Abstract
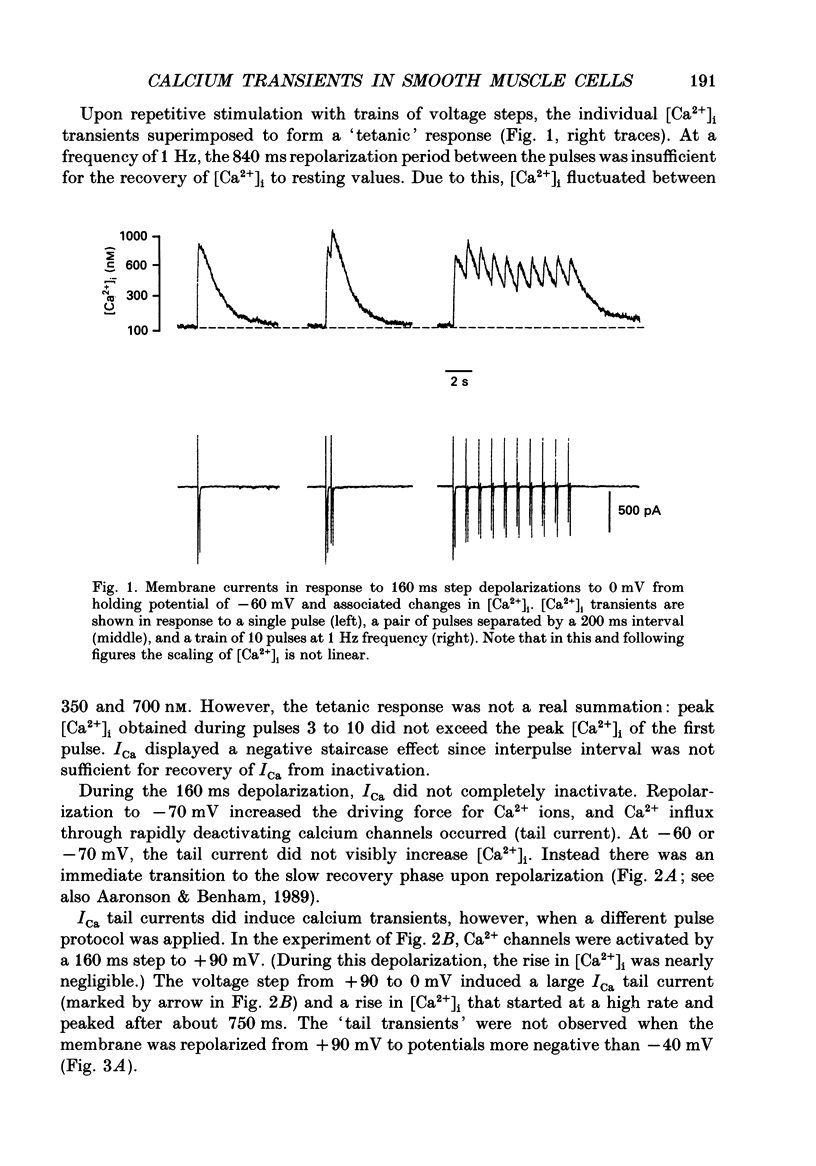
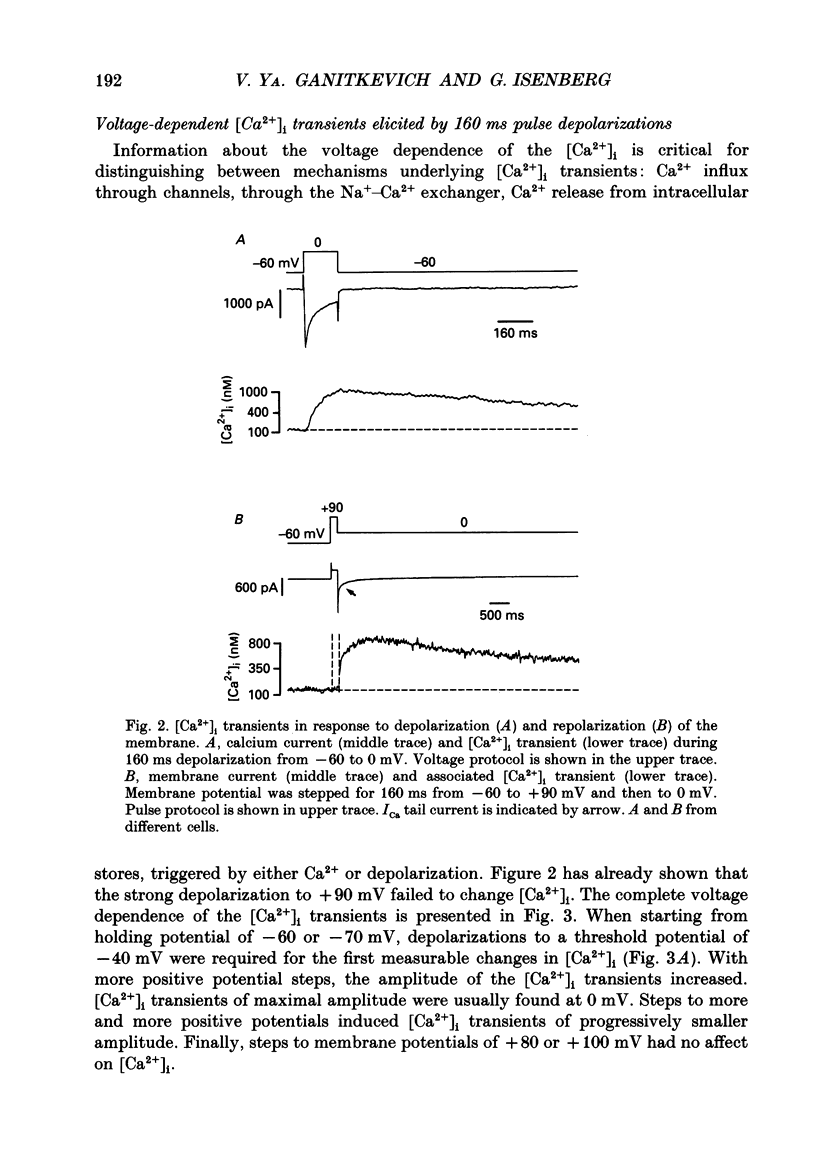
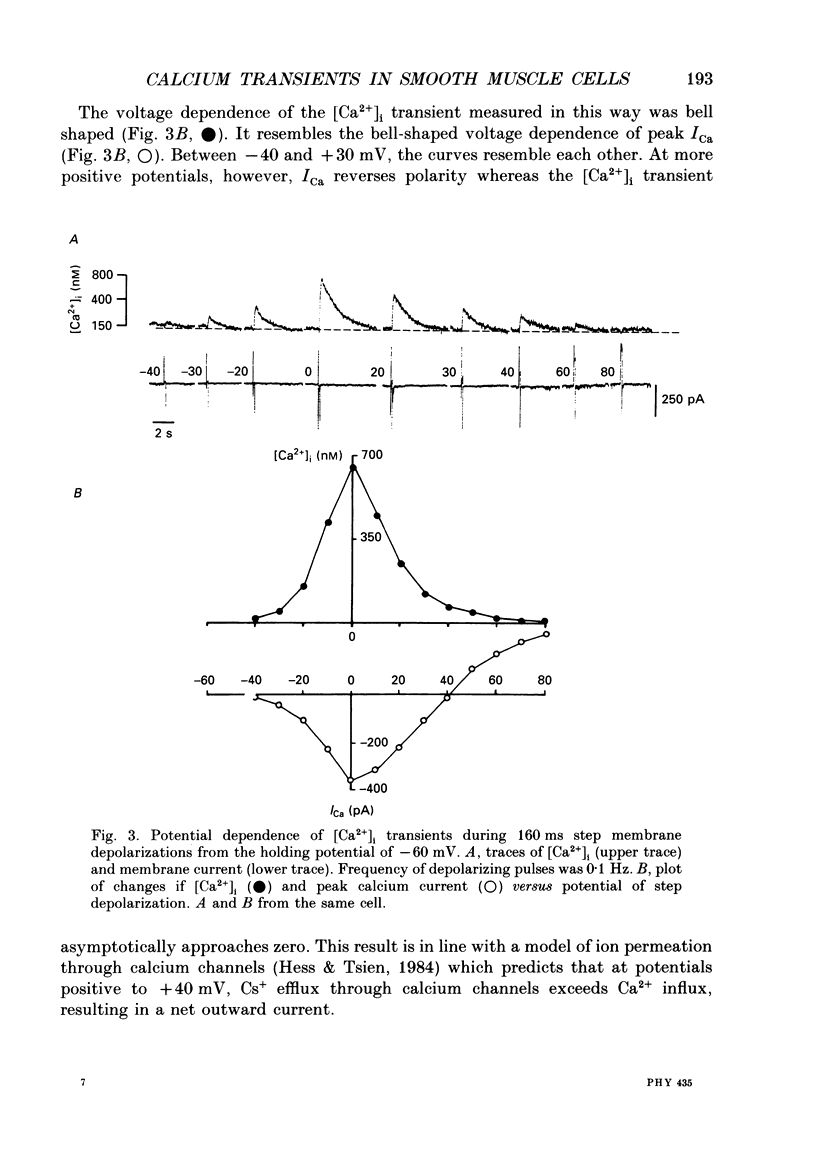
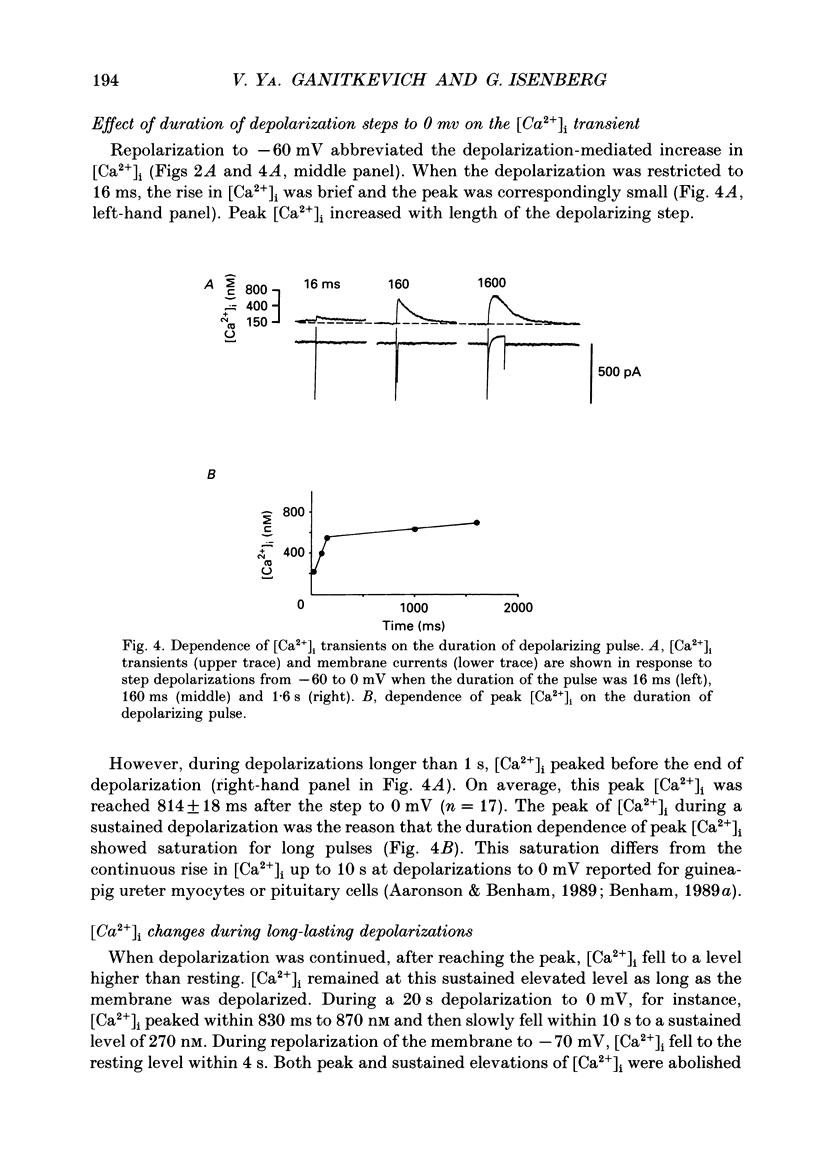
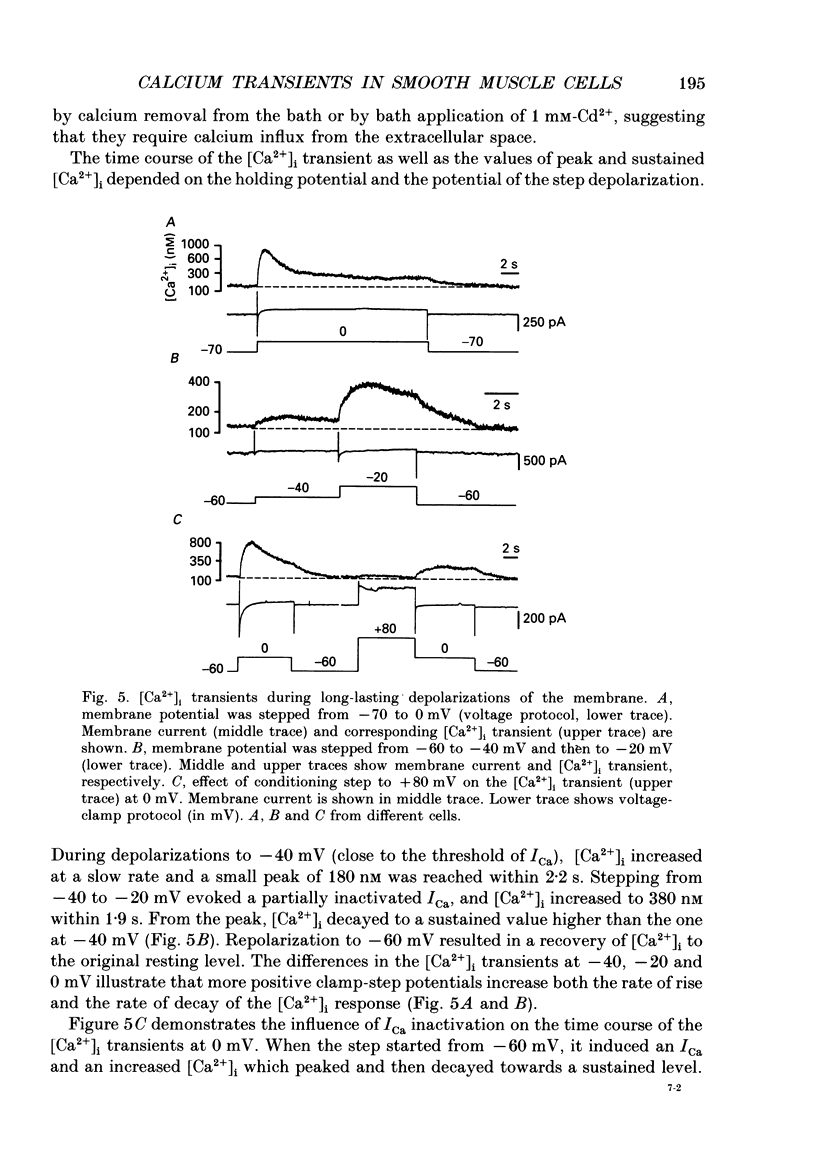
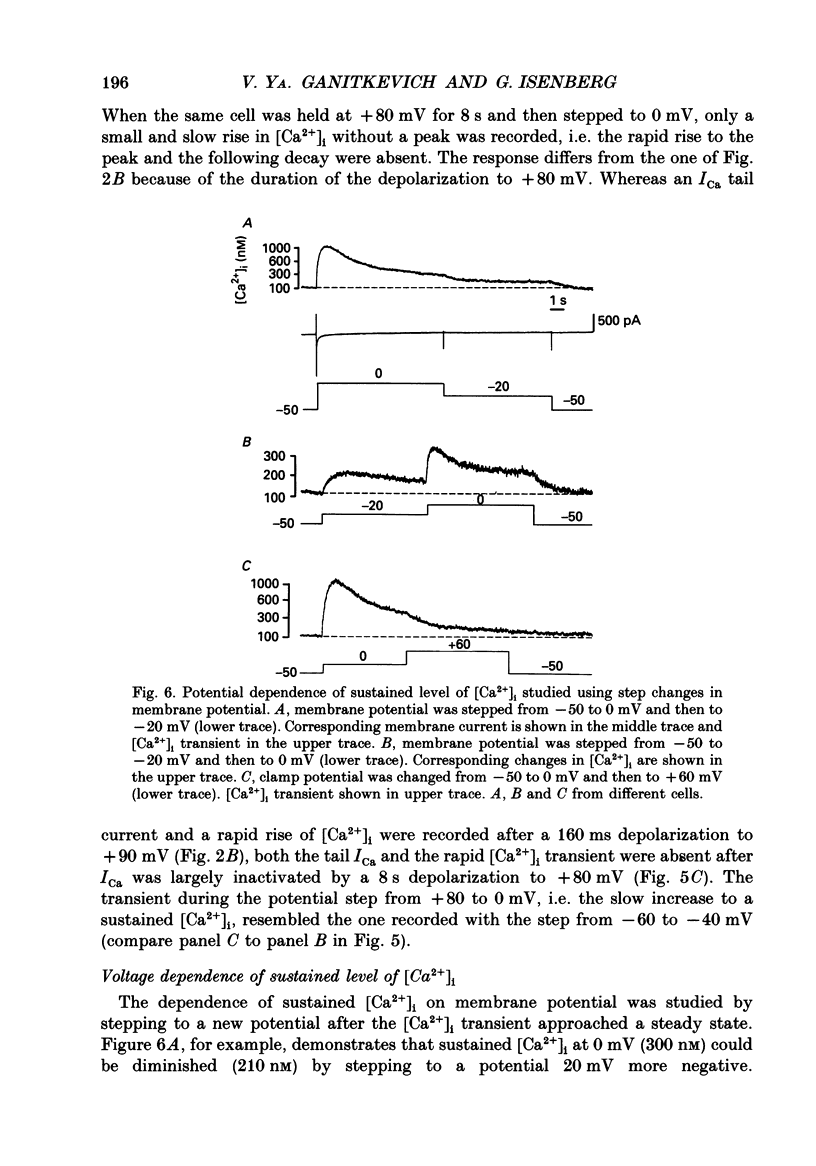
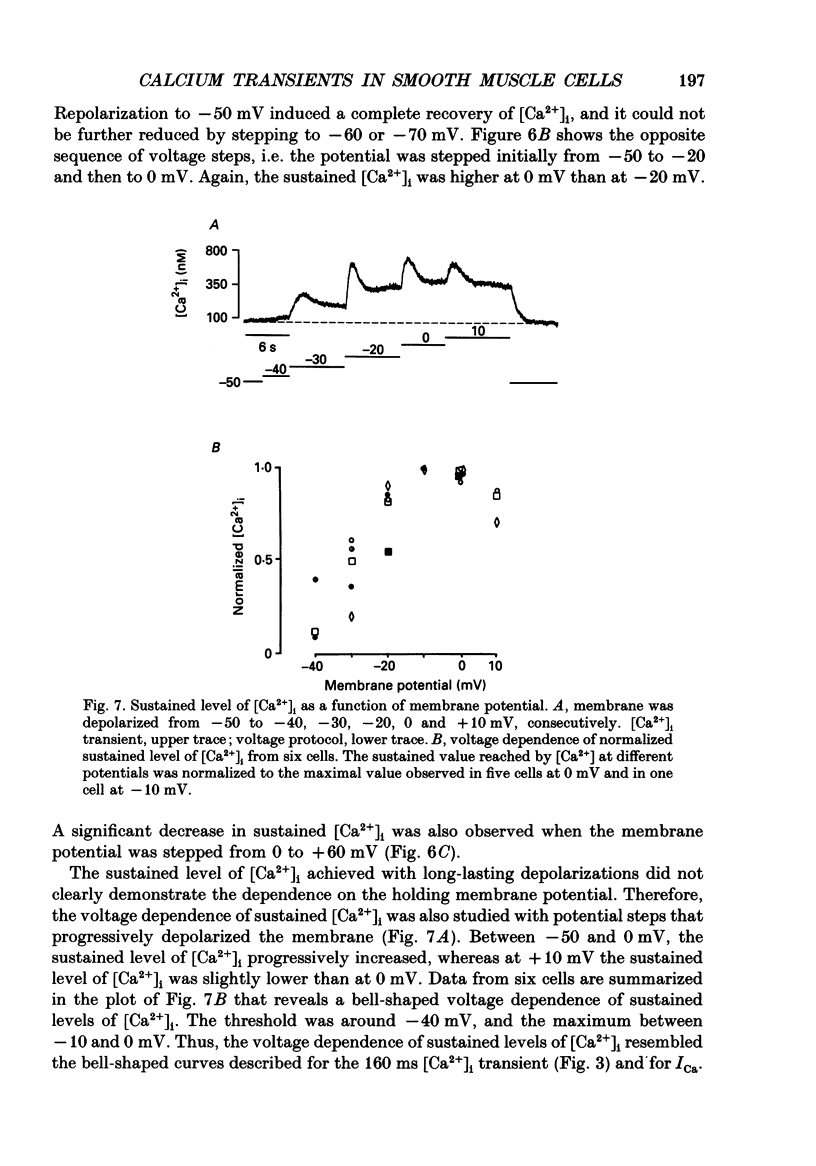
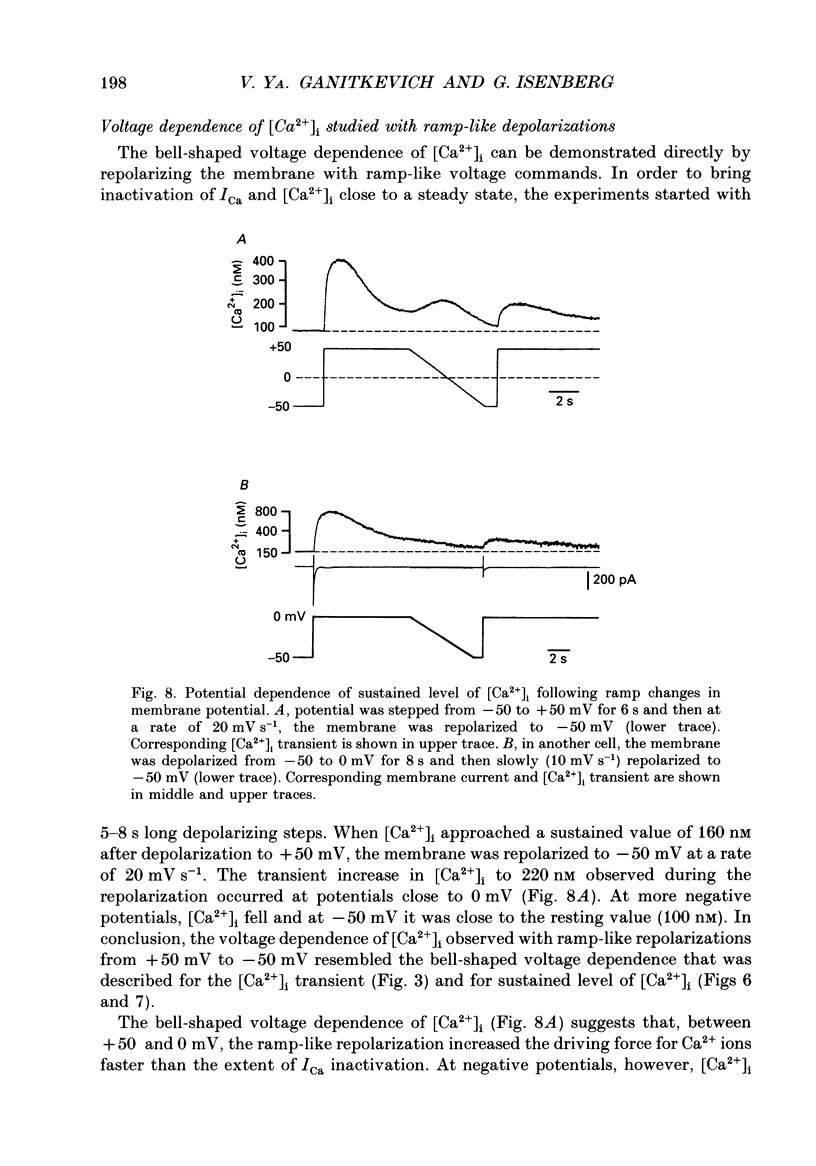
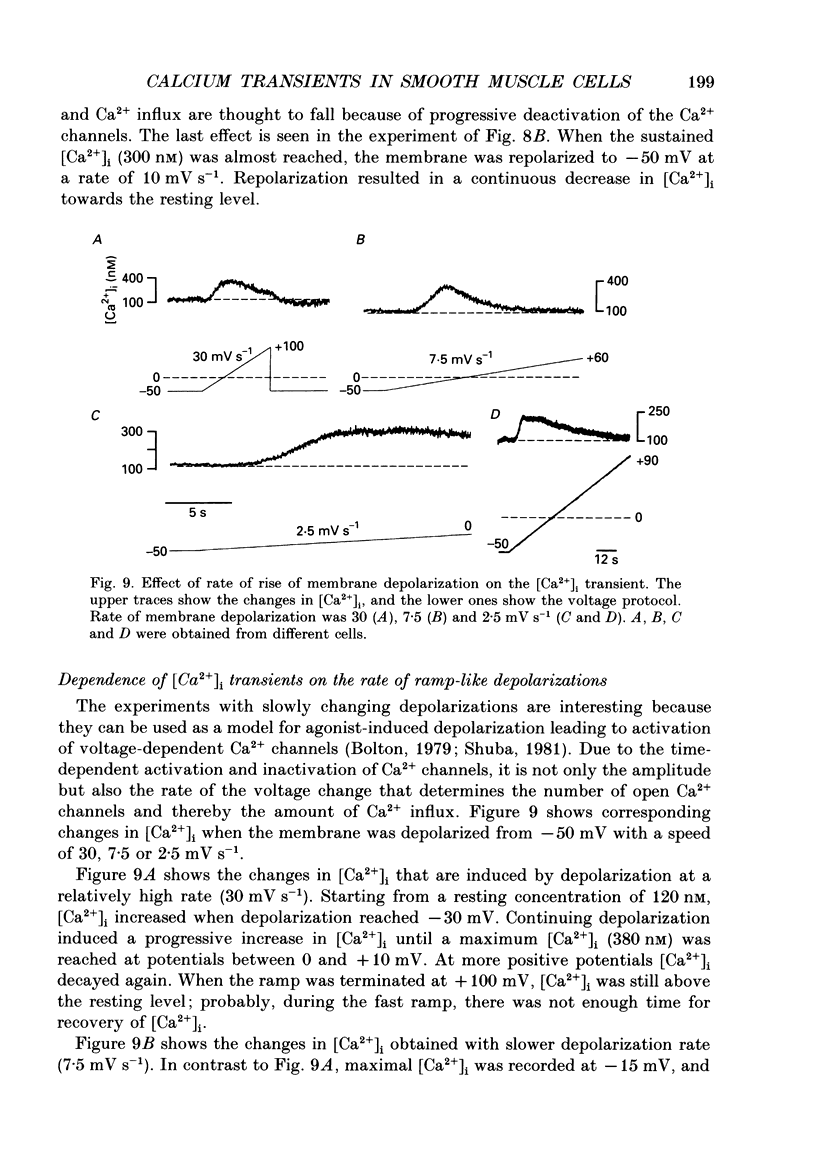
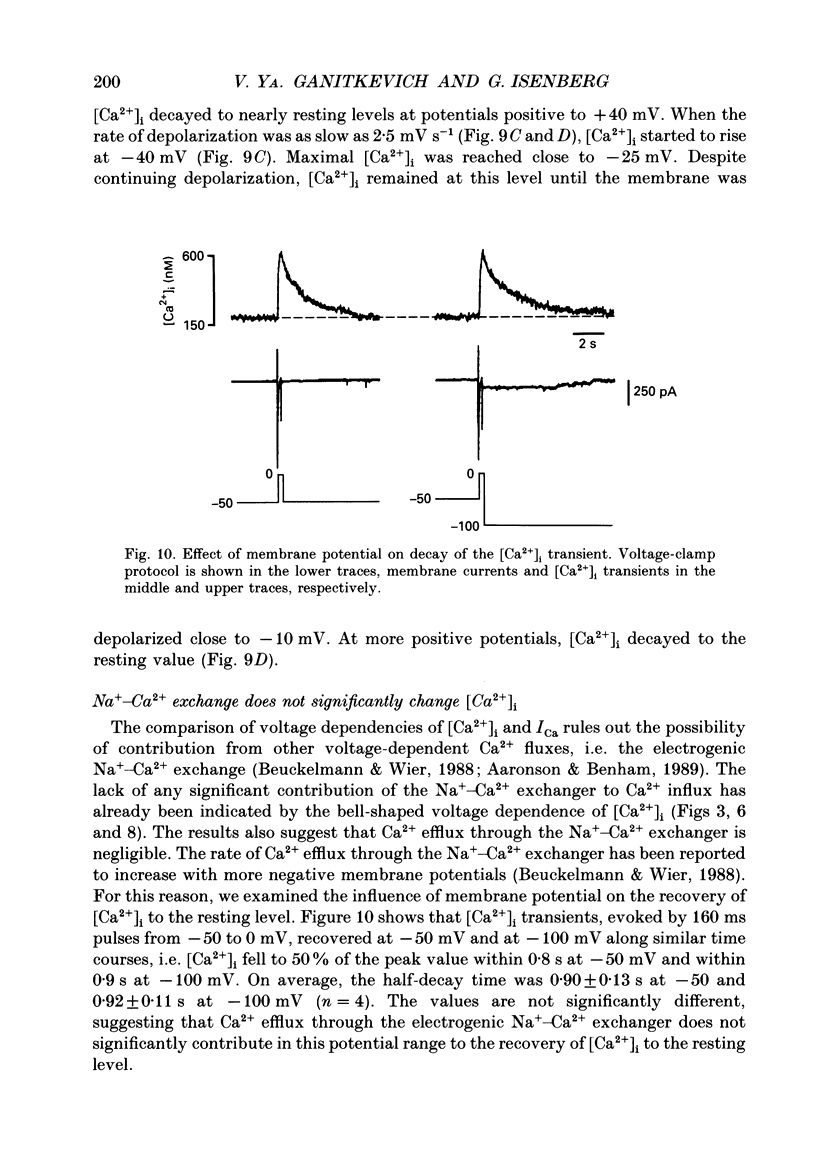
1. Free intracellular calcium concentration ([Ca2+]i) was recorded in single smooth muscle cells of the guinea-pig urinary bladder held under voltage clamp at 36 degrees C and 3.6 mM-extracellular Ca2+. The Ca2+ indicator Indo-1 was loaded into the cells through patch electrodes. To separate Ca2+ currents (ICa), superimposed K+ currents were suppressed with a Cs(+)-containing electrode solution. 2. At a holding potential of -60 mV, resting [Ca2+]i was 114 +/- 22 nM (mean +/- S.D.). During 160 ms depolarization steps to 0 mV, [Ca2+]i rose to 885 +/- 140 nM. With steps of varied duration, peak [Ca2+]i increased with the time of