Abstract
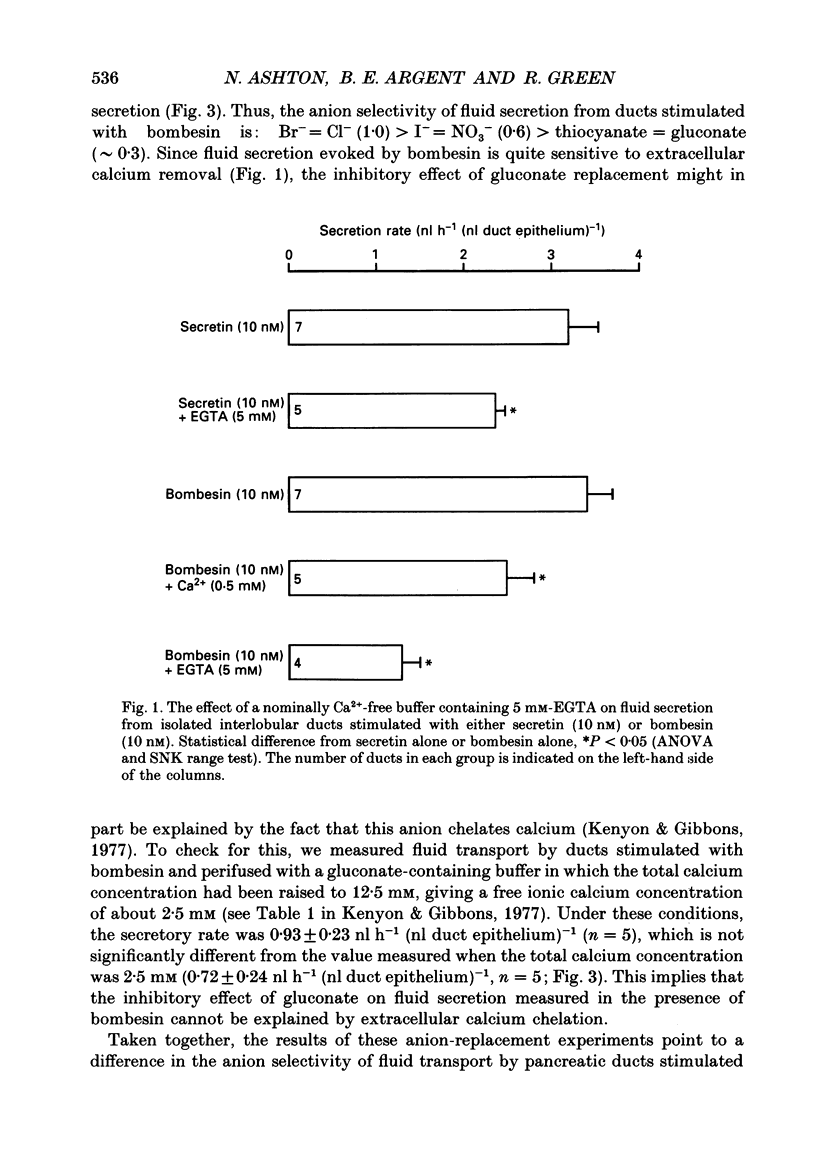
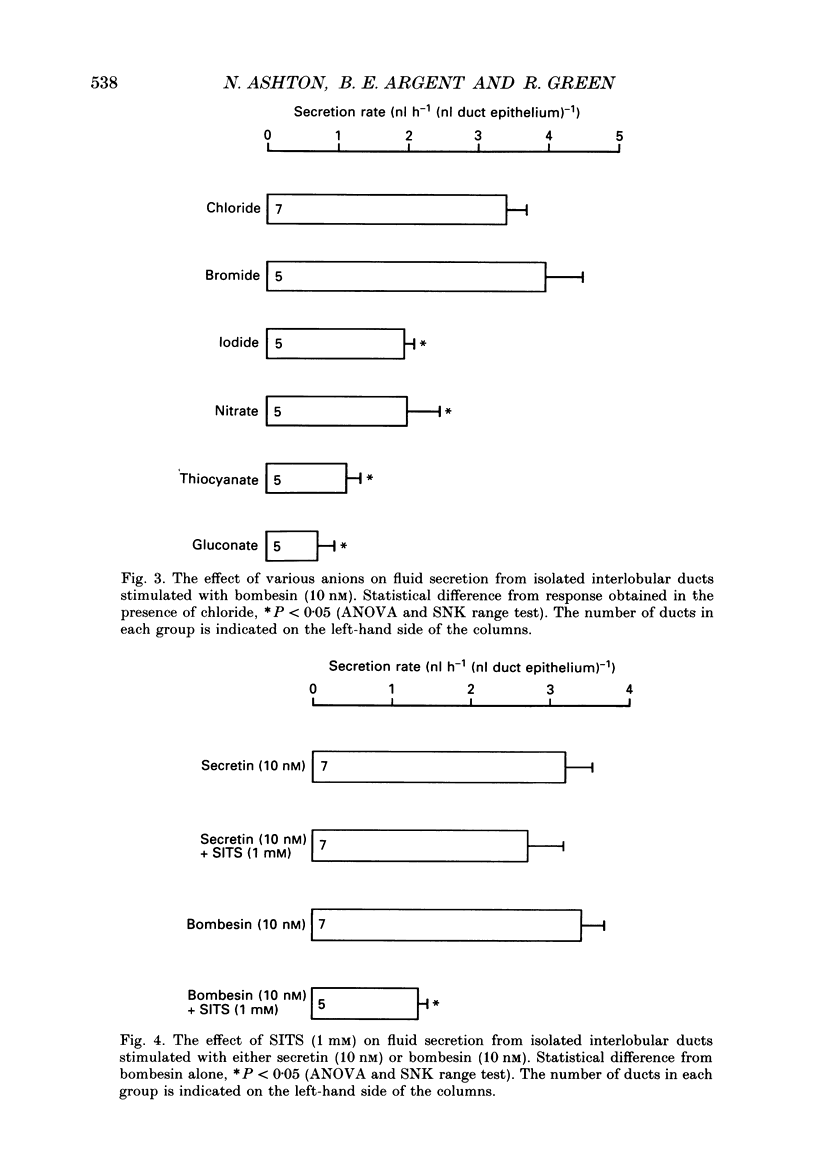
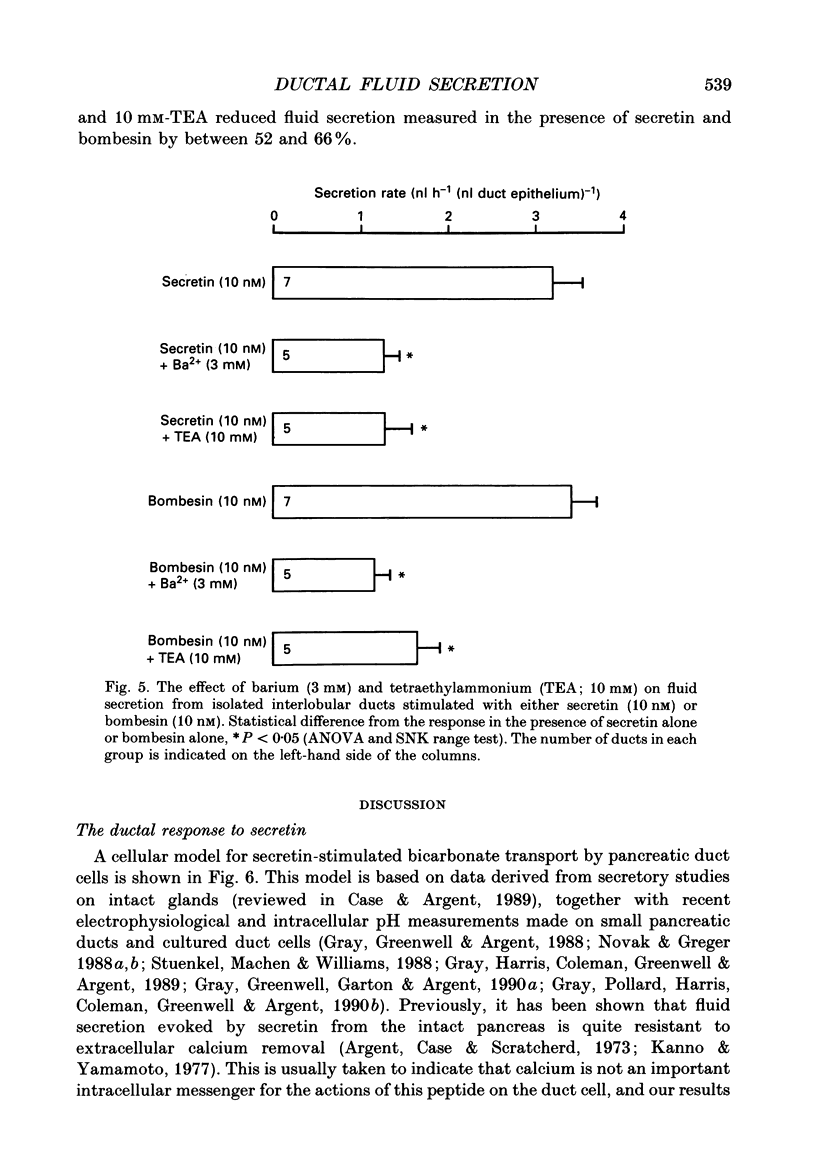
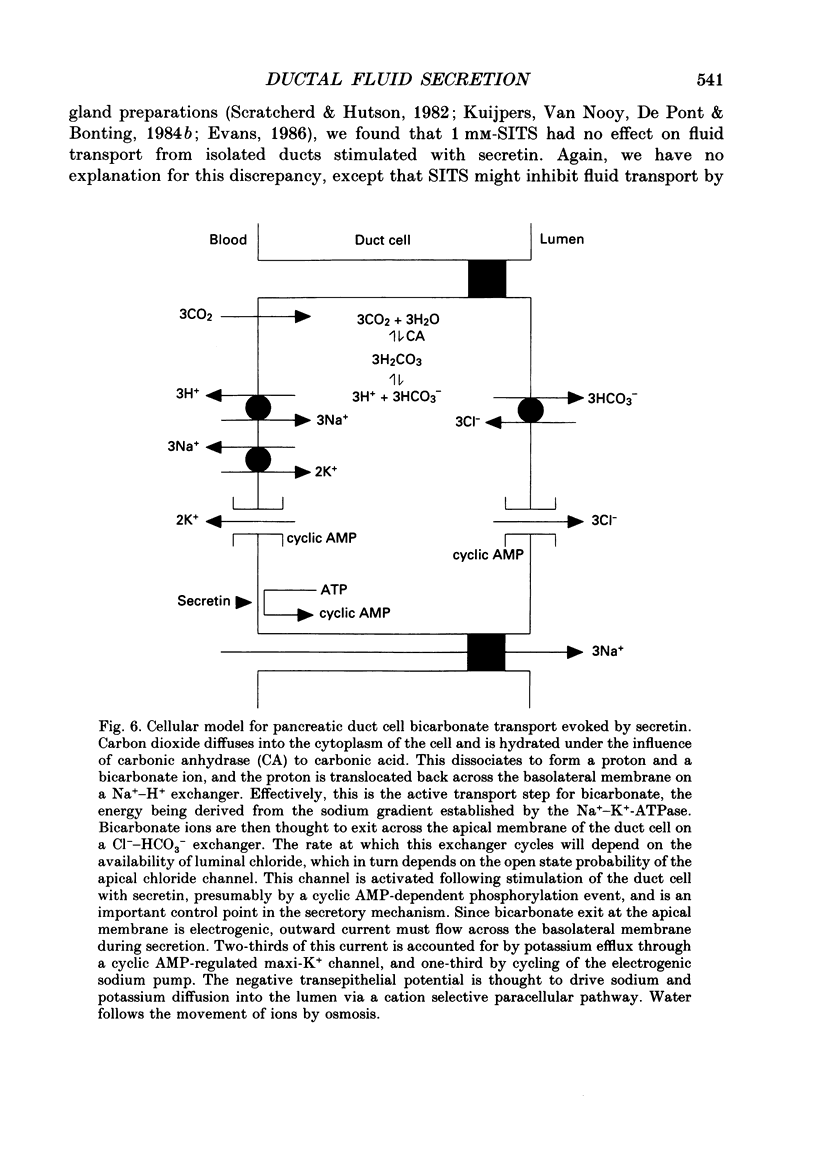
1. Micropuncture techniques were used to study the cellular mechanisms of fluid secretion by interlobular ducts isolated from the pancreas of copper-deficient rats. 2. Perifusing ducts with a calcium-free buffer containing 5 mM-EGTA reduced the volume of fluid secreted in the presence of 10 nM-bombesin by 62%, whereas fluid secretion measured in the presence of 10 nM-secretin was reduced by only 26%. 3. The anion selectivities of the fluid secretions evoked by secretin and bombesin were different. The anion sequence for secretin was: Br- = I- = NO3- = Cl- (1.0) much greater than thiocyanate = gluconate (0.3); whereas the sequence for bombesin was: Br- = Cl- (1.0) greater than I- = NO3- (0.6) greater than thiocyanate = gluconate (approximately 0.3). 4. SITS (4-acetamido-4'-isothiocyanatostilbene-2,2'-disulphonic acid; mM), reduced fluid secretion measured in the presence of bombesin by 61%, but had no effect on the response to secretin. 5. The K+ channel blockers, barium (3 mM) and tetraethylammonium (TEA; 10 mM), inhibited fluid secretion measured in the presence of both secretin and bombesin by between 52 and 66%. 6. From these results, we conclude that secretin and bombesin may utilize different intracellular signalling pathways and, furthermore, may activate different anion secretory mechanisms within the pancreatic ductal epithelium. However, the effect of the potassium channel blockers is consistent with both peptides activating secretory mechanisms which are electrogenic, and which depend for their operation on potassium efflux across the basolateral membrane of the duct cell.