Abstract
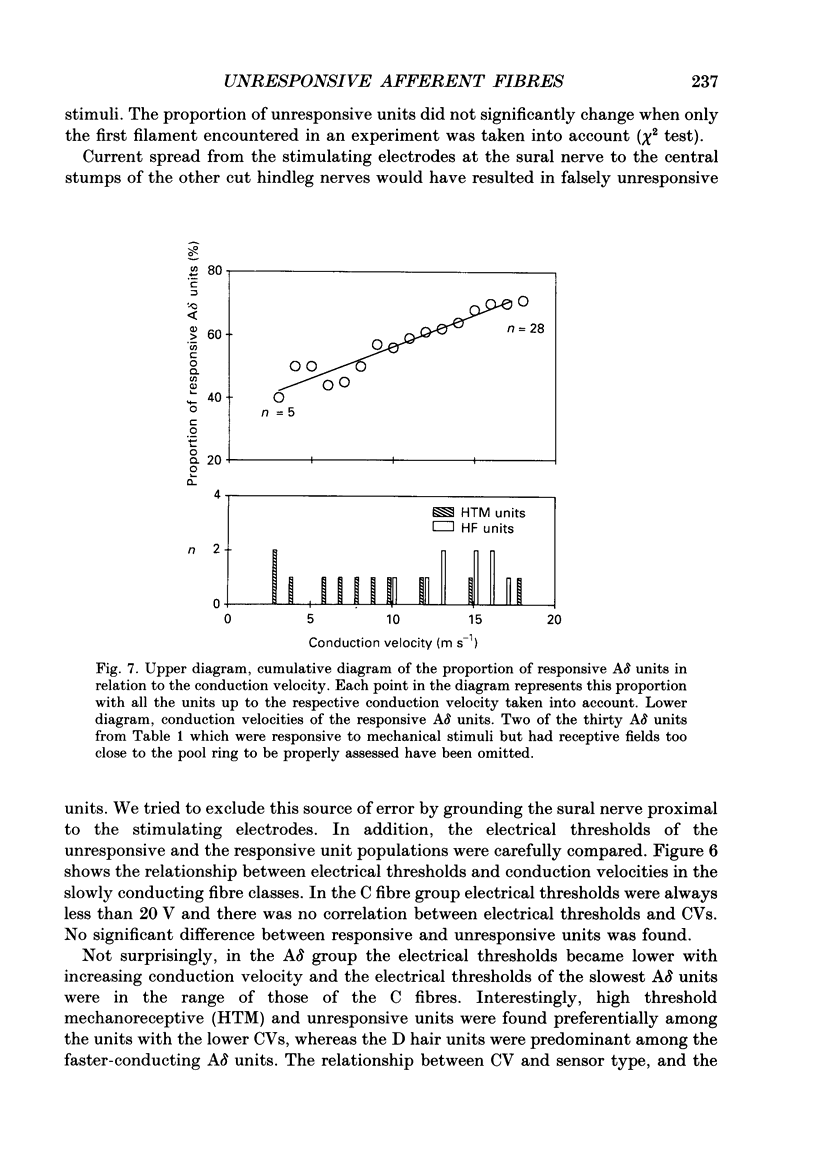
1. The proportion of primary afferent nerve fibres in a skin nerve of the rat that responded or failed to respond to mechanical or thermal stimulation of the skin in the noxious and non-noxious range was analysed. 2. Activity of afferent nerve fibres was recorded from the dorsal roots. Units projecting into the sural nerve were selected using supramaximal electrical stimulation of the nerve stem. All other hindleg nerves were cut. 3. The receptive fields were searched by carefully examining the hindleg skin with noxious and innocuous mechanical, cooling and warming stimuli. Probing of the intrinsic foot muscles and manipulation of the ankle and toe joints was employed to recruit units projecting to deeper tissues. 4. In a first series of twenty-two experiments, eighty-nine rapidly conducting myelinated A beta units, thirty slowly conducting myelinated A delta units and 101 unmyelinated C units were investigated. Most units were identified as belonging to one of the established classes of cutaneous sensory units and this was also ascertained by a collision test. 5. Two A beta, eight A delta and forty-six C fibres did not respond to any one of the stimuli. Electrical thresholds and conduction velocities of the unresponsive C fibres were not significantly different from those of the units responding to natural stimulation of their receptive fields. In the A delta group unresponsive and high threshold mechanoreceptive units were preferentially found among the units with the slowest conduction velocities. 