Abstract
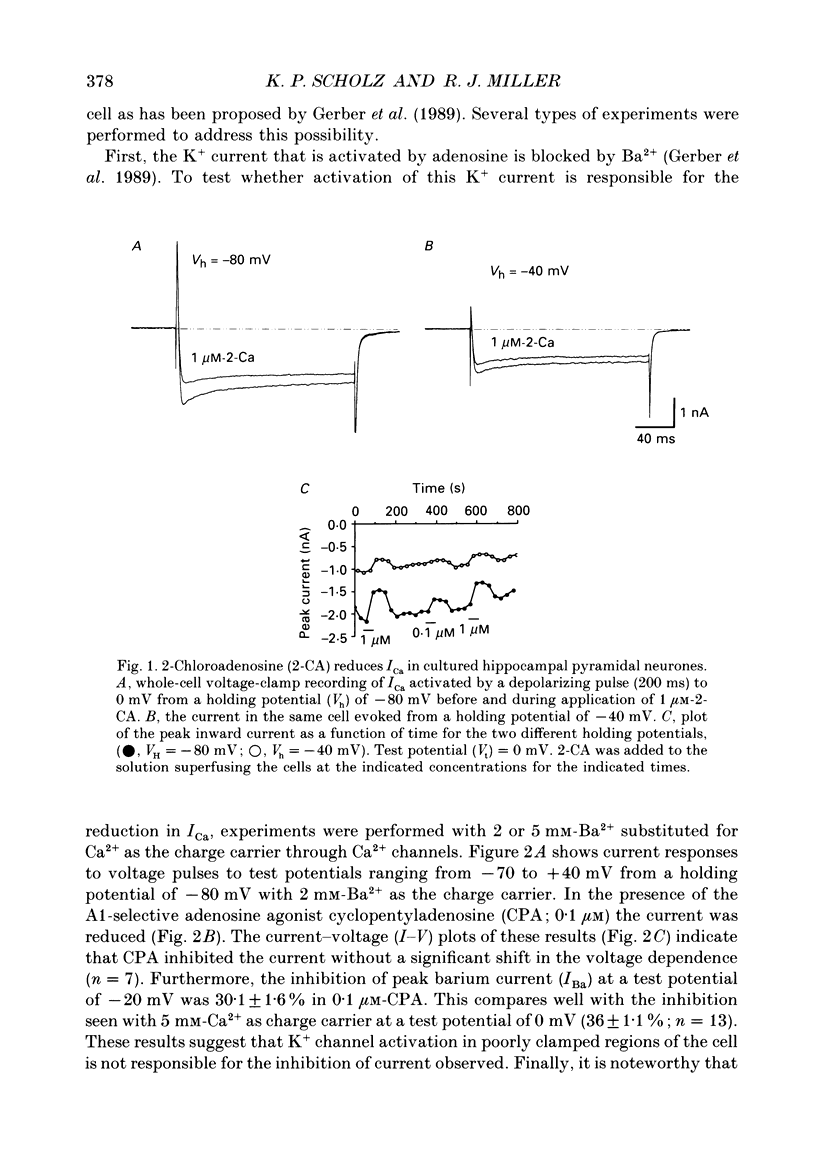
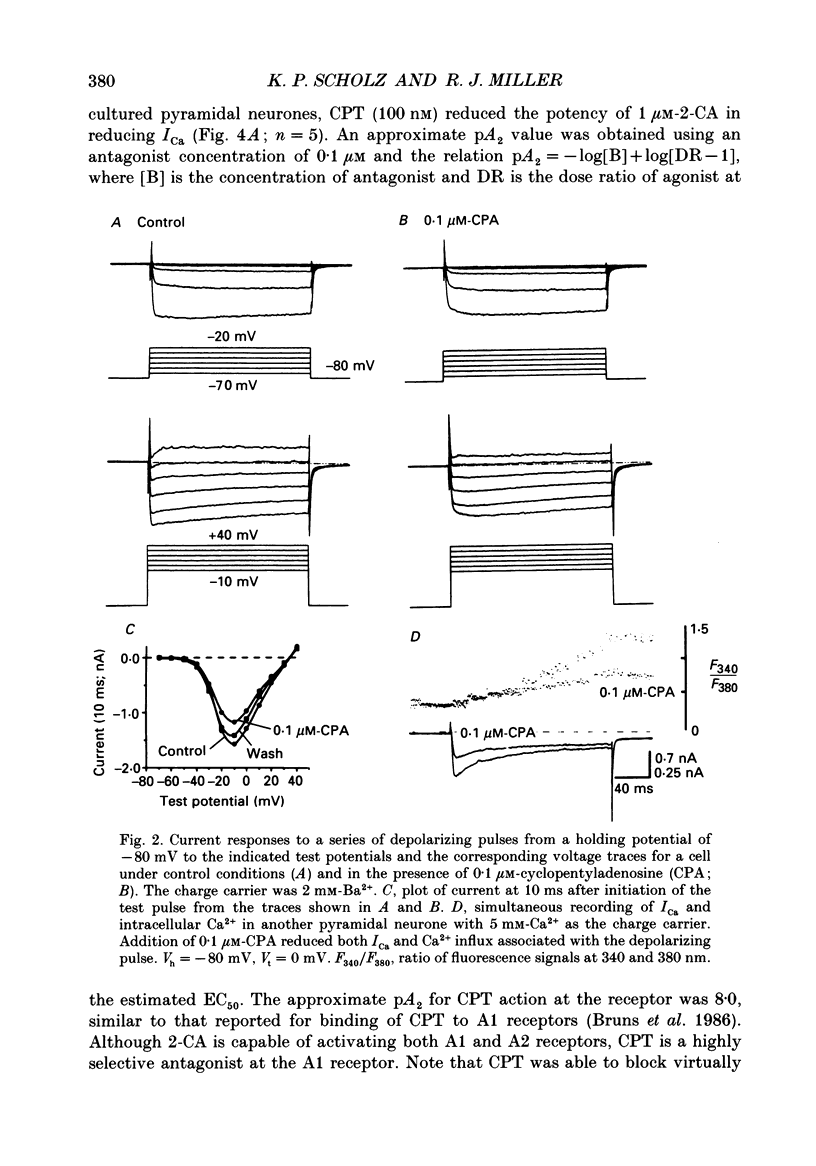
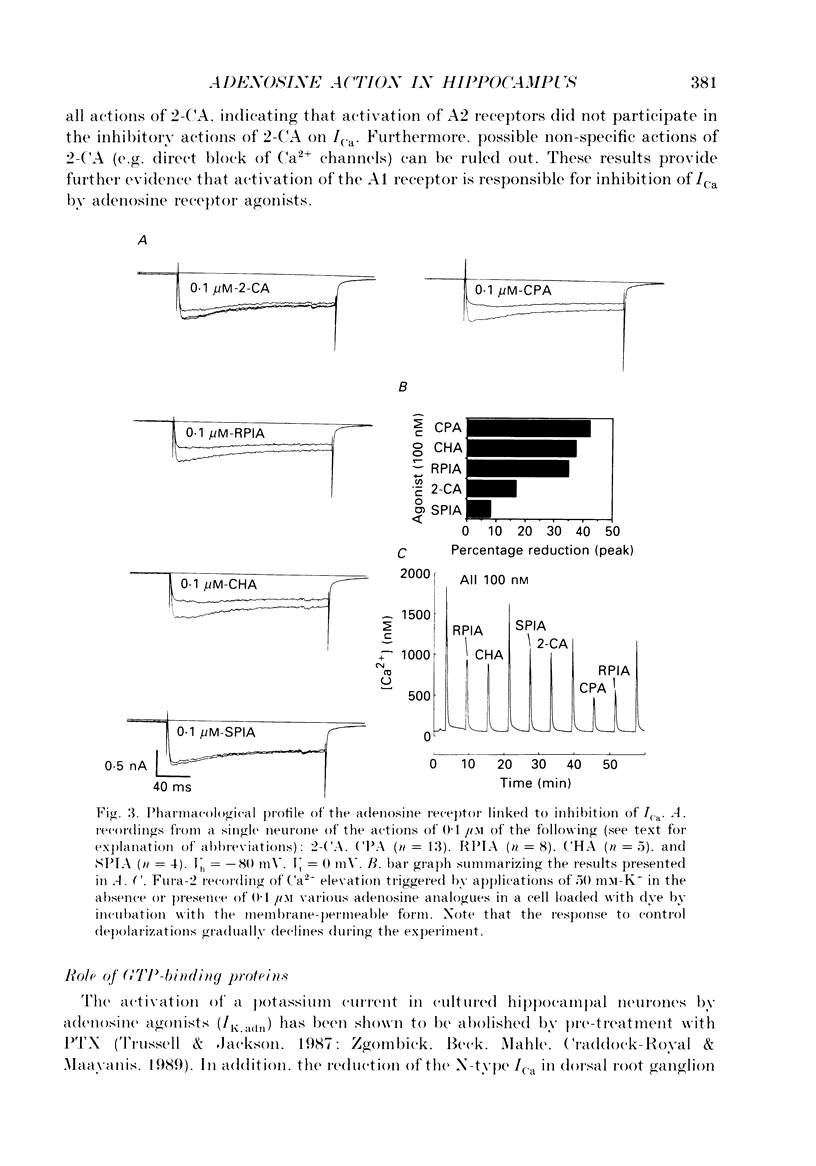
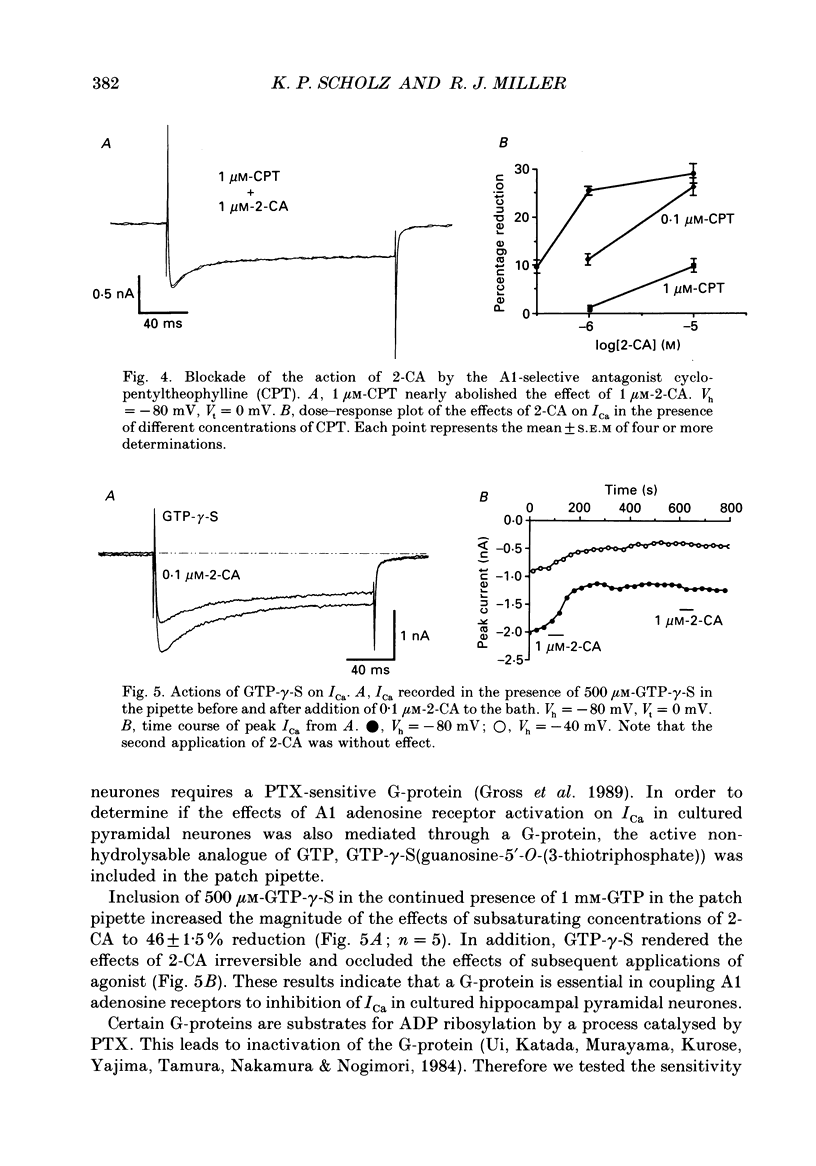
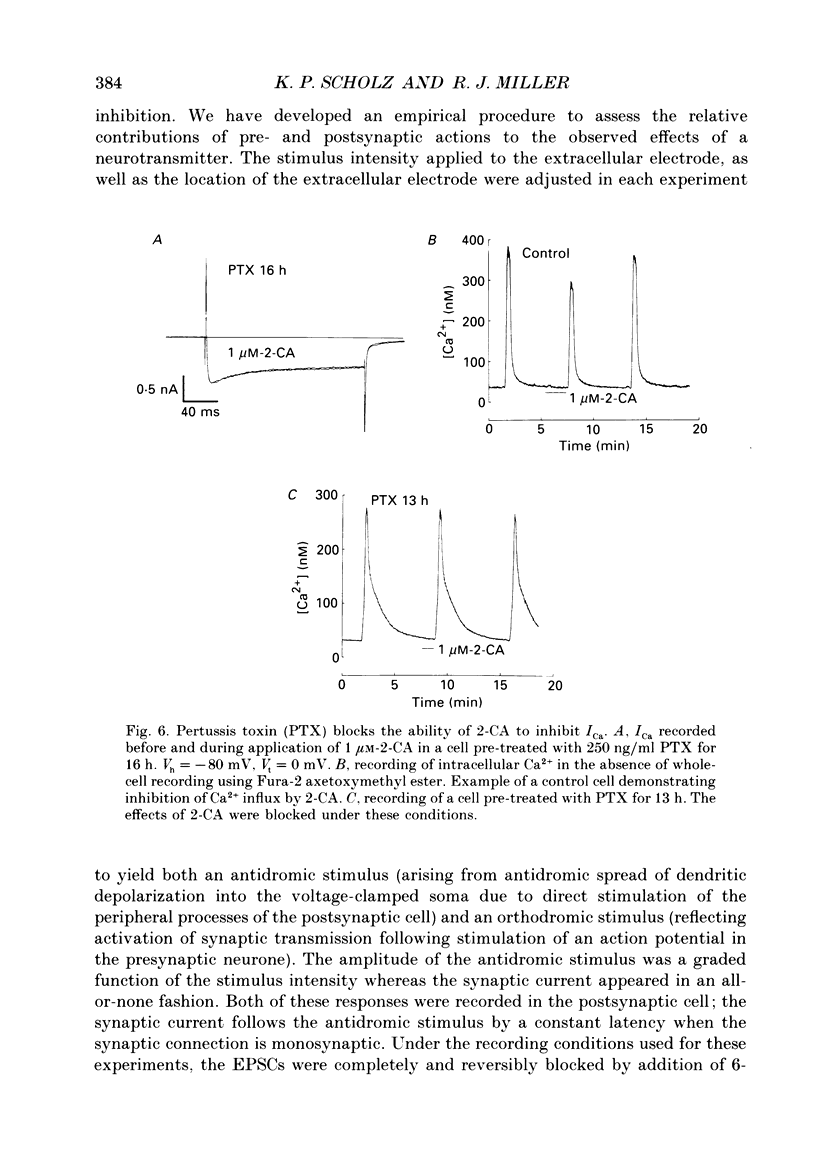
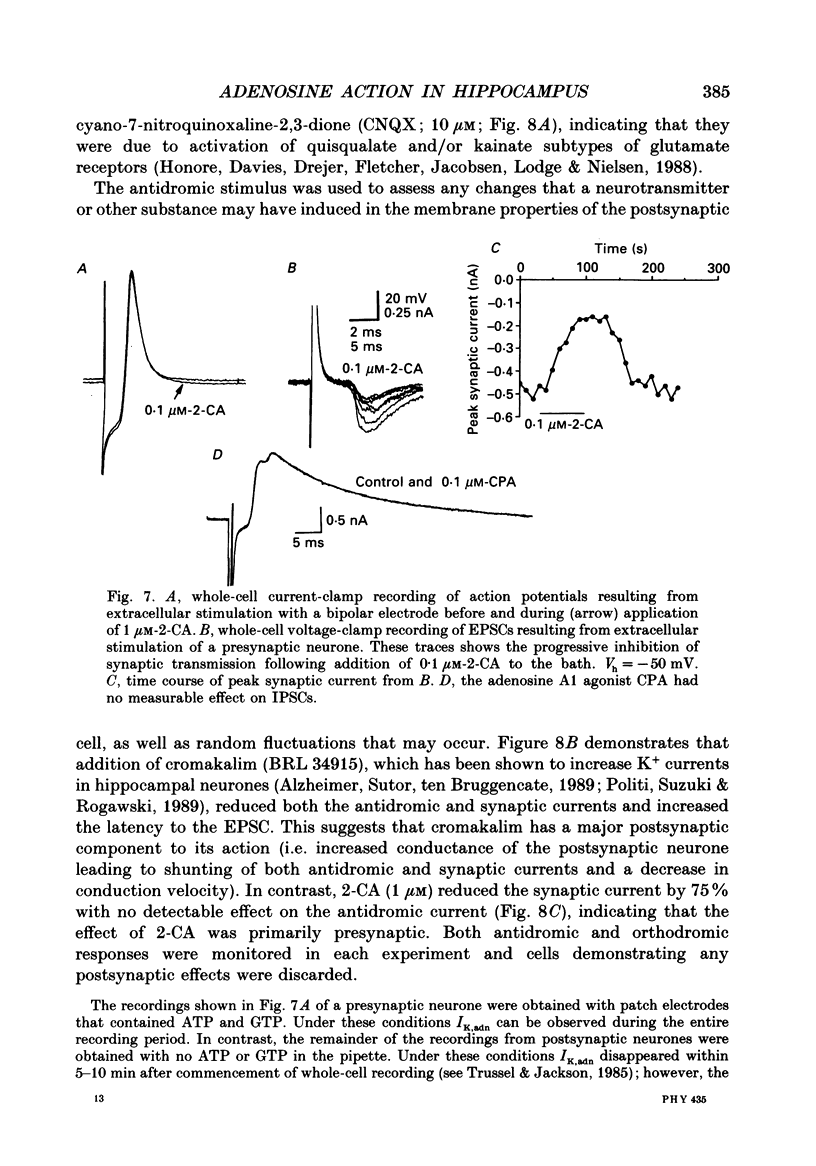
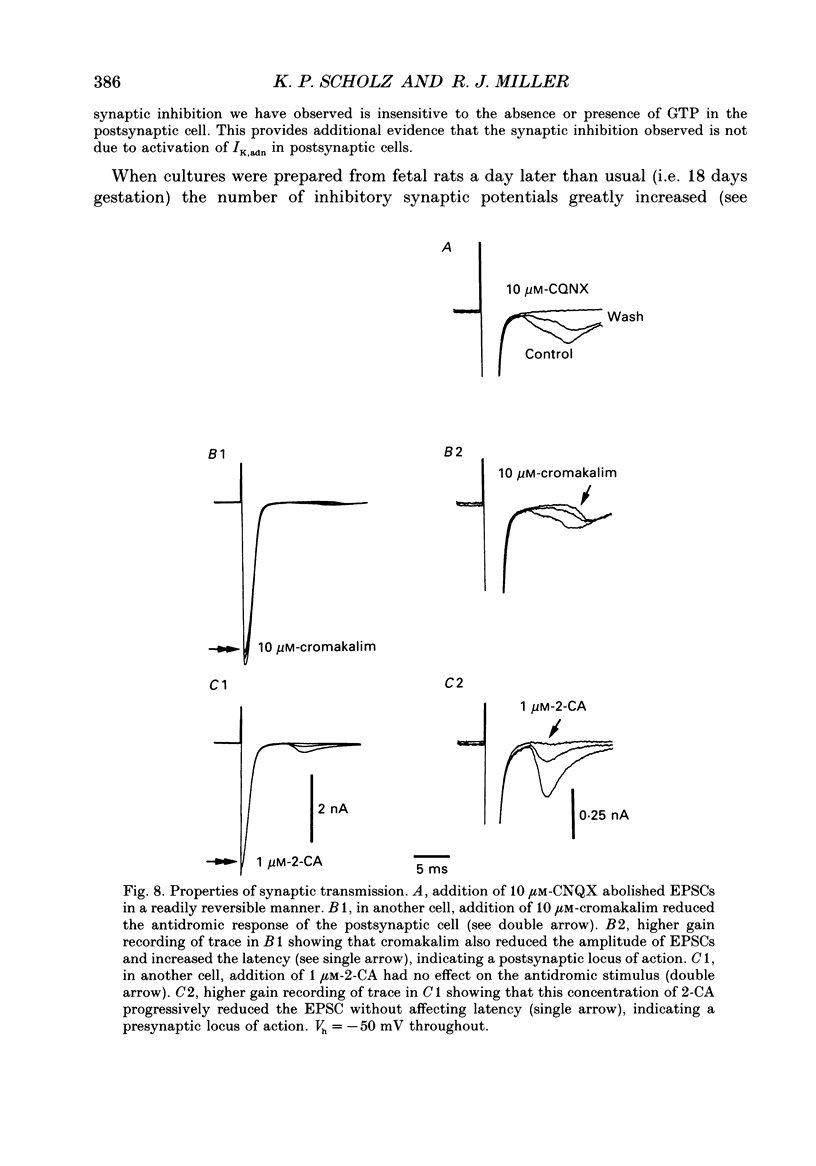
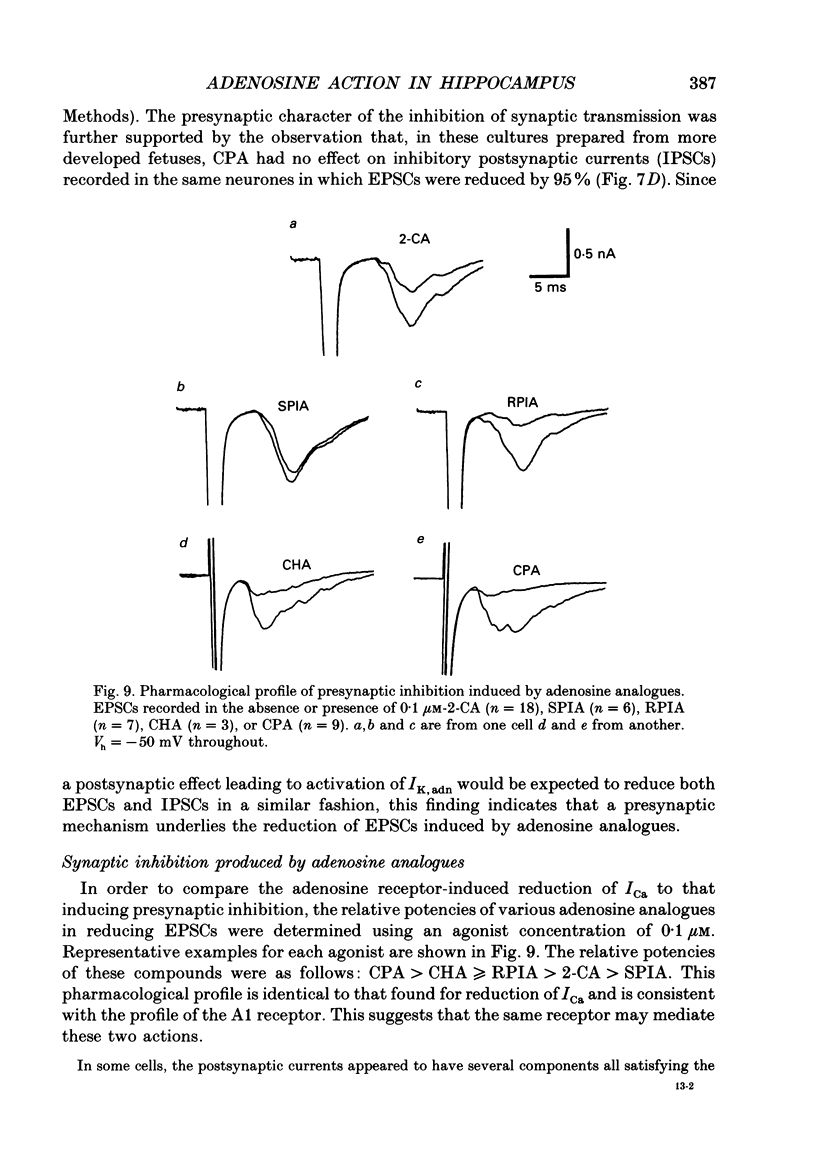
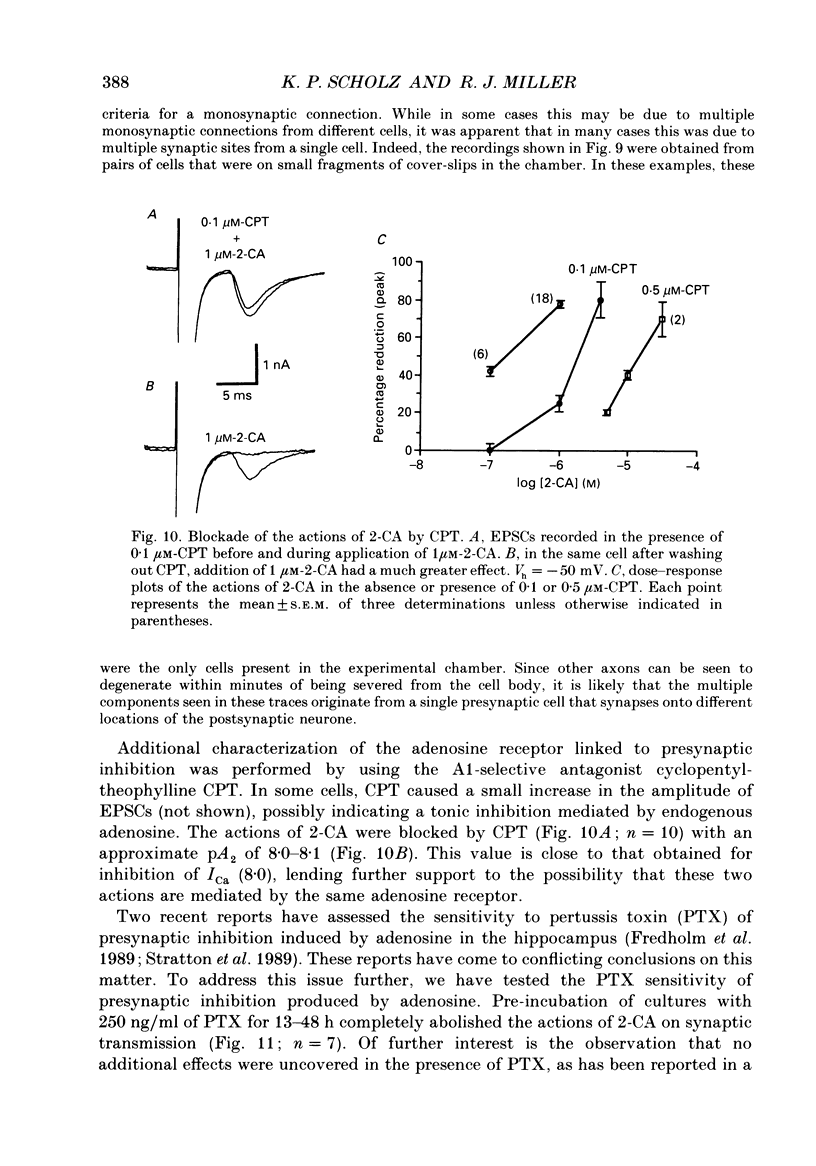
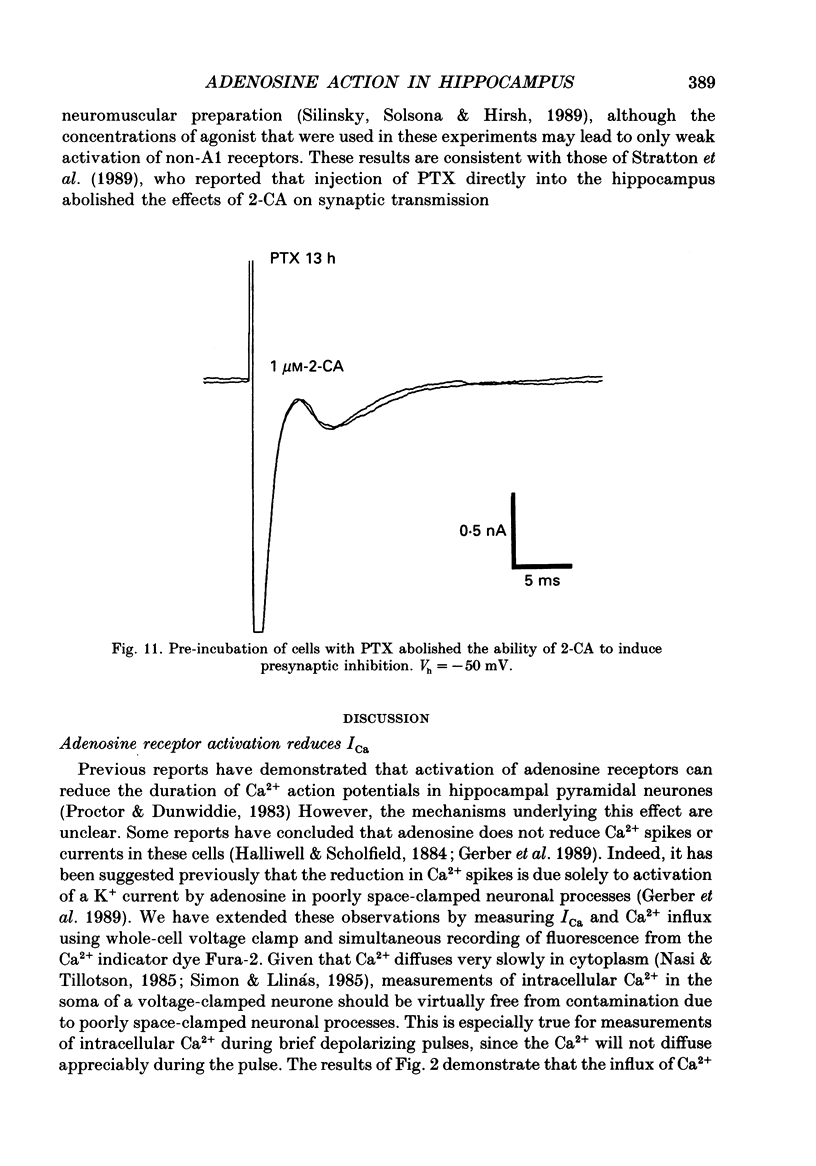
1. The role of adenosine receptors in reducing calcium currents (ICa) and in triggering presynaptic inhibition was studied using whole-cell patch-clamp techniques to record ICa and synaptic currents from the cell bodies of cultured rat hippocampal pyramidal neurones. Recordings of intracellular Ca2+ using the indicator dye Fura-2 were used to obtain further insights into the actions of adenosine agonists. 2. The adenosine analogue 2-chloroadenosine (2-CA) reduced ICa in these neurones. This action was also evident when Ba2+ was used as the charge carrier through Ca2+ channels. Adenosine also reduced the influx of Ca2+ into the cell body during a depolarizing voltage-clamp pulse as measured with Fura-2. The potency of various adenosine receptor agonists was as follows: cyclopentyladenosine greater than cyclohexyl-adenosine greater than or equal to R-phenylisopropyladenosine greater than 2-CA greater than S-phenylisopropyladenosine, consistent with the pharmacological profile of an A1 adenosine receptor. 3. The specific A1 receptor antagonist cyclopentyltheophylline (CPT) blocked the actions of 2-CA on ICa in a competitive fashion. 