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argent B. E., Arkle S., Cullen M. J., Green R. Morphological, biochemical and secretory studies on rat pancreatic ducts maintained in tissue culture. Q J Exp Physiol. 1986 Oct;71(4):633–648. doi: 10.1113/expphysiol.1986.sp003023. [DOI] [PubMed] [Google Scholar]
- Argent B. E., Case R. M., Scratcherd T. Amylase secretion by the perfused cat pancreas in relation to the secretion of calcium and other electrolytes and as influenced by the external ionic environment. J Physiol. 1973 May;230(3):575–593. doi: 10.1113/jphysiol.1973.sp010205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkle S., Lee C. M., Cullen M. J., Argent B. E. Isolation of ducts from the pancreas of copper-deficient rats. Q J Exp Physiol. 1986 Apr;71(2):249–265. doi: 10.1113/expphysiol.1986.sp002982. [DOI] [PubMed] [Google Scholar]
- Ashton N., Argent B. E., Green R. Effect of vasoactive intestinal peptide, bombesin and substance P on fluid secretion by isolated rat pancreatic ducts. J Physiol. 1990 Aug;427:471–482. doi: 10.1113/jphysiol.1990.sp018182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges R. J., Worrell R. T., Frizzell R. A., Benos D. J. Stilbene disulfonate blockade of colonic secretory Cl- channels in planar lipid bilayers. Am J Physiol. 1989 Apr;256(4 Pt 1):C902–C912. doi: 10.1152/ajpcell.1989.256.4.C902. [DOI] [PubMed] [Google Scholar]