6. In a second series of seven experiments, one single nerve filament containing responsive and unresponsive C fibres was tested repetitively at 30 min intervals. Twenty unresponsive units and seven units responding to noxious mechanical and/or heat stimuli were studied. Ten of the twenty initially unresponsive units became activated by mechanical and/or heat stimuli after observation times of 30-150 min. Some of these units had mechanical thresholds as low as 64 mN (tested with calibrated von Frey hairs), or thermal thresholds down to 42 degrees C. 7. Two of the ten C units which became responsive in the course of an experiment later lost their responsiveness again. On the other hand, two of the C units which were initially responsive to noxious heat and/or noxious mechanical stimuli became completely unresponsive after repetitive stimulation, whereas one unit initially only responding to noxious heat became responsive to mechanical stimuli, suggesting that mechanical and heat responsiveness may be separately gained or lost by sensory C fibres.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


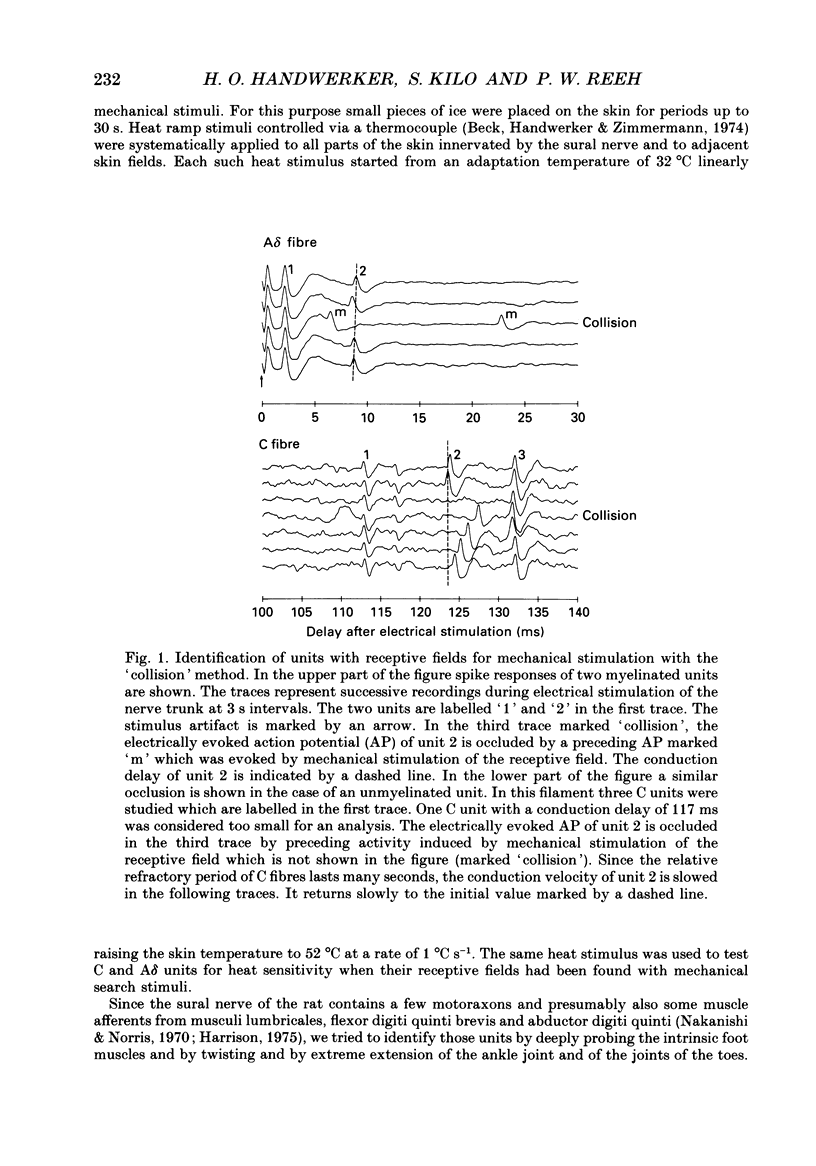

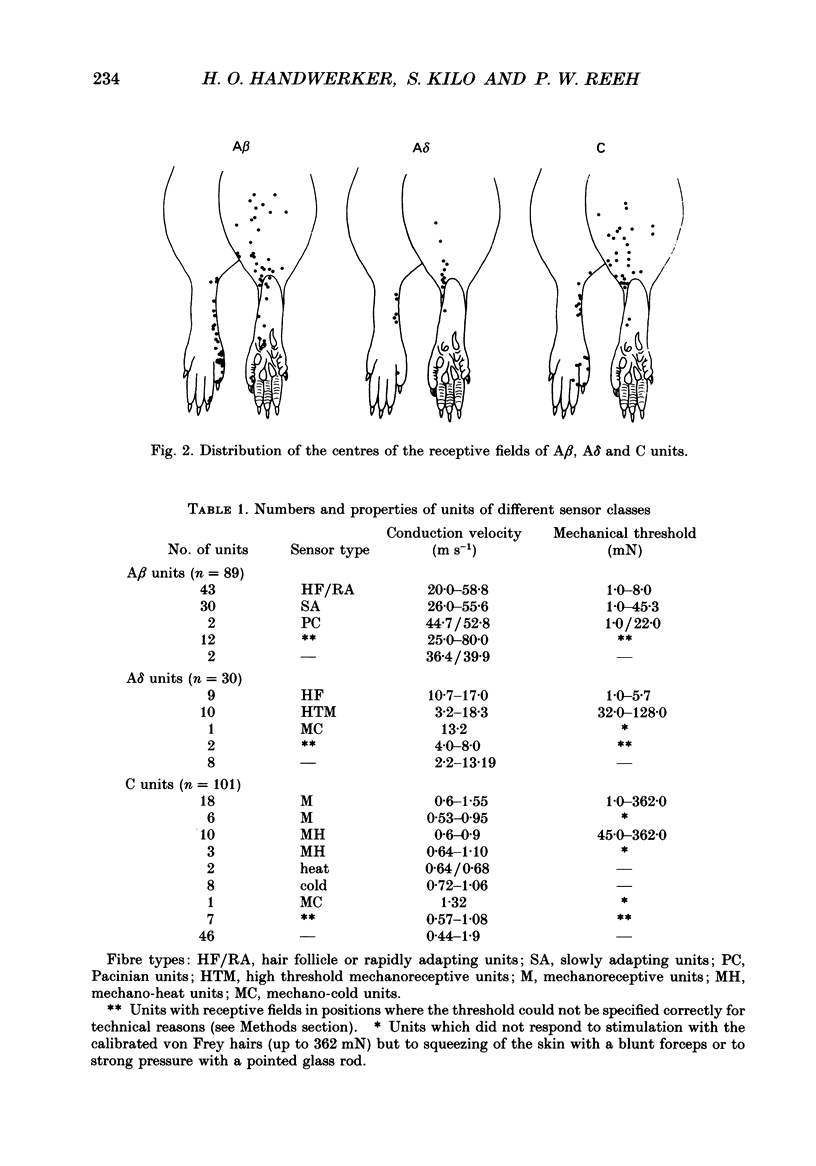
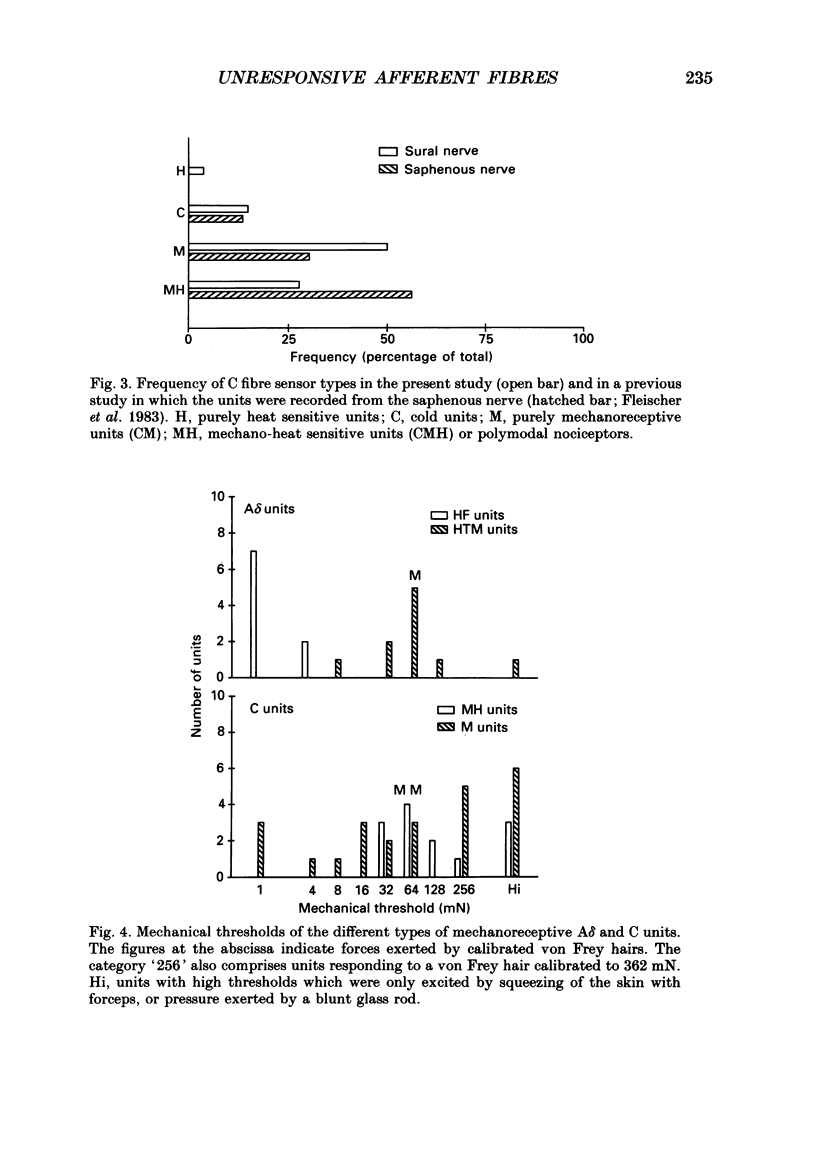
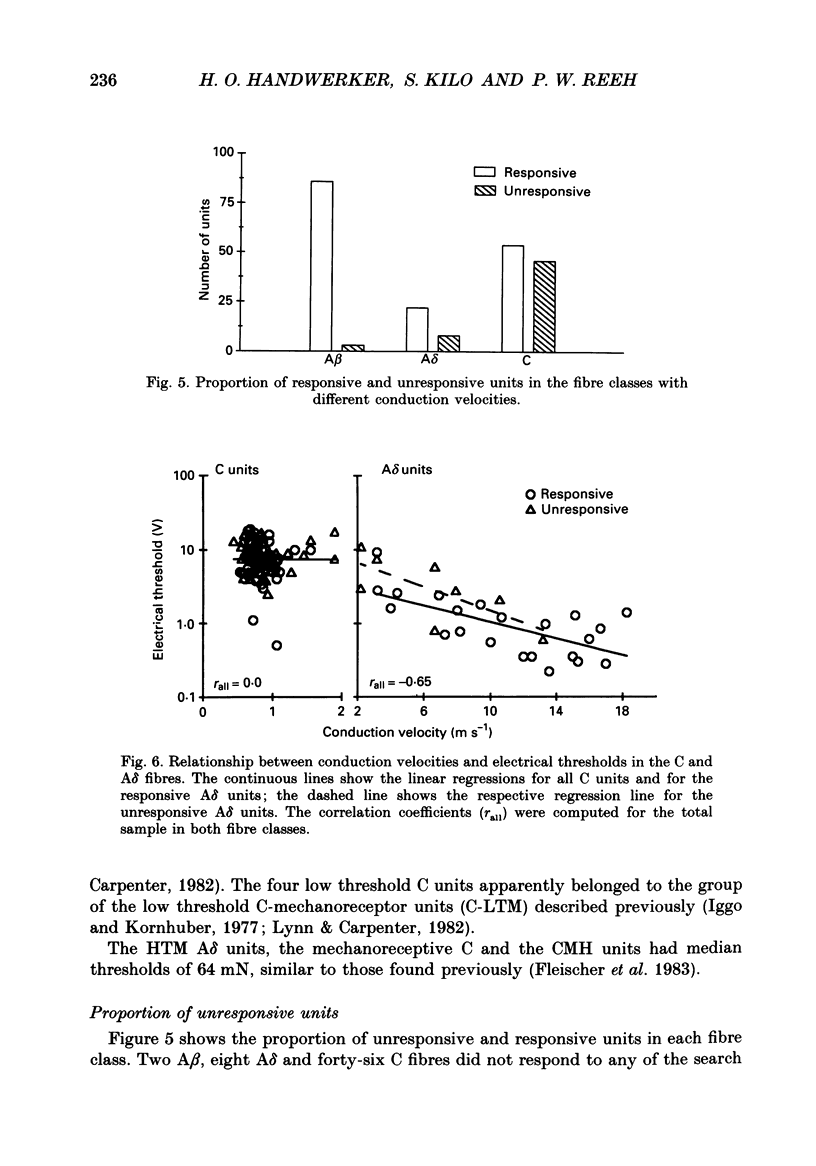





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck P. W., Handwerker H. O., Zimmermann M. Nervous outflow from the cat's foot during noxious radiant heat stimulation. Brain Res. 1974 Mar 8;67(3):373–386. doi: 10.1016/0006-8993(74)90488-0. [DOI] [PubMed] [Google Scholar]
