Abstract
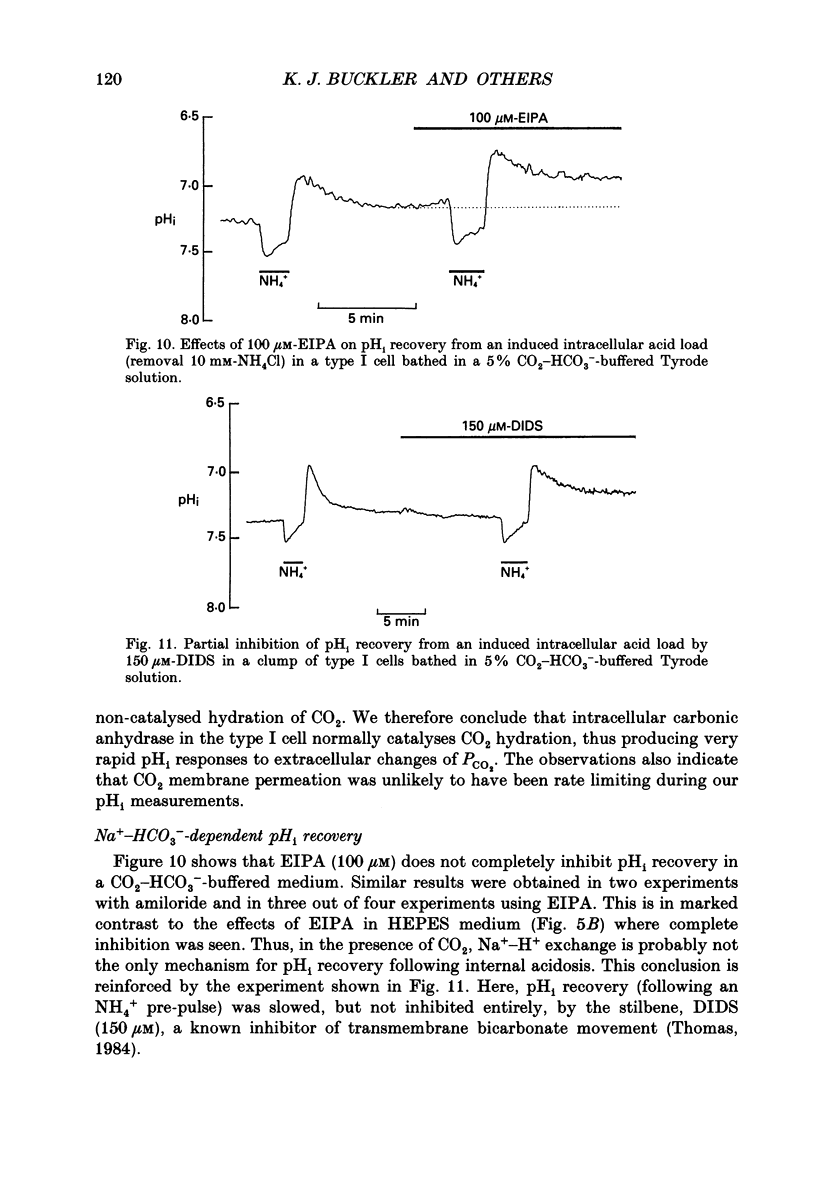
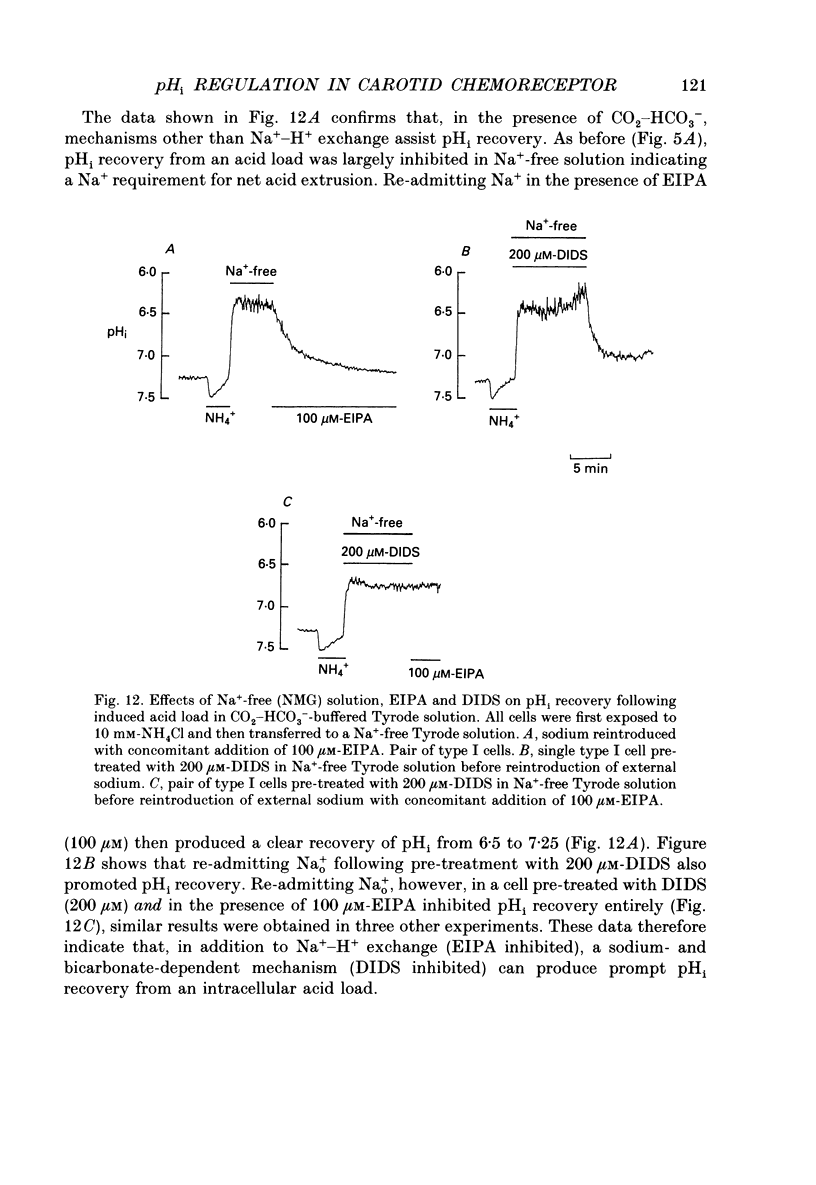
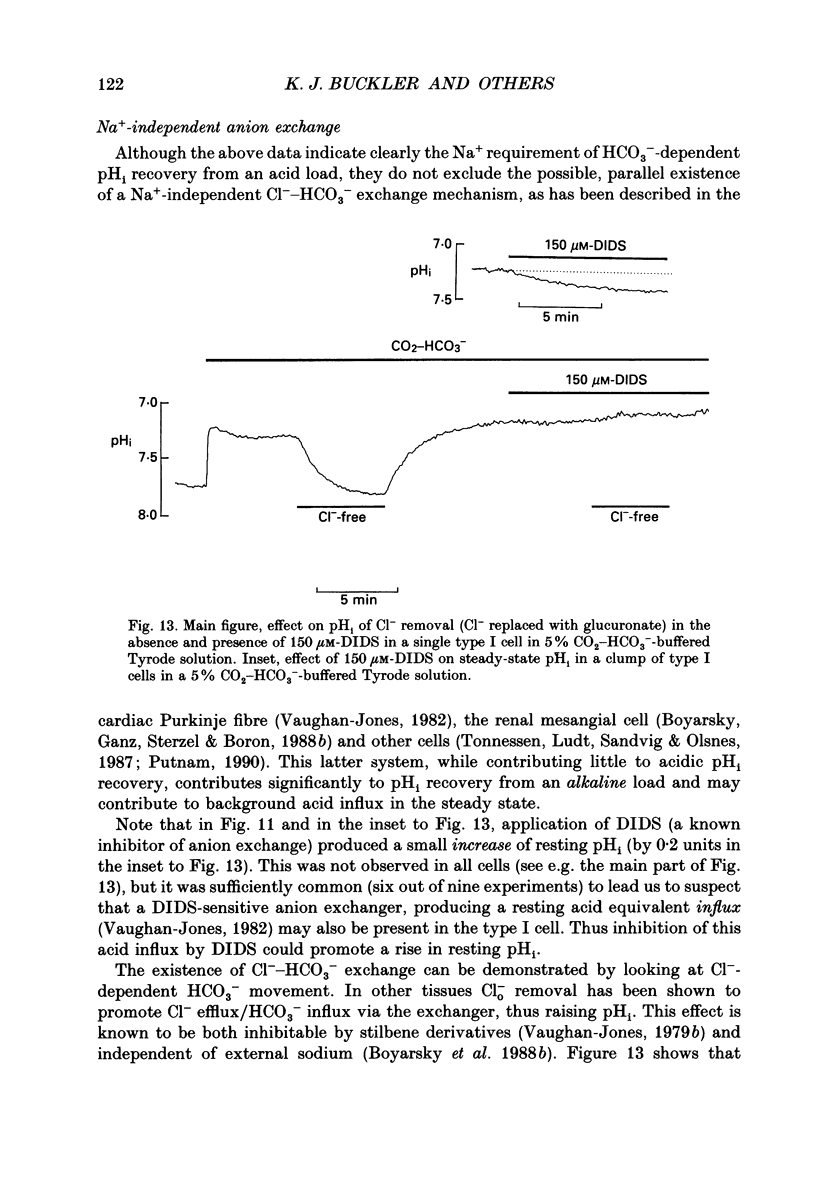
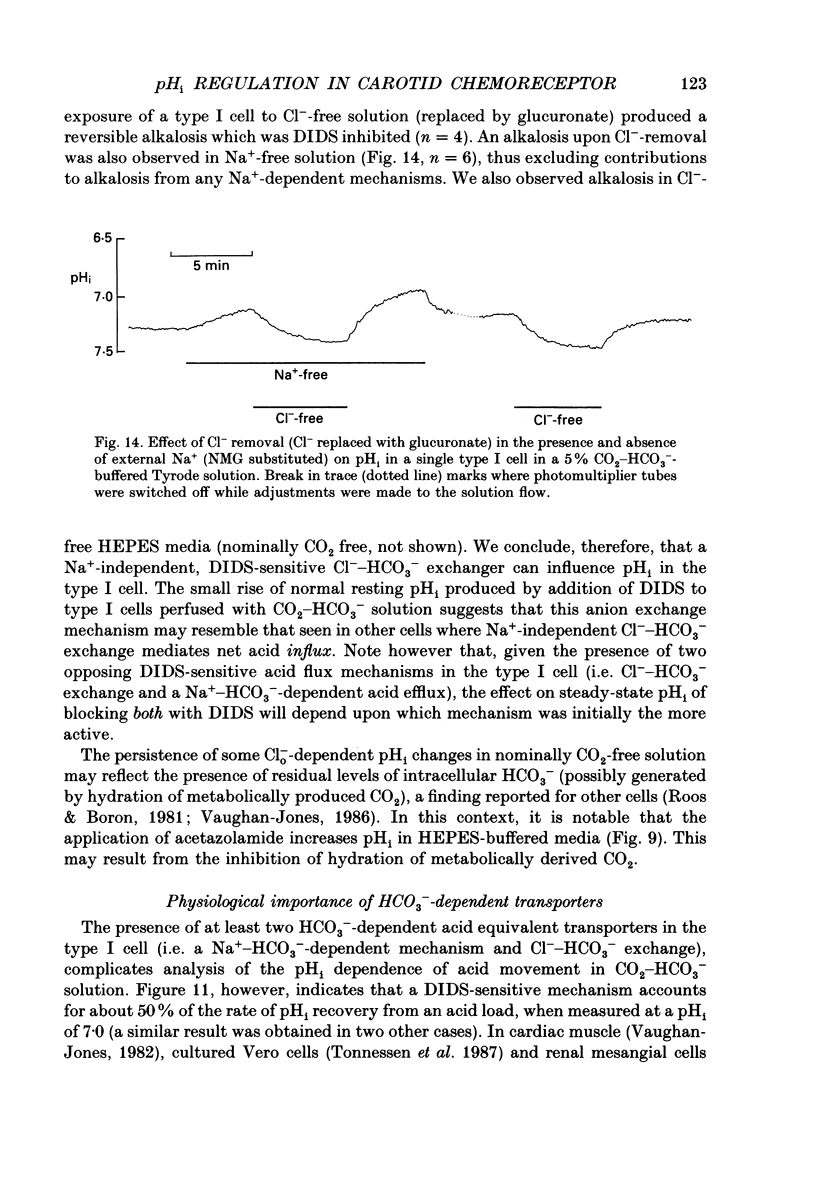
1. The dual-emission pH-sensitive fluoroprobe carboxy-SNARF-1 (carboxy-seminaptharhodofluor) was used to measure pHi in type I cells enzymically dispersed from the neonatal rat carotid body. 2. Steady-state pHi in cells bathed in a HEPES-buffered Tyrode solution (pH 7.4) was found to be remarkably alkaline (pHi = 7.77) whereas cells bathed in a CO2-HCO3(-)-buffered Tyrode solution (pH 7.4) had a more 'normal' pHi (pHi = 7.28). These observations were further substantiated by