Abstract
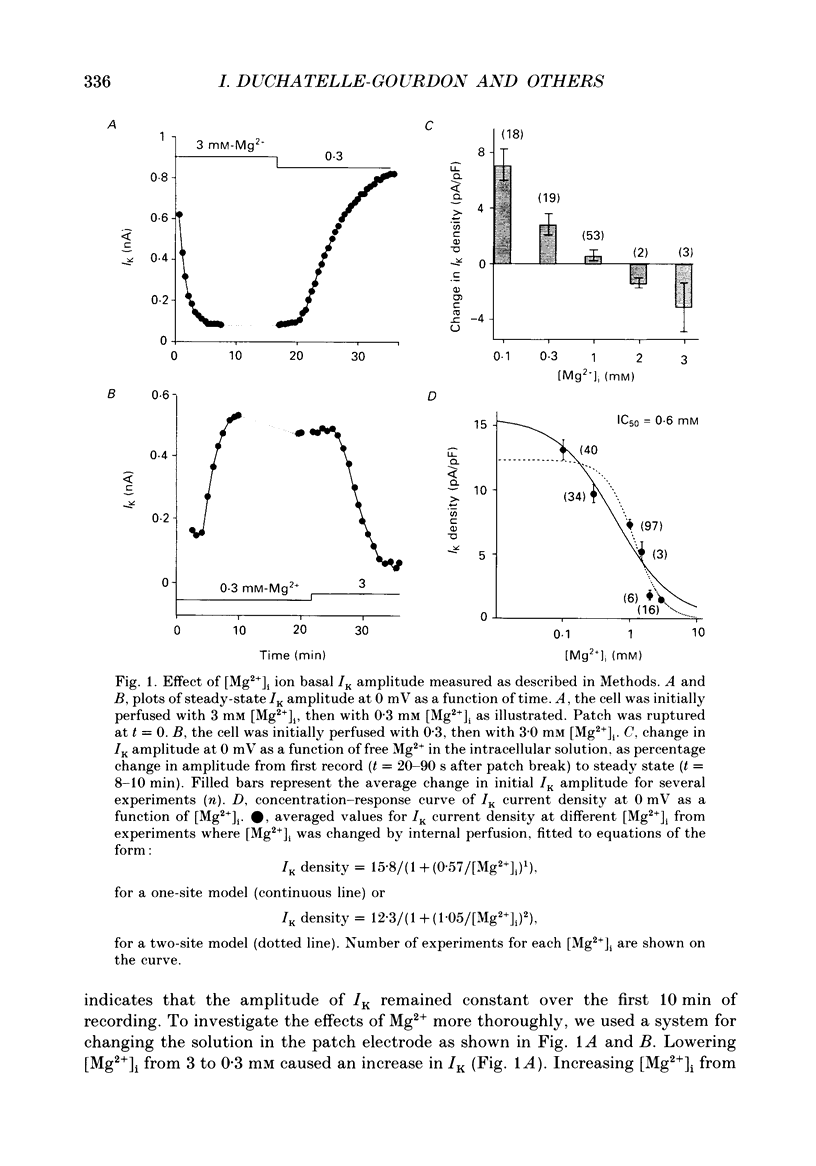
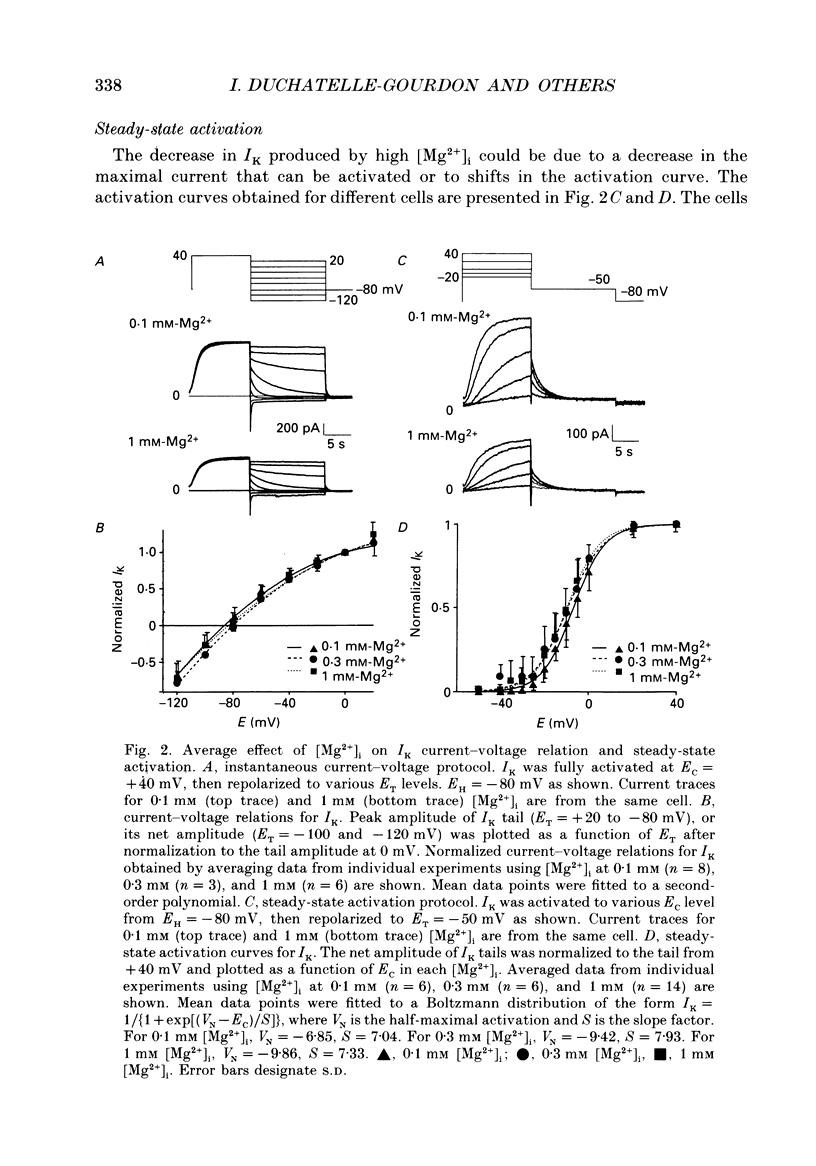
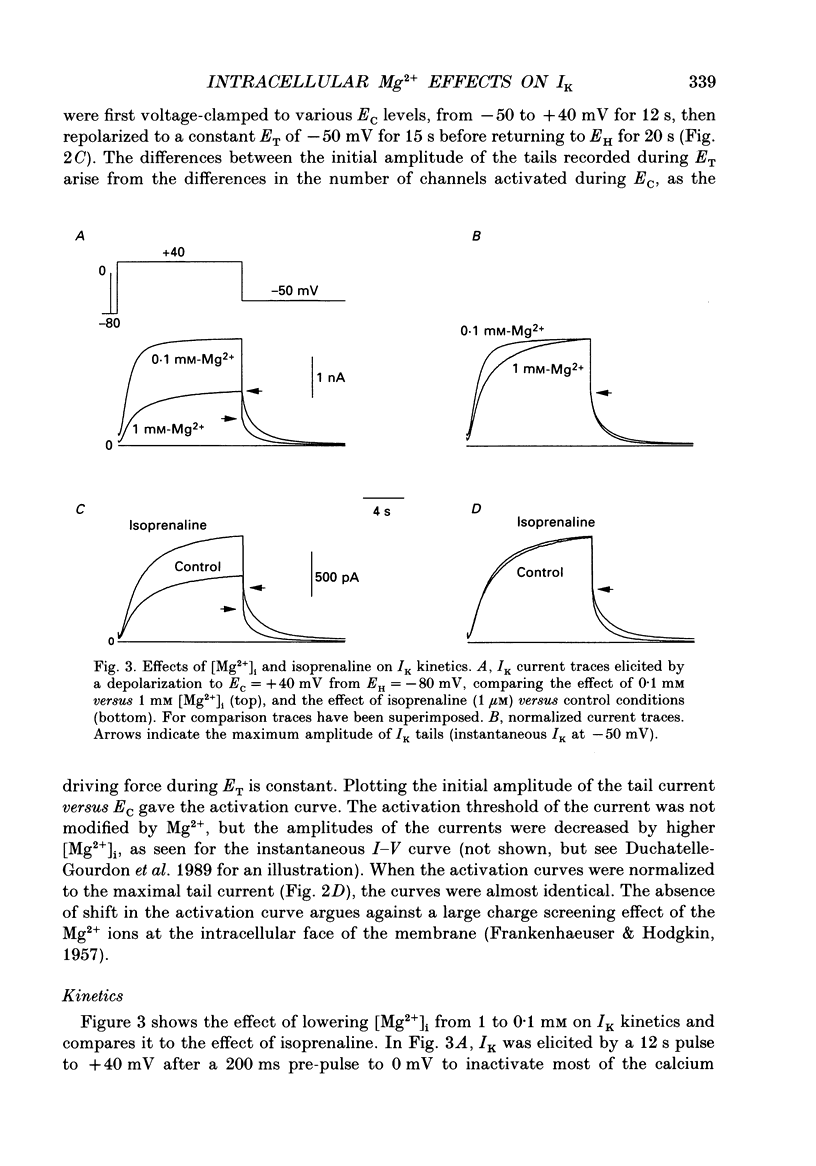
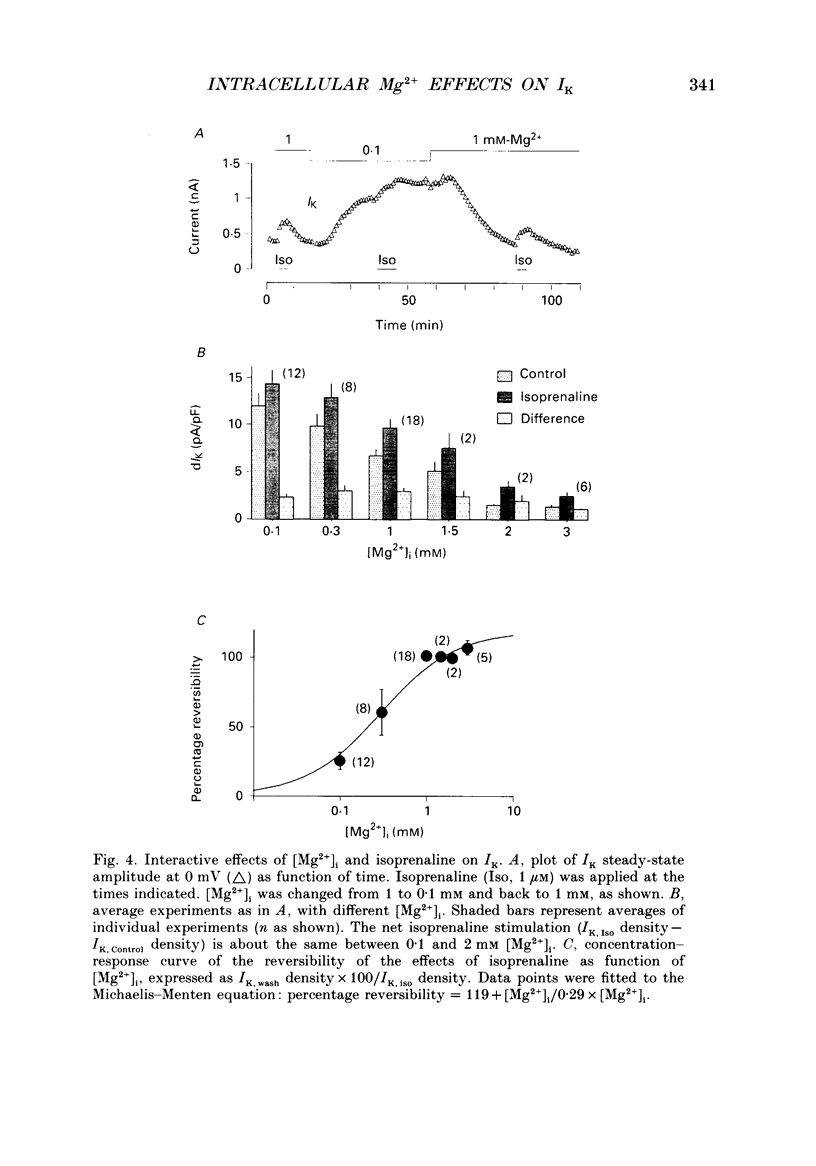
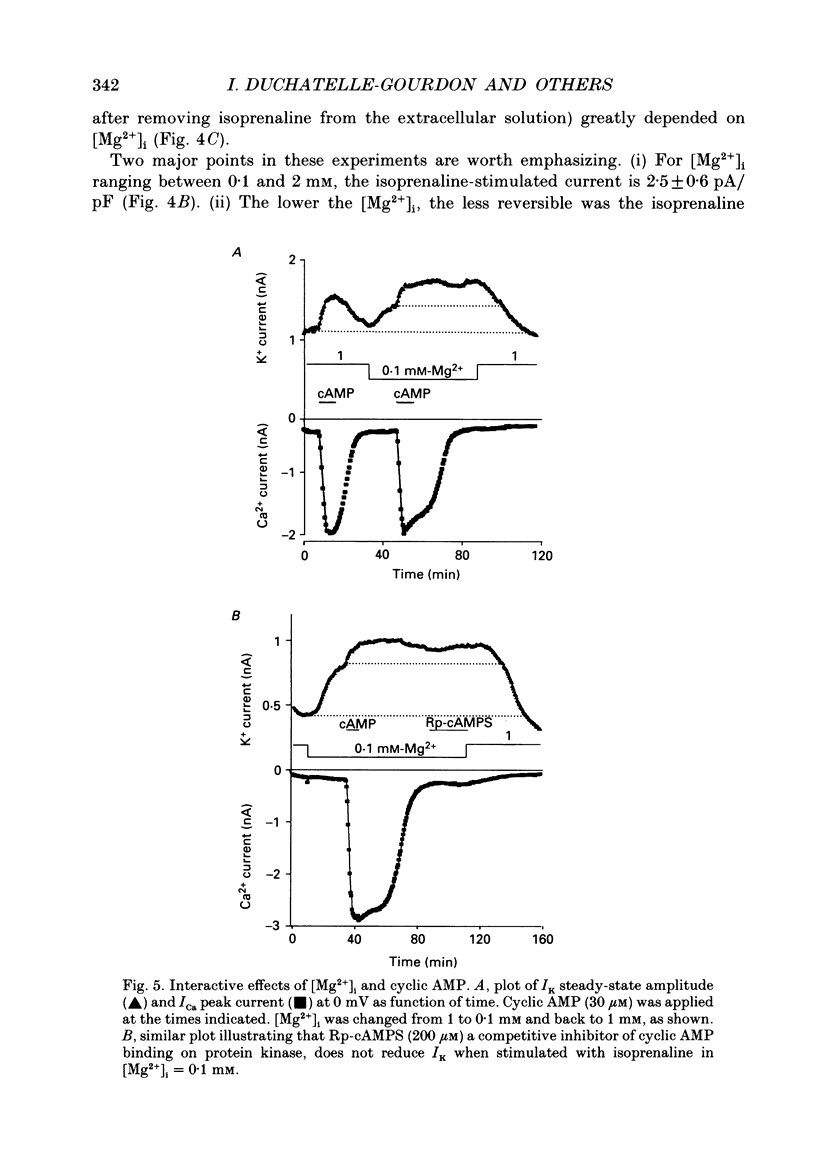
1. The effects of internal Mg2+ ions on the delayed rectifier potassium current (IK) of bull-frog atrial myocytes were studied using the whole-cell configuration of the patch-clamp technique with a perfusable patch electrode. 2. Initial variations in IK amplitude were dependent on [Mg2+]i. With [Mg2+] greater than 1 mM, the amplitude of IK usually decreased after initiating the whole-cell recording configuration (run-down); with [Mg2+]i less than 1 mM, IK usually increased (run-up). Mg2+ blocked IK with an apparent half-maximal effect of 0.6 mM [Mg2+]i. 3. The basal free [Mg2+]i, indicated by the amplitude of IK before run-up or run-down, was estimated from the relationship between [Mg2+]i and IK to be 0.8 mM. 4. The amplitude of both the activation curve and the instantaneous voltage-current relationship was decreased by increasing [Mg2+]i. Under these conditions, the voltage dependence of IK was not affected. 5. The rate of activation of the current at +40 mV was slowed by increasing [Mg2+]i with little effect on the rate of deactivation at -50 mV. This is in contrast to the effects of isoprenaline, which speeded activation and slowed deactivation. 