Abstract
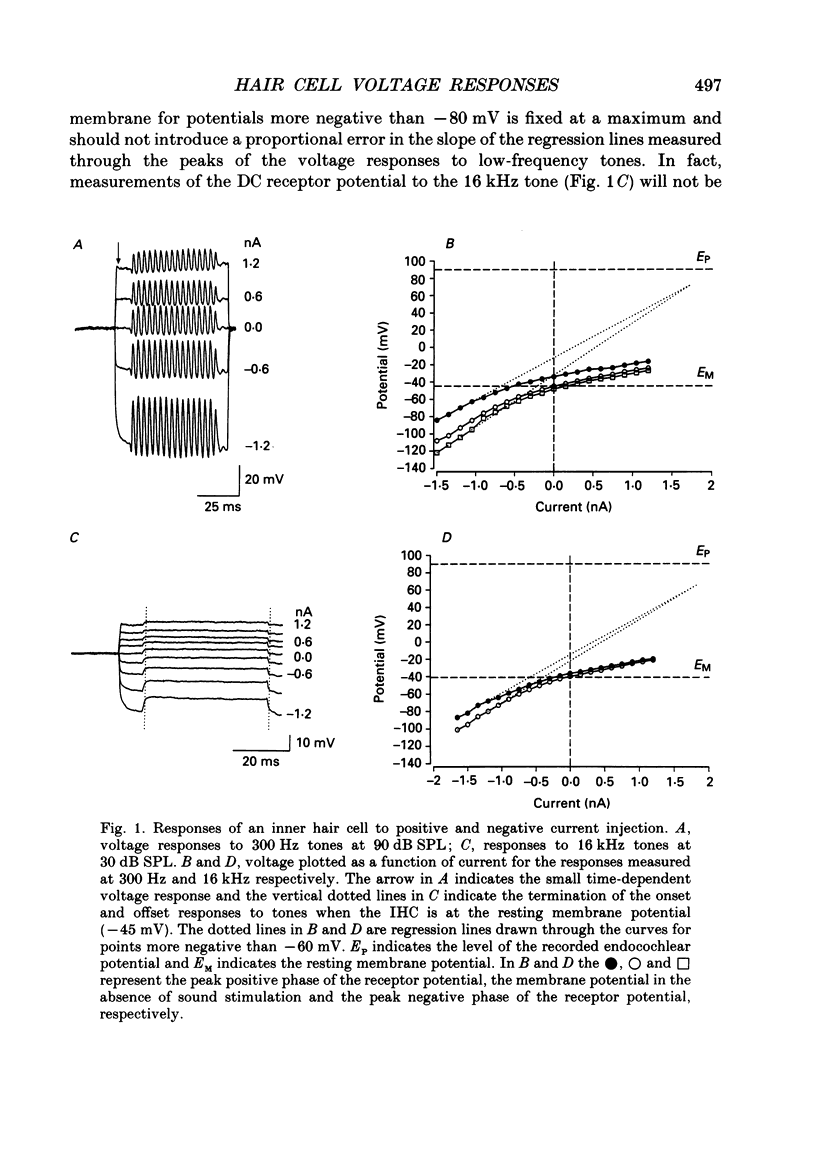
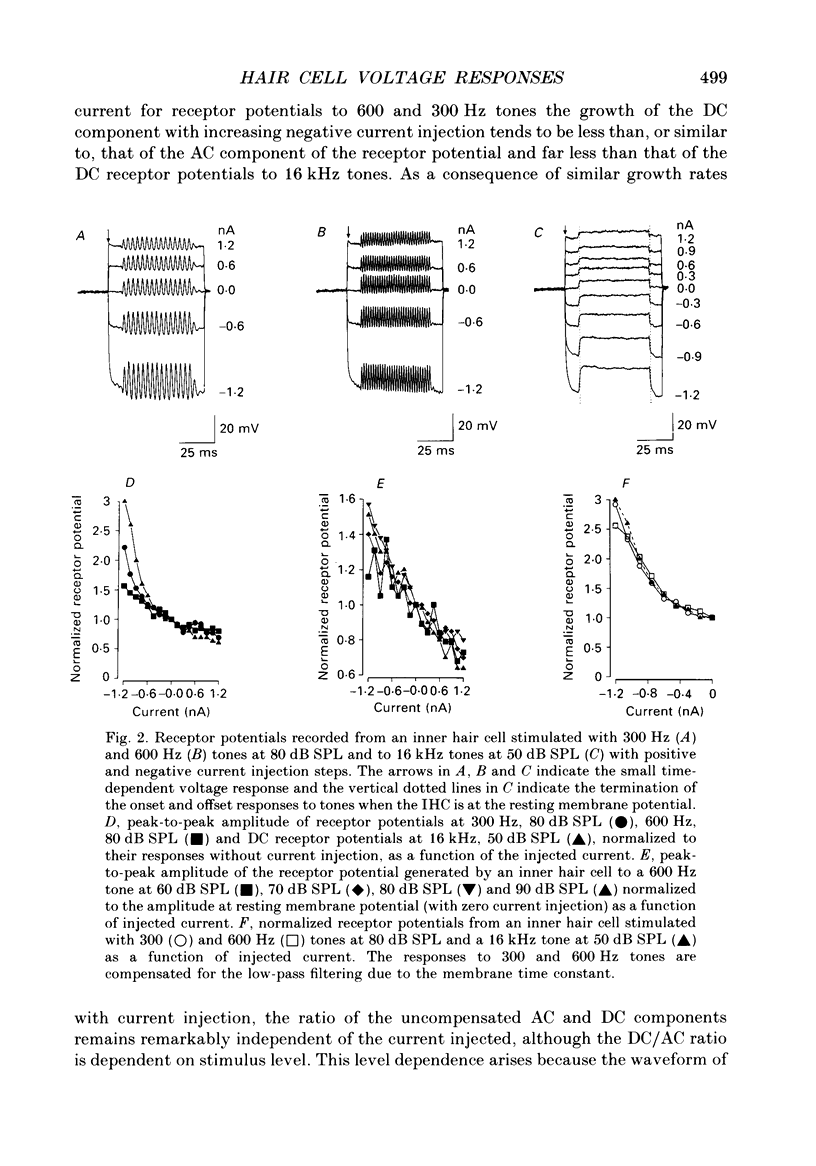
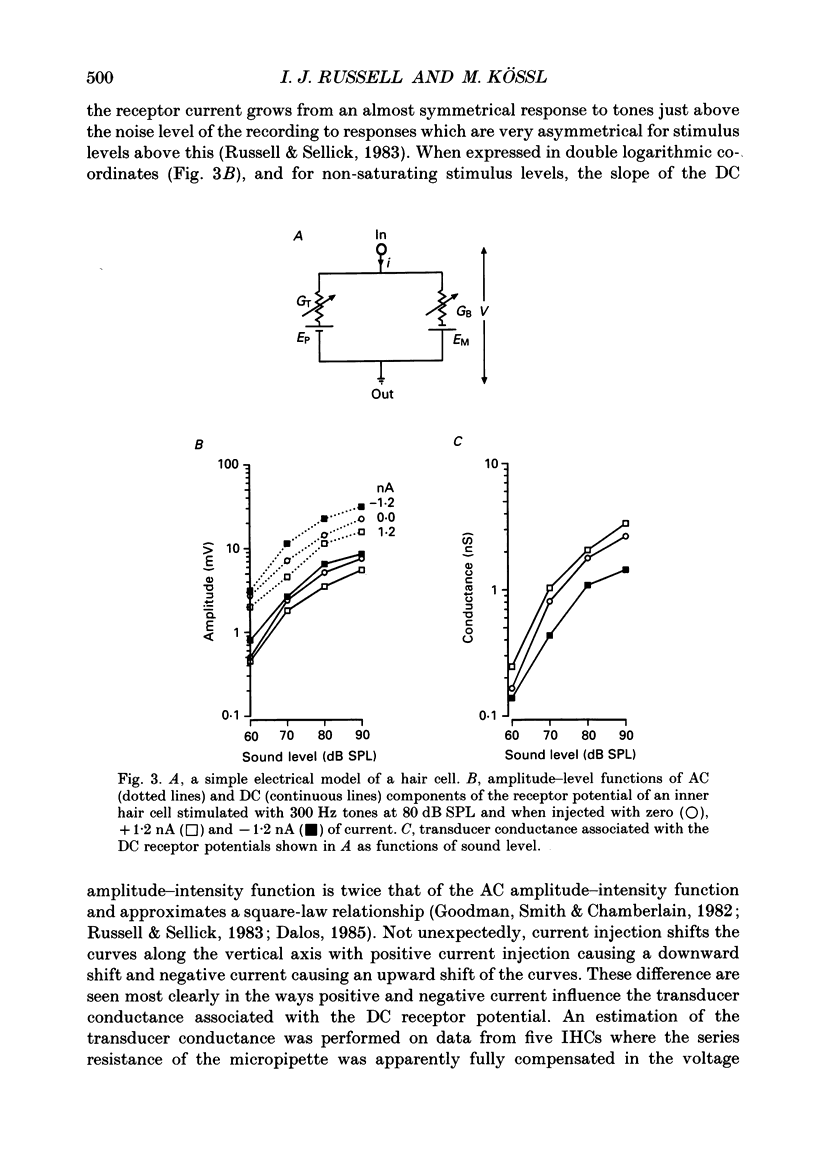
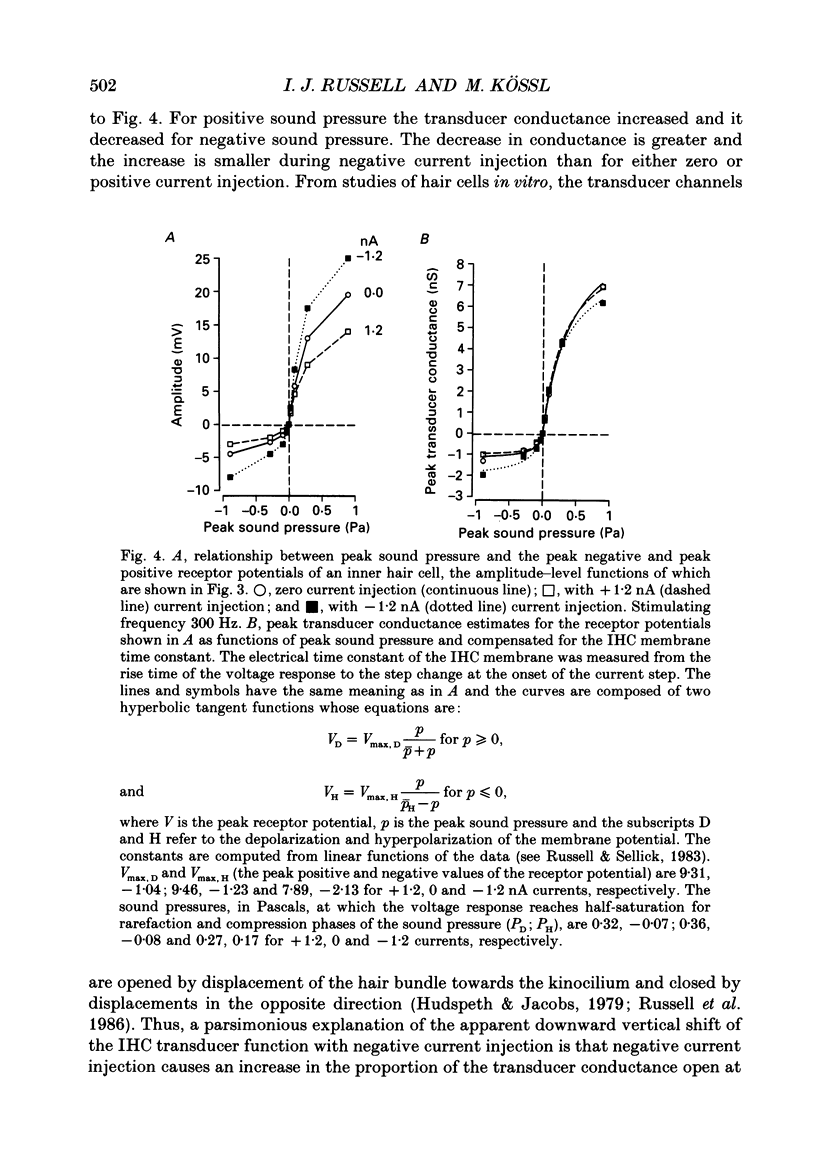
1. Intracellularly recorded voltage responses to tones and to current injection were measured from inner (IHC) and outer (OHC) hair cells in the basal turn of the guinea-pig cochlea. 2. The voltage responses of IHCs to tones are increased by negative current and decreased by positive current. For any given current strength, the decrease in the receptor potential amplitude caused by positive current is less than the increase caused by negative current. This was attributed to the voltage-dependent rectification of the membrane conductance. 3. Estimates of the reversal potential of the receptor current were based on extrapolation of the slopes of the current-voltage relations for potentials more negative than -60 mV. The estimated reversal potentials were close to the measured endolymphatic potential. 4. Negative current increased the IHC membrane time constant and increased the transducer driving voltage. When receptor potentials to low-frequency tones were adjusted for constant driving voltage and for the membrane time constant, the positive DC component of the receptor potential was decreased relative to the AC component by current injection but the DC component in response to high-frequency tones near the best frequency of the IHC (16 kHz) was unchanged by negative current. 