- Case R. M., Hotz J., Hutson D., Scratcherd T., Wynne R. D. Electrolyte secretion by the isolated cat pancreas during replacement of extracellular bicarbonate by organic anions and chloride by inorganic anions. J Physiol. 1979 Jan;286:563–576. doi: 10.1113/jphysiol.1979.sp012637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Hunter M., Novak I., Young J. A. The anionic basis of fluid secretion by the rabbit mandibular salivary gland. J Physiol. 1984 Apr;349:619–630. doi: 10.1113/jphysiol.1984.sp015177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschodt-Lanckman M., Robberecht P., De Neef P., Lammens M., Christophe J. In vitro action of bombesin and bombesin-like peptides on amylase secretion, calcium efflux, and adenylate cyclase activity in the rat pancreas: a comparison with other secretagogues. J Clin Invest. 1976 Oct;58(4):891–898. doi: 10.1172/JCI108542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenspeck G., Brodsky W. A. Effects of 4-acetamido-4'-isothiocyano-2,2-disulfonic stilbene on ion transport in turtle bladders. Biochim Biophys Acta. 1976 Feb 6;419(3):555–558. doi: 10.1016/0005-2736(76)90268-6. [DOI] [PubMed] [Google Scholar]
- Gray M. A., Greenwell J. R., Argent B. E. Secretin-regulated chloride channel on the apical plasma membrane of pancreatic duct cells. J Membr Biol. 1988 Oct;105(2):131–142. doi: 10.1007/BF02009166. [DOI] [PubMed] [Google Scholar]
- Gray M. A., Greenwell J. R., Garton A. J., Argent B. E. Regulation of maxi-K+ channels on pancreatic duct cells by cyclic AMP-dependent phosphorylation. J Membr Biol. 1990 May;115(3):203–215. doi: 10.1007/BF01868636. [DOI] [PubMed] [Google Scholar]