- Bessou P., Perl E. R. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol. 1969 Nov;32(6):1025–1043. doi: 10.1152/jn.1969.32.6.1025. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Iggo A. A quantitative study of cutaneous receptors and afferent fibres in the cat and rabbit. J Physiol. 1967 Dec;193(3):707–733. doi: 10.1113/jphysiol.1967.sp008390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess P. R., Perl E. R. Myelinated afferent fibres responding specifically to noxious stimulation of the skin. J Physiol. 1967 Jun;190(3):541–562. doi: 10.1113/jphysiol.1967.sp008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer E., Handwerker H. O., Joukhadar S. Unmyelinated nociceptive units in two skin areas of the rat. Brain Res. 1983 May 9;267(1):81–92. doi: 10.1016/0006-8993(83)91041-7. [DOI] [PubMed] [Google Scholar]
- Forster C., Handwerker H. O. Automatic classification and analysis of microneurographic spike data using a PC/AT. J Neurosci Methods. 1990 Feb;31(2):109–118. doi: 10.1016/0165-0270(90)90155-9. [DOI] [PubMed] [Google Scholar]
- Gybels J., Handwerker H. O., Van Hees J. A comparison between the discharges of human nociceptive nerve fibres and the subject's ratings of his sensations. J Physiol. 1979 Jul;292:193–206. doi: 10.1113/jphysiol.1979.sp012846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerker H. O., Anton F., Reeh P. W. Discharge patterns of afferent cutaneous nerve fibers from the rat's tail during prolonged noxious mechanical stimulation. Exp Brain Res. 1987;65(3):493–504. doi: 10.1007/BF00235972. [DOI] [PubMed] [Google Scholar]