4. The actions of 2-CA on ICa were abolished by pre-incubation of cultured cells with pertussis toxin (PTX; 250 ng/ml). Intracellular dialysis with the GTP analogue GTP-gamma-S (guanosine-5'-O-(3-thiotriphosphate] enhanced the actions of 2-CA and rendered the response irreversible. 5. Excitatory postsynaptic currents (EPSCs) were recorded from pyramidal neurones under whole-cell voltage clamp by stimulating nearby neurones with an extracellular electrode. 2-CA potently and reversibly reduced the amplitude of EPSCs. This action was shown to be due to presynaptic inhibition of neurotransmitter release. 6. The order of potency of different adenosine agonists in reducing EPSCs was as follows: cyclopentyladenosine greater than cyclohexyladenosine greater than or equal to R-phenylisopropyladenosine greater than 2-CA greater than S-phenylisopropyladenosine. CPT inhibited the action of 2-CA in a competitive fashion. 7. The effects of 2-CA on synaptic transmission were abolished by pre-treatment with 250 ng/ml PTX, indicating that a PTX-sensitive G-protein is involved in this action. 8. These results indicate that activation of adenosine receptors does induce a reduction in ICa in hippocampal pyramidal neurones. Furthermore, this effect and the reduction of excitatory synaptic transmission by adenosine analogues are both mediated by PTX-sensitive G-proteins and have identical pharmacological properties.