depolarization up to about 1 s. Upon repolarization [Ca2+]i recovered to resting levels with a half-decay time of about 1 s; recovery was not significantly changed with repolarization potentials between -50 and -100 mV. 3. The potential dependence of the above peak [Ca2+]i transients was bell shaped, with a threshold around -40 mV and a maximum at 0 mV. During depolarization steps to potentials more positive than +80 mV [Ca2+]i did not significantly rise. 4. During step depolarizations to 0 mV lasting 10 s or longer, [Ca2+]i peaked within 814 +/- 18 ms and then decayed to a sustained level of 250 +/- 60 nM. The amplitude of the [Ca2+]i peak as well as the time course of the transient depended on the amplitude of ICa. The depolarizations increased [Ca2+]i to a sustained level with no clearly defined peak when ICa was reduced by partial inactivation or during steps close to the threshold of ICa (-40 mV). 5. The sustained level of [Ca2+]i with longer depolarizations of several seconds showed a bell-shaped voltage dependence with a maximum close to 0 mV. A bell-shaped voltage dependence for [Ca2+]i was also found during ramp-like depolarizations. However, when the rate of depolarization was low (7.5 mV s-1), the peak [Ca2+]i was found at more negative potentials (-15 mV). 6. The results are compatible with the idea that Ca2+ influx through voltage-operated Ca2+ channels is the key event in depolarization-mediated changes in [Ca2+]i in smooth muscle cells from urinary bladder.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson P. I., Benham C. D. Alterations in [Ca2+]i mediated by sodium-calcium exchange in smooth muscle cells isolated from the guinea-pig ureter. J Physiol. 1989 Sep;416:1–18. doi: 10.1113/jphysiol.1989.sp017745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaronson P. I., Bolton T. B., Lang R. J., MacKenzie I. Calcium currents in single isolated smooth muscle cells from the rabbit ear artery in normal-calcium and high-barium solutions. J Physiol. 