5. When compensated for constant driving voltage and low-pass filtering due to the basolateral membrane conductance, the proportion of the transducer conductance open in IHCs at rest is increased by negative current. 6. Negative current injection reduced the positive DC components of OHC voltage responses to low-frequency tones and may make them negative. In some cases negative current injection decreased the amplitude of the OHC receptor potential while positive current injection enhanced it. From these observations it is proposed that negative current injection shifts the operating point of the OHC transducer functions towards a positive, saturating region of the relationship and positive current decreases the proportion of the transducer conductance which is open at rest.
Full text
PDF






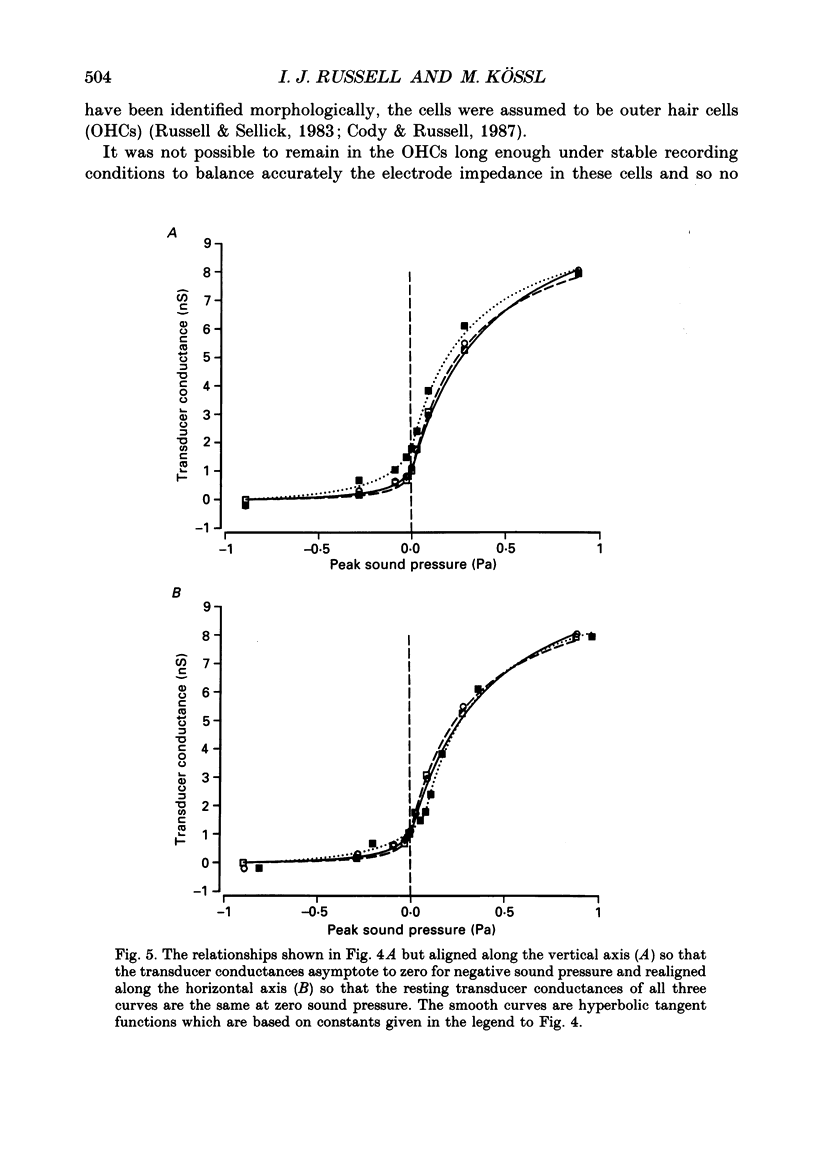
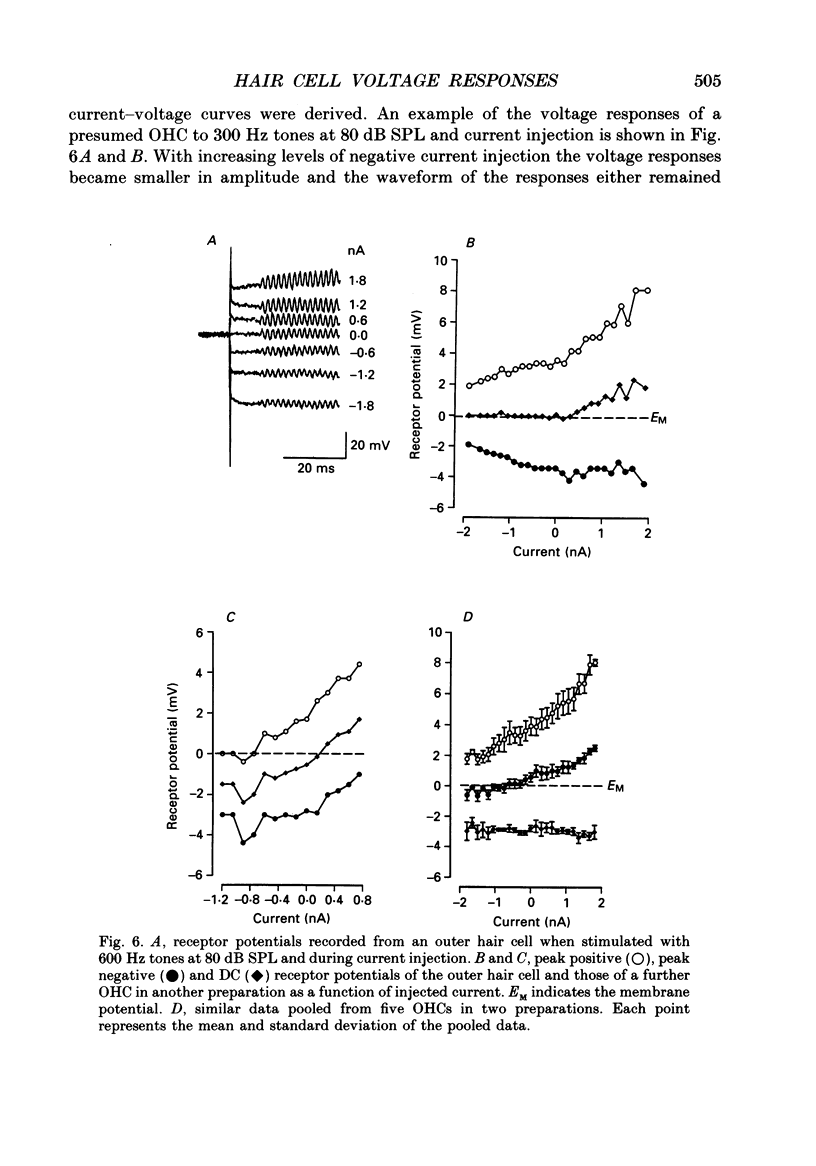






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashmore J. F. A fast motile response in guinea-pig outer hair cells: the cellular basis of the cochlear amplifier. J Physiol. 1987 Jul;388:323–347. doi: 10.1113/jphysiol.1987.sp016617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore J. F., Meech R. W. Ionic basis of membrane potential in outer hair cells of guinea pig cochlea. Nature. 1986 Jul 24;322(6077):368–371. doi: 10.1038/322368a0. [DOI] [PubMed] [Google Scholar]