6. Isoprenaline increased IK on average by about 2.5 pA/pF, whether IK had previously run down or not, and regardless of [Mg2+]i. The reversibility of isoprenaline was partially inhibited at [Mg2+]i less than 1 mM. 7. It is concluded that Mg2+ affects IK via several mechanisms that might include a Mg(2+)-dependent phosphatase.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agus Z. S., Kelepouris E., Dukes I., Morad M. Cytosolic magnesium modulates calcium channel activity in mammalian ventricular cells. Am J Physiol. 1989 Feb;256(2 Pt 1):C452–C455. doi: 10.1152/ajpcell.1989.256.2.C452. [DOI] [PubMed] [Google Scholar]
- Clay J. R., Hill C. E., Roitman D., Shrier A. Repolarization current in embryonic chick atrial heart cells. J Physiol. 1988 Sep;403:525–537. doi: 10.1113/jphysiol.1988.sp017262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Duchatelle-Gourdon I., Hartzell H. C., Lagrutta A. A. Modulation of the delayed rectifier potassium current in frog cardiomyocytes by beta-adrenergic agonists and magnesium. J Physiol. 1989 Aug;415:251–274. doi: 10.1113/jphysiol.1989.sp017721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchatelle-Gourdon I., Hartzell H. C. Single delayed rectifier channels in frog atrial cells. Effects of beta-adrenergic stimulation. Biophys J. 1990 Apr;57(4):903–909. doi: 10.1016/S0006-3495(90)82610-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischmeister R., Hartzell H. C. Cyclic guanosine 3',5'-monophosphate regulates the calcium current in single cells from frog ventricle. J Physiol. 