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alzheimer C., Sutor B., ten Bruggencate G. Effects of cromakalim (BRL 34915) on potassium conductances in CA3 neurons of the guinea-pig hippocampus in vitro. Naunyn Schmiedebergs Arch Pharmacol. 1989 Oct;340(4):465–471. doi: 10.1007/BF00167050. [DOI] [PubMed] [Google Scholar]
- Banker G. A., Cowan W. M. Further observations on hippocampal neurons in dispersed cell culture. J Comp Neurol. 1979 Oct 1;187(3):469–493. doi: 10.1002/cne.901870302. [DOI] [PubMed] [Google Scholar]
- Banker G. A., Cowan W. M. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977 May 13;126(3):397–342. doi: 10.1016/0006-8993(77)90594-7. [DOI] [PubMed] [Google Scholar]
- Banker G. A. Trophic interactions between astroglial cells and hippocampal neurons in culture. Science. 1980 Aug 15;209(4458):809–810. doi: 10.1126/science.7403847. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989 Jul 13;340(6229):153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- Bottenstein J. E., Sato G. H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979 Jan;76(1):514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns R. F., Lu G. H., Pugsley T. A. Characterization of the A2 adenosine receptor labeled by [3H]NECA in rat striatal membranes. Mol Pharmacol. 1986 Apr;29(4):331–346. [PubMed] [Google Scholar]
- Dolphin A. C., Prestwich S. A. Pertussis toxin reverses adenosine inhibition of neuronal glutamate release. Nature. 1985 Jul 11;316(6024):148–150. doi: 10.1038/316148a0. [DOI] [PubMed] [Google Scholar]