- Baden-Kristensen K., Weiss T. F. Receptor potentials of lizard hair cells with free-standing stereocilia: responses to acoustic clicks. J Physiol. 1983 Feb;335:699–721. doi: 10.1113/jphysiol.1983.sp014559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosher S. K., Warren R. L. Very low calcium content of cochlear endolymph, an extracellular fluid. Nature. 1978 Jun 1;273(5661):377–378. doi: 10.1038/273377a0. [DOI] [PubMed] [Google Scholar]
- Cody A. R., Russell I. J. The response of hair cells in the basal turn of the guinea-pig cochlea to tones. J Physiol. 1987 Feb;383:551–569. doi: 10.1113/jphysiol.1987.sp016428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey D. P., Hudspeth A. J. Ionic basis of the receptor potential in a vertebrate hair cell. Nature. 1979 Oct 25;281(5733):675–677. doi: 10.1038/281675a0. [DOI] [PubMed] [Google Scholar]
- Crawford A. C., Evans M. G., Fettiplace R. Activation and adaptation of transducer currents in turtle hair cells. J Physiol. 1989 Dec;419:405–434. doi: 10.1113/jphysiol.1989.sp017878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C., Fettiplace R. The mechanical properties of ciliary bundles of turtle cochlear hair cells. J Physiol. 1985 Jul;364:359–379. doi: 10.1113/jphysiol.1985.sp015750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS H. Transmission and transduction in the cochlea. Laryngoscope. 1958 Mar;68(3):359–382. doi: 10.1002/lary.5540680314. [DOI] [PubMed] [Google Scholar]
- Dallos P. Membrane potential and response changes in mammalian cochlear hair cells during intracellular recording. J Neurosci. 1985 Jun;5(6):1609–1615. doi: 10.1523/JNEUROSCI.05-06-01609.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P. Neurobiology of cochlear inner and outer hair cells: intracellular recordings. Hear Res. 1986;22:185–198. doi: 10.1016/0378-5955(86)90095-x. [DOI] [PubMed] [Google Scholar]