1987 Jun;387:453–472. doi: 10.1113/jphysiol.1987.sp016584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles W., Nakajima T., Ono K., Shibata E. F. Modulation of the delayed rectifier K+ current by isoprenaline in bull-frog atrial myocytes. J Physiol. 1989 Aug;415:233–249. doi: 10.1113/jphysiol.1989.sp017720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt R. E., Lindley B. D. Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog. J Gen Physiol. 1982 Aug;80(2):279–297. doi: 10.1085/jgp.80.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., White R. E. Effects of magnesium on inactivation of the voltage-gated calcium current in cardiac myocytes. J Gen Physiol. 1989 Oct;94(4):745–767. doi: 10.1085/jgp.94.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. D., Hume J. R. Autonomic regulation of delayed rectifier K+ current in mammalian heart involves G proteins. Am J Physiol. 1989 Sep;257(3 Pt 2):H818–H823. doi: 10.1152/ajpheart.1989.257.3.H818. [DOI] [PubMed] [Google Scholar]
- Horie M., Irisawa H. Dual effects of intracellular magnesium on muscarinic potassium channel current in single guinea-pig atrial cells. J Physiol. 1989 Jan;408:313–332. doi: 10.1113/jphysiol.1989.sp017461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie M., Irisawa H., Noma A. Voltage-dependent magnesium block of adenosine-triphosphate-sensitive potassium channel in guinea-pig ventricular cells. J Physiol. 1987 Jun;387:251–272. doi: 10.1113/jphysiol.1987.sp016572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie M., Irisawa H. Rectification of muscarinic K+ current by magnesium ion in guinea pig atrial cells. Am J Physiol. 1987 Jul;253(1 Pt 2):H210–H214. doi: 10.1152/ajpheart.1987.253.1.H210. [DOI] [PubMed] [Google Scholar]
- Hume J. R. Do catecholamines directly modulate the delayed plateau potassium current in frog atrium? J Mol Cell Cardiol. 1985 Aug;17(8):813–816. doi: 10.1016/s0022-2828(85)80043-2. [DOI] [PubMed] [Google Scholar]
- Hume J. R., Giles W., Robinson K., Shibata E. F., Nathan R. D., Kanai K., Rasmusson R. A time- and voltage-dependent K+ current in single cardiac cells from bullfrog atrium. J Gen Physiol. 1986 Dec;88(6):777–798. doi: 10.1085/jgp.88.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara K., Mitsuiye T., Noma A., Takano M. The Mg2+ block and intrinsic gating underlying inward rectification of the K+ current in guinea-pig cardiac myocytes. J Physiol. 