- Harrison L. M. Fiber diameter spectrum of the motor fibers of rat sural nerve. Exp Neurol. 1975 May;47(2):364–366. doi: 10.1016/0014-4886(75)90266-6. [DOI] [PubMed] [Google Scholar]
- Häbler H. J., Jänig W., Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol. 1990 Jun;425:545–562. doi: 10.1113/jphysiol.1990.sp018117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IGGO A. The electrophysiological identification of single nerve fibres, with particular reference to the slowest-conducting vagal afferent fibres in the cat. J Physiol. 1958 Jun 18;142(1):110–126. doi: 10.1113/jphysiol.1958.sp006002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A. Cutaneous and subcutaneous sense organs. Br Med Bull. 1977 May;33(2):97–102. doi: 10.1093/oxfordjournals.bmb.a071432. [DOI] [PubMed] [Google Scholar]
- Iggo A., Kornhuber H. H. A quantitative study of C-mechanoreceptors in hairy skin of the cat. J Physiol. 1977 Oct;271(2):549–565. doi: 10.1113/jphysiol.1977.sp012014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte R. H., Thalhammer J. G., Robinson C. J. Peripheral neural correlates of magnitude of cutaneous pain and hyperalgesia: a comparison of neural events in monkey with sensory judgments in human. J Neurophysiol. 1983 Jul;50(1):1–26. doi: 10.1152/jn.1983.50.1.1. [DOI] [PubMed] [Google Scholar]