- Dunwiddie T. V., Fredholm B. B. Adenosine A1 receptors inhibit adenylate cyclase activity and neurotransmitter release and hyperpolarize pyramidal neurons in rat hippocampus. J Pharmacol Exp Ther. 1989 Apr;249(1):31–37. [PubMed] [Google Scholar]
- Dunwiddie T. V., Haas H. L. Adenosine increases synaptic facilitation in the in vitro rat hippocampus: evidence for a presynaptic site of action. J Physiol. 1985 Dec;369:365–377. doi: 10.1113/jphysiol.1985.sp015907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie T. V. Interactions between the effects of adenosine and calcium on synaptic responses in rat hippocampus in vitro. J Physiol. 1984 May;350:545–559. doi: 10.1113/jphysiol.1984.sp015217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe I. D., Westbrook G. L., Mayer M. L. Modulation of excitatory synaptic transmission by glycine and zinc in cultures of mouse hippocampal neurons. J Neurosci. 1988 Oct;8(10):3733–3741. doi: 10.1523/JNEUROSCI.08-10-03733.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm B. B., Proctor W., Van der Ploeg I., Dunwiddie T. V. In vivo pertussis toxin treatment attenuates some, but not all, adenosine A1 effects in slices of the rat hippocampus. Eur J Pharmacol. 1989 Aug 15;172(3):249–262. doi: 10.1016/0922-4106(89)90055-2. [DOI] [PubMed] [Google Scholar]