1988 Nov;405:57–75. doi: 10.1113/jphysiol.1988.sp017321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., Neher E. The Ca signal from fura-2 loaded mast cells depends strongly on the method of dye-loading. FEBS Lett. 1985 Nov 11;192(1):13–18. doi: 10.1016/0014-5793(85)80033-8. [DOI] [PubMed] [Google Scholar]
- Barcenas-Ruiz L., Beuckelmann D. J., Wier W. G. Sodium-calcium exchange in heart: membrane currents and changes in [Ca2+]i. Science. 1987 Dec 18;238(4834):1720–1722. doi: 10.1126/science.3686010. [DOI] [PubMed] [Google Scholar]
- Barcenas-Ruiz L., Wier W. G. Voltage dependence of intracellular [Ca2+]i transients in guinea pig ventricular myocytes. Circ Res. 1987 Jul;61(1):148–154. doi: 10.1161/01.res.61.1.148. [DOI] [PubMed] [Google Scholar]
- Becker P. L., Singer J. J., Walsh J. V., Jr, Fay F. S. Regulation of calcium concentration in voltage-clamped smooth muscle cells. Science. 1989 Apr 14;244(4901):211–214. doi: 10.1126/science.2704996. [DOI] [PubMed] [Google Scholar]
- Benham C. D. ATP-activated channels gate calcium entry in single smooth muscle cells dissociated from rabbit ear artery. J Physiol. 