- Dallos P., Santos-Sacchi J., Flock A. Intracellular recordings from cochlear outer hair cells. Science. 1982 Nov 5;218(4572):582–584. doi: 10.1126/science.7123260. [DOI] [PubMed] [Google Scholar]
- Dallos P., Schoeny Z. G., Worthington D. W., Cheatham M. A. Cochlear distortion: effect of direct-current polarization. Science. 1969 Apr 25;164(3878):449–451. doi: 10.1126/science.164.3878.449. [DOI] [PubMed] [Google Scholar]
- Dallos P. Some electrical circuit properties of the organ of Corti. II. Analysis including reactive elements. Hear Res. 1984 Jun;14(3):281–291. doi: 10.1016/0378-5955(84)90055-8. [DOI] [PubMed] [Google Scholar]
- Davis H. An active process in cochlear mechanics. Hear Res. 1983 Jan;9(1):79–90. doi: 10.1016/0378-5955(83)90136-3. [DOI] [PubMed] [Google Scholar]
- Evans E. F. Neuroleptanesthesia for the guinea pig. An ideal anesthetic procedure for long-term physiological studies of the cochlea. Arch Otolaryngol. 1979 Apr;105(4):185–186. doi: 10.1001/archotol.1979.00790160019004. [DOI] [PubMed] [Google Scholar]
- Goodman D. A., Smith R. L., Chamberlain S. C. Intracellular and extracellular responses in the organ of Corti of the gerbil. Hear Res. 1982 Jul;7(2):161–169. doi: 10.1016/0378-5955(82)90012-0. [DOI] [PubMed] [Google Scholar]