- Gerber U., Greene R. W., Haas H. L., Stevens D. R. Characterization of inhibition mediated by adenosine in the hippocampus of the rat in vitro. J Physiol. 1989 Oct;417:567–578. doi: 10.1113/jphysiol.1989.sp017819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R. A., Macdonald R. L., Ryan-Jastrow T. 2-Chloroadenosine reduces the N calcium current of cultured mouse sensory neurones in a pertussis toxin-sensitive manner. J Physiol. 1989 Apr;411:585–595. doi: 10.1113/jphysiol.1989.sp017592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Halliwell J. V., Scholfield C. N. Somatically recorded Ca-currents in guinea-pig hippocampal and olfactory cortex neurones are resistant to adenosine action. Neurosci Lett. 1984 Sep 7;50(1-3):13–18. doi: 10.1016/0304-3940(84)90454-3. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Honoré T., Davies S. N., Drejer J., Fletcher E. J., Jacobsen P., Lodge D., Nielsen F. E. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988 Aug 5;241(4866):701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- Kasai H., Aosaki T. Modulation of Ca-channel current by an adenosine analog mediated by a GTP-binding protein in chick sensory neurons. Pflugers Arch. 1989 Jun;414(2):145–149. doi: 10.1007/BF00580956. [DOI] [PubMed] [Google Scholar]
- Kay A. R., Wong R. K. Calcium current activation kinetics in isolated pyramidal neurones of the Ca1 region of the mature guinea-pig hippocampus. J Physiol. 1987 Nov;392:603–616. doi: 10.1113/jphysiol.1987.sp016799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald R. L., Skerritt J. H., Werz M. A. Adenosine agonists reduce voltage-dependent calcium conductance of mouse sensory neurones in cell culture. J Physiol. 