1989 Dec;419:689–701. doi: 10.1113/jphysiol.1989.sp017893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D. Voltage-gated and agonist-mediated rises in intracellular Ca2+ in rat clonal pituitary cells (GH3) held under voltage clamp. J Physiol. 1989 Aug;415:143–158. doi: 10.1113/jphysiol.1989.sp017716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuckelmann D. J., Wier W. G. Mechanism of release of calcium from sarcoplasmic reticulum of guinea-pig cardiac cells. J Physiol. 1988 Nov;405:233–255. doi: 10.1113/jphysiol.1988.sp017331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Bond M., Shuman H., Somlyo A. P., Somlyo A. V. Total cytoplasmic calcium in relaxed and maximally contracted rabbit portal vein smooth muscle. J Physiol. 1984 Dec;357:185–201. doi: 10.1113/jphysiol.1984.sp015496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed K. E. Effects of ions and drugs on the smooth muscle cell membrane of the guinea-pig urinary bladder. Pflugers Arch. 1971;326(2):127–141. doi: 10.1007/BF00586905. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983 Jul;245(1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Ganitkevich VYa, Shuba M. F., Smirnov S. V. Calcium-dependent inactivation of potential-dependent calcium inward current in an isolated guinea-pig smooth muscle cell. J Physiol. 1987 Nov;392:431–449. doi: 10.1113/jphysiol.1987.sp016789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman W. F., Wier W. G., Blaustein M. P. Effects of activation on distribution of Ca2+ in single arterial smooth muscle cells. Determination with fura-2 digital imaging microscopy. Circ Res. 1989 May;64(5):1019–1029. doi: 10.1161/01.res.64.5.1019. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Himpens B., Somlyo A. P. Free-calcium and force transients during depolarization and pharmacomechanical coupling in guinea-pig smooth muscle. J Physiol. 1988 Jan;395:507–530. doi: 10.1113/jphysiol.1988.sp016932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi Y., Muraki K., Takeda M., Watanabe M. Measurement and simulation of noninactivating Ca current in smooth muscle cells. Am J Physiol. 1989 Apr;256(4 Pt 1):C880–C885. doi: 10.1152/ajpcell.1989.256.4.C880. [DOI] [PubMed] [Google Scholar]