using an independent nullpoint test method to determine pHi. 3. Intracellular intrinsic buffering (beta, determined by acidifying the cell using an NH4Cl pre-pulse) was in the range 7-20 mM per pH unit and appeared to be dependent upon pHi with beta increasing as pHi decreased. 4. In cells bathed in a HEPES-buffered Tyrode solution, pHi recovery from an induced intracellular acid load (10 mM-NH4Cl pre-pulse) was inhibited by the Na(+)-H+ exchange inhibitor ethyl isopropyl amiloride (EIPA; 150 microM) or substitution of Nao+ with N-methyl-D-glucamine (NMG). Both EIPA and Nao+ removal also caused a rapid intracellular acidification, which in the case of Nao+ removal, was readily reversible. The rate of this acidification was similar for both Nao+ removal and EIPA addition. 5. Transferring cells from a HEPES-buffered Tyrode solution to one buffered with 5% CO2-HCO3- resulted in an intracellular acidification which was partially, or wholly, sustained. The rate of acidification upon transfer to CO2-HCO3- was considerably slowed by the membrane permeant carbonic anhydrase inhibitor, acetazolamide, thus indicating the presence of the enzyme in these cells. 6. In CO2-HCO3(-)-buffered Tyrode solution, pHi recovery from an intracellular acidosis (NH4+ pre-pulse) was only partially inhibited by