1986 Jan;370:75–90. doi: 10.1113/jphysiol.1986.sp015923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasi E., Tillotson D. The rate of diffusion of Ca2+ and Ba2+ in a nerve cell body. Biophys J. 1985 May;47(5):735–738. doi: 10.1016/S0006-3495(85)83972-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P. G., Pun R. Y., Westbrook G. L. Synaptic excitation in cultures of mouse spinal cord neurones: receptor pharmacology and behaviour of synaptic currents. J Physiol. 1986 Mar;372:169–190. doi: 10.1113/jphysiol.1986.sp016003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Ozawa S. Inhibitory action of adenosine on synaptic transmission in the hippocampus of the guinea pig in vitro. Eur J Pharmacol. 1980 Dec 19;68(4):483–492. doi: 10.1016/0014-2999(80)90424-0. [DOI] [PubMed] [Google Scholar]
- Politi D. M., Suzuki S., Rogawski M. A. BRL 34915 (cromakalim) enhances voltage-dependent K+ current in cultured rat hippocampal neurons. Eur J Pharmacol. 1989 Sep 1;168(1):7–14. doi: 10.1016/0014-2999(89)90626-2. [DOI] [PubMed] [Google Scholar]
- Proctor W. R., Dunwiddie T. V. Adenosine inhibits calcium spikes in hippocampal pyramidal neurons in vitro. Neurosci Lett. 1983 Feb 21;35(2):197–201. doi: 10.1016/0304-3940(83)90550-5. [DOI] [PubMed] [Google Scholar]