- LaMotte R. H., Thalhammer J. G., Torebjörk H. E., Robinson C. J. Peripheral neural mechanisms of cutaneous hyperalgesia following mild injury by heat. J Neurosci. 1982 Jun;2(6):765–781. doi: 10.1523/JNEUROSCI.02-06-00765.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn B., Carpenter S. E. Primary afferent units from the hairy skin of the rat hind limb. Brain Res. 1982 Apr 22;238(1):29–43. doi: 10.1016/0006-8993(82)90768-5. [DOI] [PubMed] [Google Scholar]
- McMahon S. B., Koltzenburg M. Novel classes of nociceptors: beyond Sherrington. Trends Neurosci. 1990 Jun;13(6):199–201. doi: 10.1016/0166-2236(90)90159-8. [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Campbell J. N. Myelinated nociceptive afferents account for the hyperalgesia that follows a burn to the hand. Science. 1981 Sep 25;213(4515):1527–1529. doi: 10.1126/science.7280675. [DOI] [PubMed] [Google Scholar]
- Nakanishi T., Norris F. H., Jr Motor fibers in rat sural nerve. Exp Neurol. 1970 Feb;26(2):433–435. doi: 10.1016/0014-4886(70)90138-x. [DOI] [PubMed] [Google Scholar]
- Perl E. R. Myelinated afferent fibres innervating the primate skin and their response to noxious stimuli. J Physiol. 1968 Aug;197(3):593–615. doi: 10.1113/jphysiol.1968.sp008576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible H. G., Schmidt R. F. Excitation and sensitization of fine articular afferents from cat's knee joint by prostaglandin E2. J Physiol. 