- Gray M. A., Harris A., Coleman L., Greenwell J. R., Argent B. E. Two types of chloride channel on duct cells cultured from human fetal pancreas. Am J Physiol. 1989 Aug;257(2 Pt 1):C240–C251. doi: 10.1152/ajpcell.1989.257.2.C240. [DOI] [PubMed] [Google Scholar]
- Gray M. A., Pollard C. E., Harris A., Coleman L., Greenwell J. R., Argent B. E. Anion selectivity and block of the small-conductance chloride channel on pancreatic duct cells. Am J Physiol. 1990 Nov;259(5 Pt 1):C752–C761. doi: 10.1152/ajpcell.1990.259.5.C752. [DOI] [PubMed] [Google Scholar]
- Greger R., Schlatter E., Wang F., Forrest J. N., Jr Mechanism of NaCl secretion in rectal gland tubules of spiny dogfish (Squalus acanthias). III. Effects of stimulation of secretion by cyclic AMP. Pflugers Arch. 1984 Dec;402(4):376–384. doi: 10.1007/BF00583938. [DOI] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. In vitro action of bombesin on amylase secretion, membrane potential, and membrane resistance in rat and mouse pancreatic acinar cells. A comparison with other secretagogues. J Clin Invest. 1978 Jan;61(1):41–46. doi: 10.1172/JCI108923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno T., Yamamoto M. Differentiation between the calcium-dependent effects of cholecystokinin-pancreaozymin and the bicarbonate-dependent effects of secretin in exocrine secretion of the rat pancreas. J Physiol. 1977 Jan;264(3):787–799. doi: 10.1113/jphysiol.1977.sp011694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon J. L., Gibbons W. R. Effects of low-chloride solutions on action potentials of sheep cardiac Purkinje fibers. J Gen Physiol. 1977 Nov;70(5):635–660. doi: 10.1085/jgp.70.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers G. A., Van Nooy I. G., De Pont J. J., Bonting S. L. Anion secretion by the isolated rabbit pancreas. Biochim Biophys Acta. 1984 Jul 25;774(2):269–276. doi: 10.1016/0005-2736(84)90301-8. [DOI] [PubMed] [Google Scholar]
