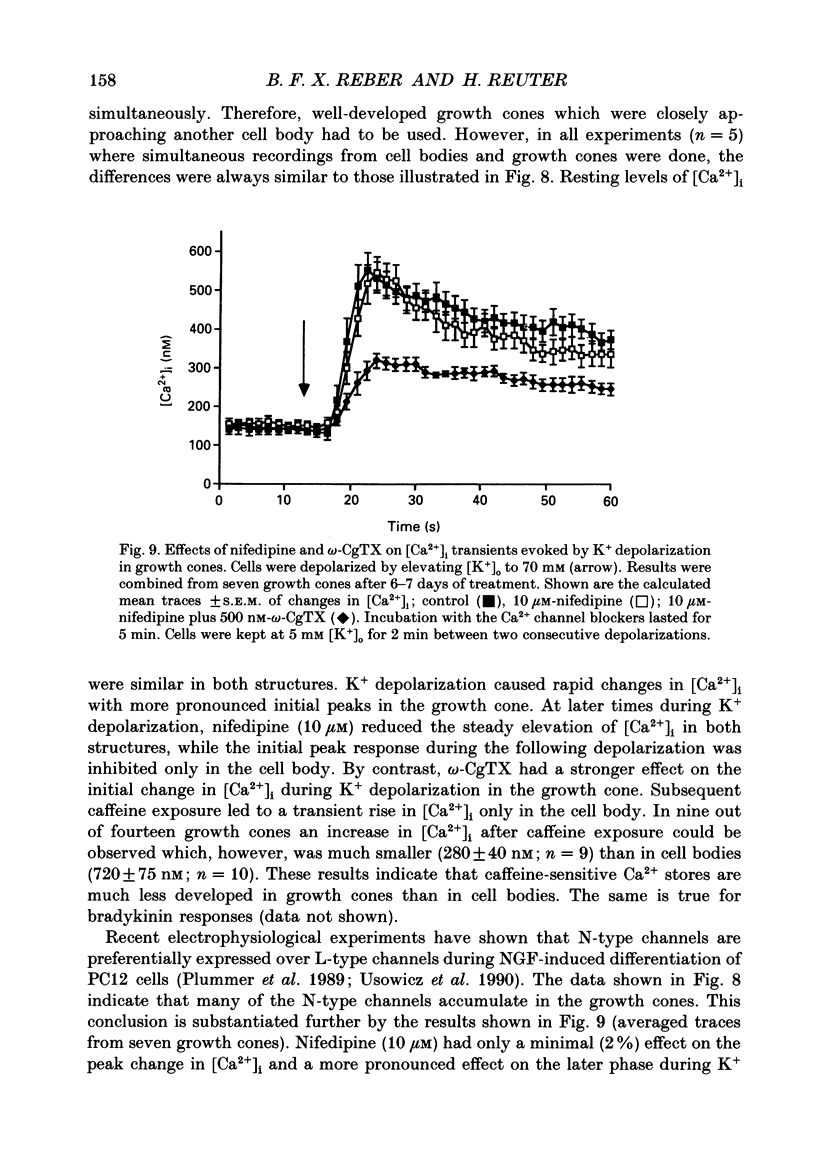
1. The rat clonal pheochromocytoma cell line (PC12) was used to study changes in the free intracellular Ca2+ concentration [( Ca2+]i) that are related to the distribution of L-type (dihydropyridine-sensitive) and N-type (omega-conotoxin-sensitive) calcium channels during nerve growth factor (NGF)-induced outgrowth of neurites. Changes in [Ca2+]i during K+ depolarization were recorded by means of Fura-2 single-cell microfluorimetry. 2. The basal [Ca2+]i of cells at rest was not altered by long-term treatment with NGF, neither in the cell bodies nor in the growth cones. K+ depolarization of the cells caused a rise in [Ca2+]i. 3. The dihydropyridine (DHP) nifedipine alone, or together with omega-conotoxin (omega-CgTX), were similarly effective in inhibiting the K(+)-induced increase in [Ca2+]i in untreated and NGF-treated cell bodies, arguing for a preferential distribution of L-type Ca2+ channels in this cell area. By contrast, after 6-7 days exposure to NGF the K(+)-induced initial transient rise of [Ca2+]i in growth cones was very sensitive to omega-CgTX, whereas nifedipine affected only the sustained rise. 4. PC12 cells also contain caffeine- and inositol trisphosphate (IP3)-sensitive intracellular Ca2+ stores. Addition of 30 mM-caffeine caused a fast transient rise in [Ca2+]i. The extent of filling of the caffeine-sensitive pool affected basal [Ca2+]i. These Ca2+ storage sites were empty under normal culture conditions. However, a single K+ depolarization caused filling of the stores, followed by spontaneous depletion (50% in about 5 min) after wash-out of high [K+]o. When the caffeine-sensitive stores were empty, the rise in [Ca2+]i was attenuated during submaximal depolarization. Caffeine-sensitive Ca2+ stores were also present in some growth cones, though with much smaller capacities than in cell bodies. 5. Mobilization of Ca2+ from the IP3-sensitive store, by bradykinin exposure, was found to be independent of the caffeine-sensitive pool. There was no apparent 'cross-talk' between both Ca2+ pools. 6. We conclude that changes in [Ca2+]i in cell bodies depend on both membrane Ca2+ channels and intracellular Ca2+ stores. During NGF-induced differentiation there is a predominance of N-type Ca2+ channels in growth cones, while Ca2+ stores are of minor importance in these structures.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aletta J. M., Greene L. A. Growth cone configuration and advance: a time-lapse study using video-enhanced differential interference contrast microscopy. J Neurosci. 1988 Apr;8(4):1425–1435. doi: 10.1523/JNEUROSCI.08-04-01425.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appell K. C., Barefoot D. S. Neurotransmitter release from bradykinin-stimulated PC12 cells. Stimulation of cytosolic calcium and neurotransmitter release. Biochem J. 1989 Oct 1;263(1):11–18. doi: 10.1042/bj2630011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P. Calcium transport and buffering in neurons. Trends Neurosci. 1988 Oct;11(10):438–443. doi: 10.1016/0166-2236(88)90195-6. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Cheek T. R., Morgan A., O'Sullivan A. J., Moreton R. B., Berridge M. J., Mata A. M., Colyer J., Lee A. G., East J. M. Distribution of two distinct Ca2+-ATPase-like proteins and their relationships to the agonist-sensitive calcium store in adrenal chromaffin cells. Nature. 1989 Nov 2;342(6245):72–74. doi: 10.1038/342072a0. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Fasolato C., Pandiella A., Meldolesi J., Pozzan T. Generation of inositol phosphates, cytosolic Ca2+, and ionic fluxes in PC12 cells treated with bradykinin. J Biol Chem. 1988 Nov 25;263(33):17350–17359. [PubMed] [Google Scholar]