1989 Dec;419:297–320. doi: 10.1113/jphysiol.1989.sp017874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy L. A., Murphy E., Raju B., London R. E. Measurement of cytosolic free magnesium ion concentration by 19F NMR. Biochemistry. 1988 May 31;27(11):4041–4048. doi: 10.1021/bi00411a021. [DOI] [PubMed] [Google Scholar]
- Llano I., Webb C. K., Bezanilla F. Potassium conductance of the squid giant axon. Single-channel studies. J Gen Physiol. 1988 Aug;92(2):179–196. doi: 10.1085/jgp.92.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H. Open-state substructure of inwardly rectifying potassium channels revealed by magnesium block in guinea-pig heart cells. J Physiol. 1988 Mar;397:237–258. doi: 10.1113/jphysiol.1988.sp016998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H., Saigusa A., Irisawa H. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+. Nature. 1987 Jan 8;325(7000):156–159. doi: 10.1038/325156a0. [DOI] [PubMed] [Google Scholar]
- Matsuura H., Ehara T., Imoto Y. An analysis of the delayed outward current in single ventricular cells of the guinea-pig. Pflugers Arch. 1987 Dec;410(6):596–603. doi: 10.1007/BF00581319. [DOI] [PubMed] [Google Scholar]
- Mazzanti M., DeFelice L. J. K channel kinetics during the spontaneous heart beat in embryonic chick ventricle cells. Biophys J. 1988 Dec;54(6):1139–1148. doi: 10.1016/S0006-3495(88)83048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrier A., Clay J. R. Repolarization currents in