- Kuijpers G. A., Van Nooy I. G., De Pont J. J., Bonting S. L. The mechanism of fluid secretion in the rabbit pancreas studied by means of various inhibitors. Biochim Biophys Acta. 1984 Dec 5;778(2):324–331. doi: 10.1016/0005-2736(84)90376-6. [DOI] [PubMed] [Google Scholar]
- Latorre R., Miller C. Conduction and selectivity in potassium channels. J Membr Biol. 1983;71(1-2):11–30. doi: 10.1007/BF01870671. [DOI] [PubMed] [Google Scholar]
- MADDY A. H. A FLUORESCENT LABEL FOR THE OUTER COMPONENTS OF THE PLASMA MEMBRANE. Biochim Biophys Acta. 1964 Sep 25;88:390–399. doi: 10.1016/0926-6577(64)90194-9. [DOI] [PubMed] [Google Scholar]
- Novak I., Greger R. Electrophysiological study of transport systems in isolated perfused pancreatic ducts: properties of the basolateral membrane. Pflugers Arch. 1988 Jan;411(1):58–68. doi: 10.1007/BF00581647. [DOI] [PubMed] [Google Scholar]
- Novak I., Greger R. Properties of the luminal membrane of isolated perfused rat pancreatic ducts. Effect of cyclic AMP and blockers of chloride transport. Pflugers Arch. 1988 May;411(5):546–553. doi: 10.1007/BF00582376. [DOI] [PubMed] [Google Scholar]
- Petersen O. H. Calcium-activated potassium channels and fluid secretion by exocrine glands. Am J Physiol. 1986 Jul;251(1 Pt 1):G1–13. doi: 10.1152/ajpgi.1986.251.1.G1. [DOI] [PubMed] [Google Scholar]
- Pralong W. F., Wollheim C. B., Bruzzone R. Measurement of cytosolic free Ca2+ in individual pancreatic acini. FEBS Lett. 1988 Dec 19;242(1):79–84. doi: 10.1016/0014-5793(88)80989-x. [DOI] [PubMed] [Google Scholar]