- Hacohen N., Assad J. A., Smith W. J., Corey D. P. Regulation of tension on hair-cell transduction channels: displacement and calcium dependence. J Neurosci. 1989 Nov;9(11):3988–3997. doi: 10.1523/JNEUROSCI.09-11-03988.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holton T., Hudspeth A. J. The transduction channel of hair cells from the bull-frog characterized by noise analysis. J Physiol. 1986 Jun;375:195–227. doi: 10.1113/jphysiol.1986.sp016113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J., Hudspeth A. J. Mechanical relaxation of the hair bundle mediates adaptation in mechanoelectrical transduction by the bullfrog's saccular hair cell. Proc Natl Acad Sci U S A. 1987 May;84(9):3064–3068. doi: 10.1073/pnas.84.9.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth A. J., Jacobs R. Stereocilia mediate transduction in vertebrate hair cells (auditory system/cilium/vestibular system). Proc Natl Acad Sci U S A. 1979 Mar;76(3):1506–1509. doi: 10.1073/pnas.76.3.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kros C. J., Crawford A. C. Potassium currents in inner hair cells isolated from the guinea-pig cochlea. J Physiol. 1990 Feb;421:263–291. doi: 10.1113/jphysiol.1990.sp017944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountain D. C. Changes in endolymphatic potential and crossed olivocochlear bundle stimulation alter cochlear mechanics. Science. 1980 Oct 3;210(4465):71–72. doi: 10.1126/science.7414321. [DOI] [PubMed] [Google Scholar]
- Nuttall A. L. Influence of direct current on dc receptor potentials from cochlear inner hair cells in the guinea pig. J Acoust Soc Am. 1985 Jan;77(1):165–175. doi: 10.1121/1.392282. [DOI] [PubMed] [Google Scholar]