embryonic chick atrial heart cell aggregates. Biophys J. 1986 Nov;50(5):861–874. doi: 10.1016/S0006-3495(86)83527-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons M. A., Creazzo T., Hartzell H. C. A time-dependent and voltage-sensitive K+ current in single cells from frog atrium. J Gen Physiol. 1986 Dec;88(6):739–755. doi: 10.1085/jgp.88.6.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg C. A. Inward rectification of a potassium channel in cardiac ventricular cells depends on internal magnesium ions. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2560–2564. doi: 10.1073/pnas.84.8.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. E., Hartzell H. C. Effects of intracellular free magnesium on calcium current in isolated cardiac myocytes. Science. 1988 Feb 12;239(4841 Pt 1):778–780. doi: 10.1126/science.2448878. [DOI] [PubMed] [Google Scholar]
- White R. E., Hartzell H. C. Magnesium ions in cardiac function. Regulator of ion channels and second messengers. Biochem Pharmacol. 1989 Mar 15;38(6):859–867. doi: 10.1016/0006-2952(89)90272-4. [DOI] [PubMed] [Google Scholar]
- Yazawa K., Kameyama M. Mechanism of receptor-mediated modulation of the delayed outward potassium current in guinea-pig ventricular myocytes. J Physiol. 1990 Feb;421:135–150. doi: 10.1113/jphysiol.1990.sp017937. [DOI] [PMC free article] [PubMed] [Google Scholar]