- Preisig P. A., Alpern R. J. Basolateral membrane H-OH-HCO3 transport in the proximal tubule. Am J Physiol. 1989 May;256(5 Pt 2):F751–F765. doi: 10.1152/ajprenal.1989.256.5.F751. [DOI] [PubMed] [Google Scholar]
- Rothman S. S., Brooks F. P. Pancreatic secretion in vitro in "Cl-free," "Co-2-free," and low-Na+environment. Am J Physiol. 1965 Oct;209(4):790–796. doi: 10.1152/ajplegacy.1965.209.4.790. [DOI] [PubMed] [Google Scholar]
- Seow K. T., Lingard J. M., Young J. A. Anionic basis of fluid secretion by rat pancreatic acini in vitro. Am J Physiol. 1986 Feb;250(2 Pt 1):G140–G148. doi: 10.1152/ajpgi.1986.250.2.G140. [DOI] [PubMed] [Google Scholar]
- Silva P., Stoff J., Field M., Fine L., Forrest J. N., Epstein F. H. Mechanism of active chloride secretion by shark rectal gland: role of Na-K-ATPase in chloride transport. Am J Physiol. 1977 Oct;233(4):F298–F306. doi: 10.1152/ajprenal.1977.233.4.F298. [DOI] [PubMed] [Google Scholar]
- Stuenkel E. L., Machen T. E., Williams J. A. pH regulatory mechanisms in rat pancreatic ductal cells. Am J Physiol. 1988 Jun;254(6 Pt 1):G925–G930. doi: 10.1152/ajpgi.1988.254.6.G925. [DOI] [PubMed] [Google Scholar]
- White J. F., Imon M. A. A role for basolateral anion exchange in active jejunal absorption of HCO-3. Am J Physiol. 1983 Apr;244(4):G397–G405. doi: 10.1152/ajpgi.1983.244.4.G397. [DOI] [PubMed] [Google Scholar]
- Wieth J. O. Effect of some monovalent anions on chloride and sulphate permeability of human red cells. J Physiol. 1970 May;207(3):581–609. doi: 10.1113/jphysiol.1970.sp009082. [DOI] [PMC free article] [PubMed] [Google Scholar]