- Ohmori H. Mechano-electrical transduction currents in isolated vestibular hair cells of the chick. J Physiol. 1985 Feb;359:189–217. doi: 10.1113/jphysiol.1985.sp015581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell I. J., Cody A. R., Richardson G. P. The responses of inner and outer hair cells in the basal turn of the guinea-pig cochlea and in the mouse cochlea grown in vitro. Hear Res. 1986;22:199–216. doi: 10.1016/0378-5955(86)90096-1. [DOI] [PubMed] [Google Scholar]
- Russell I. J. Origin of the receptor potential in inner hair cells of the mammalian cochlea--evidence for Davis' theory. Nature. 1983 Jan 27;301(5898):334–336. doi: 10.1038/301334a0. [DOI] [PubMed] [Google Scholar]
- Russell I. J., Richardson G. P., Kössl M. The responses of cochlear hair cells to tonic displacements of the sensory hair bundle. Hear Res. 1989 Dec;43(1):55–69. doi: 10.1016/0378-5955(89)90059-2. [DOI] [PubMed] [Google Scholar]
- Russell I. J., Richardson G. P. The morphology and physiology of hair cells in organotypic cultures of the mouse cochlea. Hear Res. 1987 Nov;31(1):9–24. doi: 10.1016/0378-5955(87)90210-3. [DOI] [PubMed] [Google Scholar]
- Russell I. J., Sellick P. M. Intracellular studies of hair cells in the mammalian cochlea. J Physiol. 1978 Nov;284:261–290. doi: 10.1113/jphysiol.1978.sp012540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell I. J., Sellick P. M. Low-frequency characteristics of intracellularly recorded receptor potentials in guinea-pig cochlear hair cells. J Physiol. 