1988 Sep;403:91–104. doi: 10.1113/jphysiol.1988.sp017240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible H. G., Schmidt R. F. Time course of mechanosensitivity changes in articular afferents during a developing experimental arthritis. J Neurophysiol. 1988 Dec;60(6):2180–2195. doi: 10.1152/jn.1988.60.6.2180. [DOI] [PubMed] [Google Scholar]
- Swett J. E., Woolf C. J. The somatotopic organization of primary afferent terminals in the superficial laminae of the dorsal horn of the rat spinal cord. J Comp Neurol. 1985 Jan 1;231(1):66–77. doi: 10.1002/cne.902310106. [DOI] [PubMed] [Google Scholar]
- Szolcsanyi J., Anton F., Reeh P. W., Handwerker H. O. Selective excitation by capsaicin of mechano-heat sensitive nociceptors in rat skin. Brain Res. 1988 Apr 19;446(2):262–268. doi: 10.1016/0006-8993(88)90885-2. [DOI] [PubMed] [Google Scholar]
- Torebjörk H. E., LaMotte R. H., Robinson C. J. Peripheral neural correlates of magnitude of cutaneous pain and hyperalgesia: simultaneous recordings in humans of sensory judgments of pain and evoked responses in nociceptors with C-fibers. J Neurophysiol. 1984 Feb;51(2):325–339. doi: 10.1152/jn.1984.51.2.325. [DOI] [PubMed] [Google Scholar]
- Vallbo A. B., Hagbarth K. E., Torebjörk H. E., Wallin B. G. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979 Oct;59(4):919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Welk E., Petsche U., Fleischer E., Handwerker H. O. Altered excitability of afferent C-fibres of the rat distal to a nerve site exposed to capsaicin. Neurosci Lett. 1983 Aug 8;38(3):245–250. doi: 10.1016/0304-3940(83)90376-2. [DOI] [PubMed] [Google Scholar]