- Kimura J., Miyamae S., Noma A. Identification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J Physiol. 1987 Mar;384:199–222. doi: 10.1113/jphysiol.1987.sp016450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Action potentials and net membrane currents of isolated smooth muscle cells (urinary bladder of the guinea-pig). Pflugers Arch. 1985 Dec;405(4):329–339. doi: 10.1007/BF00595685. [DOI] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Calcium currents of cesium loaded isolated smooth muscle cells (urinary bladder of the guinea pig). Pflugers Arch. 1985 Dec;405(4):340–348. doi: 10.1007/BF00595686. [DOI] [PubMed] [Google Scholar]
- Lang R. J. The whole-cell Ca2+ channel current in single smooth muscle cells of the guinea-pig ureter. J Physiol. 1990 Apr;423:453–473. doi: 10.1113/jphysiol.1990.sp018033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W., Schneider M. F., Simon B. J., Szucs G. Intramembrane charge movement and calcium release in frog skeletal muscle. J Physiol. 1986 Apr;373:481–511. doi: 10.1113/jphysiol.1986.sp016059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Vascular smooth muscle: the first recorded Ca2+ transients. Pflugers Arch. 1982 Oct;395(1):75–77. doi: 10.1007/BF00584972. [DOI] [PubMed] [Google Scholar]
- Thayer S. A., Hirning L. D., Miller R. J. The role of caffeine-sensitive calcium stores in the regulation of the intracellular free calcium concentration in rat sympathetic neurons in vitro. Mol Pharmacol. 1988 Nov;34(5):664–673. [PubMed] [Google Scholar]
- Yagi S., Becker P. L., Fay F. S. Relationship between force and Ca2+ concentration in smooth muscle as revealed by measurements on single cells. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4109–4113. doi: 10.1073/pnas.85.11.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H. Recording of intracellular Ca2+ from smooth muscle cells by sub-micron tip, double-barrelled CA2+-selective microelectrodes. Cell Calcium. 1986 Aug;7(4):203–219. doi: 10.1016/0143-4160(86)90001-1. [DOI] [PubMed] [Google Scholar]