1983 May;338:179–206. doi: 10.1113/jphysiol.1983.sp014668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüsch A., Thurm U. Spontaneous and electrically induced movements of ampullary kinocilia and stereovilli. Hear Res. 1990 Oct;48(3):247–263. doi: 10.1016/0378-5955(90)90065-w. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J. Asymmetry in voltage-dependent movements of isolated outer hair cells from the organ of Corti. J Neurosci. 1989 Aug;9(8):2954–2962. doi: 10.1523/JNEUROSCI.09-08-02954.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J., Dilger J. P. Whole cell currents and mechanical responses of isolated outer hair cells. Hear Res. 1988 Sep 15;35(2-3):143–150. doi: 10.1016/0378-5955(88)90113-x. [DOI] [PubMed] [Google Scholar]
- Sellick P. M., Patuzzi R., Johnstone B. M. Measurement of basilar membrane motion in the guinea pig using the Mössbauer technique. J Acoust Soc Am. 1982 Jul;72(1):131–141. doi: 10.1121/1.387996. [DOI] [PubMed] [Google Scholar]
- TASAKI I., FERNANDEZ C. Modification of cochlear microphonics and action potentials by KC1 solution and by direct currents. J Neurophysiol. 1952 Nov;15(6):497–512. doi: 10.1152/jn.1952.15.6.497. [DOI] [PubMed] [Google Scholar]
- Weiss T. F., Mulroy M. J., Altmann D. W. Intracellular responses to acoustic clicks in the inner ear of the alligator lizard. J Acoust Soc Am. 1974 Mar;55(3):606–619. doi: 10.1121/1.1914571. [DOI] [PubMed] [Google Scholar]