- Scholz W. K., Baitinger C., Schulman H., Kelly P. T. Developmental changes in Ca2+/calmodulin-dependent protein kinase II in cultures of hippocampal pyramidal neurons and astrocytes. J Neurosci. 1988 Mar;8(3):1039–1051. doi: 10.1523/JNEUROSCI.08-03-01039.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M. On the mechanism by which adenosine receptor activation inhibits the release of acetylcholine from motor nerve endings. J Physiol. 1984 Jan;346:243–256. doi: 10.1113/jphysiol.1984.sp015019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M., Solsona C., Hirsh J. K. Pertussis toxin prevents the inhibitory effect of adenosine and unmasks adenosine-induced excitation of mammalian motor nerve endings. Br J Pharmacol. 1989 May;97(1):16–18. doi: 10.1111/j.1476-5381.1989.tb11918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. M., Llinás R. R. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys J. 1985 Sep;48(3):485–498. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. H. Adenosine as a neuromodulator. Annu Rev Neurosci. 1985;8:103–124. doi: 10.1146/annurev.ne.08.030185.000535. [DOI] [PubMed] [Google Scholar]
- Stratton K. R., Cole A. J., Pritchett J., Eccles C. U., Worley P. F., Baraban J. M. Intrahippocampal injection of pertussis toxin blocks adenosine suppression of synaptic responses. Brain Res. 1989 Aug 14;494(2):359–364. doi: 10.1016/0006-8993(89)90604-5. [DOI] [PubMed] [Google Scholar]
- Thayer S. A., Sturek M., Miller R. J. Measurement of neuronal Ca2+ transients using simultaneous microfluorimetry and electrophysiology. Pflugers Arch. 1988 Jul;412(1-2):216–223. doi: 10.1007/BF00583753. [DOI] [PubMed] [Google Scholar]