- Garber S. S., Hoshi T., Aldrich R. W. Regulation of ionic currents in pheochromocytoma cells by nerve growth factor and dexamethasone. J Neurosci. 1989 Nov;9(11):3976–3987. doi: 10.1523/JNEUROSCI.09-11-03976.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti G., Madeddu L., Pandiella A., Pozzan T., Meldolesi J. Second-messenger generation in PC12 cells. Interactions between cyclic AMP and Ca2+ signals. Biochem J. 1988 Nov 1;255(3):753–760. doi: 10.1042/bj2550753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbeter A., Dupont G., Berridge M. J. Minimal model for signal-induced Ca2+ oscillations and for their frequency encoding through protein phosphorylation. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1461–1465. doi: 10.1073/pnas.87.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Kongsamut S., Miller R. J. Nerve growth factor modulates the drug sensitivity of neurotransmitter release from PC-12 cells. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2243–2247. doi: 10.1073/pnas.83.7.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscombe D., Madison D. V., Poenie M., Reuter H., Tsien R. W., Tsien R. Y. Imaging of cytosolic Ca2+ transients arising from Ca2+ stores and Ca2+ channels in sympathetic neurons. Neuron. 1988 Jul;1(5):355–365. doi: 10.1016/0896-6273(88)90185-7. [DOI] [PubMed] [Google Scholar]
- Lipscombe D., Madison D. V., Poenie M., Reuter H., Tsien R. Y., Tsien R. W. Spatial distribution of calcium channels and cytosolic calcium transients in growth cones and cell bodies of sympathetic neurons. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2398–2402. doi: 10.1073/pnas.85.7.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldolesi J., Volpe P., Pozzan T. The intracellular distribution of calcium. Trends Neurosci. 1988 Oct;11(10):449–452. doi: 10.1016/0166-2236(88)90197-x. [DOI] [PubMed] [Google Scholar]
- Miller R. J. Calcium signalling in neurons. Trends Neurosci. 1988 Oct;11(10):415–419. doi: 10.1016/0166-2236(88)90191-9. [DOI] [PubMed] [Google Scholar]
- Miller R. J. Multiple calcium channels and neuronal function. Science. 1987 Jan 2;235(4784):46–52. doi: 10.1126/science.2432656. [DOI] [PubMed] [Google Scholar]
- Neering I. R., McBurney R. N. Role for microsomal Ca storage in mammalian neurones? Nature. 1984 May 10;309(5964):158–160. doi: 10.1038/309158a0. [DOI] [PubMed] [Google Scholar]
- Plummer M. R., Logothetis D. E., Hess P. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron. 1989 May;2(5):1453–1463. doi: 10.1016/0896-6273(89)90191-8. [DOI] [PubMed] [Google Scholar]
- Streit J., Lux H. D. Distribution of calcium currents in sprouting PC12 cells. J Neurosci. 1989 Dec;9(12):4190–4199. doi: 10.1523/JNEUROSCI.09-12-04190.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit J., Lux H. D. Voltage dependent calcium currents in PC12 growth cones and cells during NGF-induced cell growth. Pflugers Arch. 1987 May;408(6):634–641. doi: 10.1007/BF00581167. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Tsukui H., Hatanaka H. Neuronal differentiation of Ca2+ channel by nerve growth factor. Brain Res. 1985 Aug 26;341(2):381–384. doi: 10.1016/0006-8993(85)91079-0. [DOI] [PubMed] [Google Scholar]
- Thayer S. A., Hirning L. D., Miller R. J. The role of caffeine-sensitive calcium stores in the regulation of the intracellular free calcium concentration in rat sympathetic neurons in vitro. Mol Pharmacol. 1988 Nov;34(5):664–673. [PubMed] [Google Scholar]
- Thayer S. A., Perney T. M., Miller R. J. Regulation of calcium homeostasis in sensory neurons by bradykinin. J Neurosci. 1988 Nov;8(11):4089–4097. doi: 10.1523/JNEUROSCI.08-11-04089.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W., Lipscombe D., Madison D. V., Bley K. R., Fox A. P. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988 Oct;11(10):431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- Usowicz M. M., Porzig H., Becker C., Reuter H. Differential expression by nerve growth factor of two types of Ca2+ channels in rat phaeochromocytoma cell lines. J Physiol. 1990 Jul;426:95–116. doi: 10.1113/jphysiol.1990.sp018128. [DOI] [PMC free article] [PubMed] [Google Scholar]