- Trussell L. O., Jackson M. B. Adenosine-activated potassium conductance in cultured striatal neurons. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4857–4861. doi: 10.1073/pnas.82.14.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell L. O., Jackson M. B. Dependence of an adenosine-activated potassium current on a GTP-binding protein in mammalian central neurons. J Neurosci. 1987 Oct;7(10):3306–3316. doi: 10.1523/JNEUROSCI.07-10-03306.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W., Lipscombe D., Madison D. V., Bley K. R., Fox A. P. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988 Oct;11(10):431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- Ui M., Katada T., Murayama T., Kurose H., Yajima M., Tamura M., Nakamura T., Nogimori K. Islet-activating protein, pertussis toxin: a specific uncoupler of receptor-mediated inhibition of adenylate cyclase. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:145–151. [PubMed] [Google Scholar]
- Wakade A. R., Wakade T. D. Inhibition of noradrenaline release by adenosine. J Physiol. 1978 Sep;282:35–49. doi: 10.1113/jphysiol.1978.sp012446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgombick J. M., Beck S. G., Mahle C. D., Craddock-Royal B., Maayani S. Pertussis toxin-sensitive guanine nucleotide-binding protein(S) couple adenosine A1 and 5-hydroxytryptamine1A receptors to the same effector systems in rat hippocampus: biochemical and electrophysiological studies. Mol Pharmacol. 1989 Apr;35(4):484–494. [PubMed] [Google Scholar]