EIPA or amiloride whereas Nao+ removal completely inhibited the recovery. The stilbene DIDS (4,4-diisothiocyanatostilbenedisulphonic acid, 200 microM) also partially inhibited pHi recovery following an induced intracellular acidosis. Furthermore, the pre-treatment with 200 microM-DIDS of a pre-acidified cell in Na(+)-free Tyrode solution completely inhibited pHi recovery when Nao+ was reintroduced together with concomitant addition of 150 microM-EIPA. We conclude, that in the presence of CO2-HCO3-, a Na(+)- and HCO3-dependent (DIDS inhibitable) mechanism aids acid extrusion.(ABSTRACT TRUNCATED AT 400 WORDS)
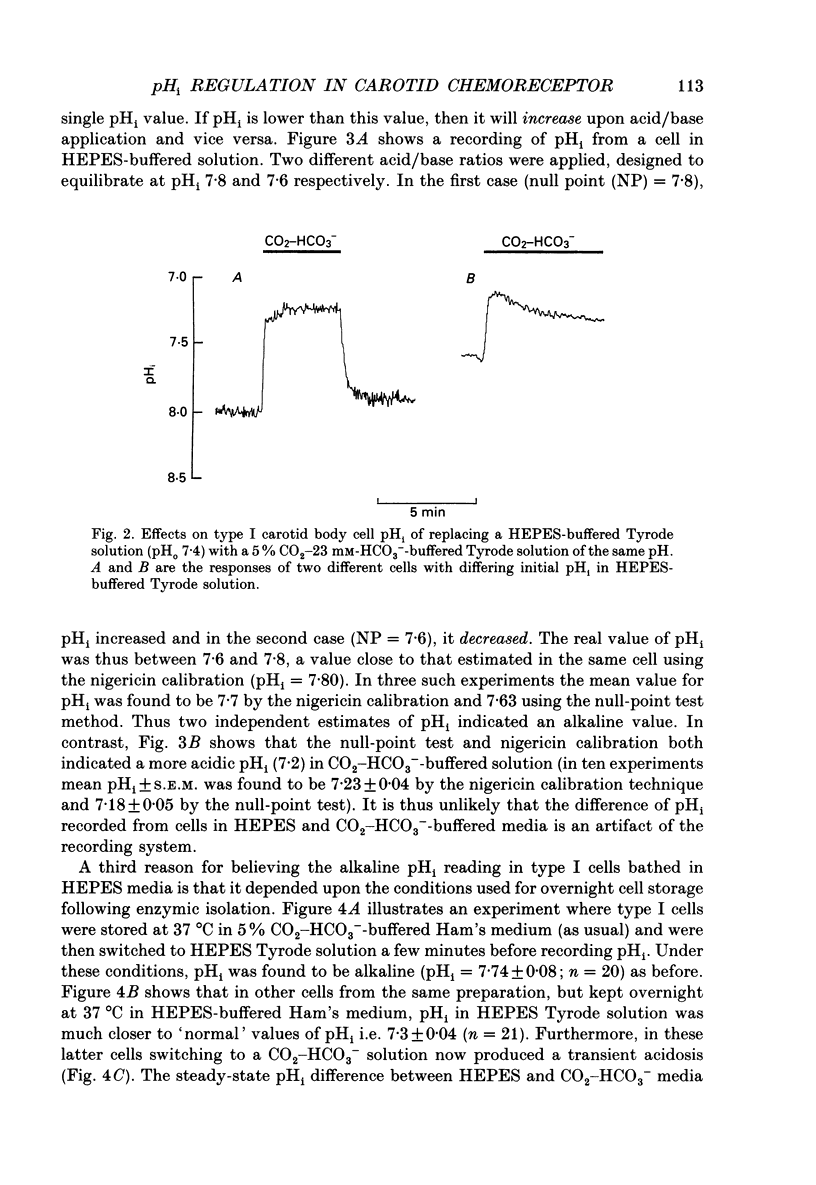
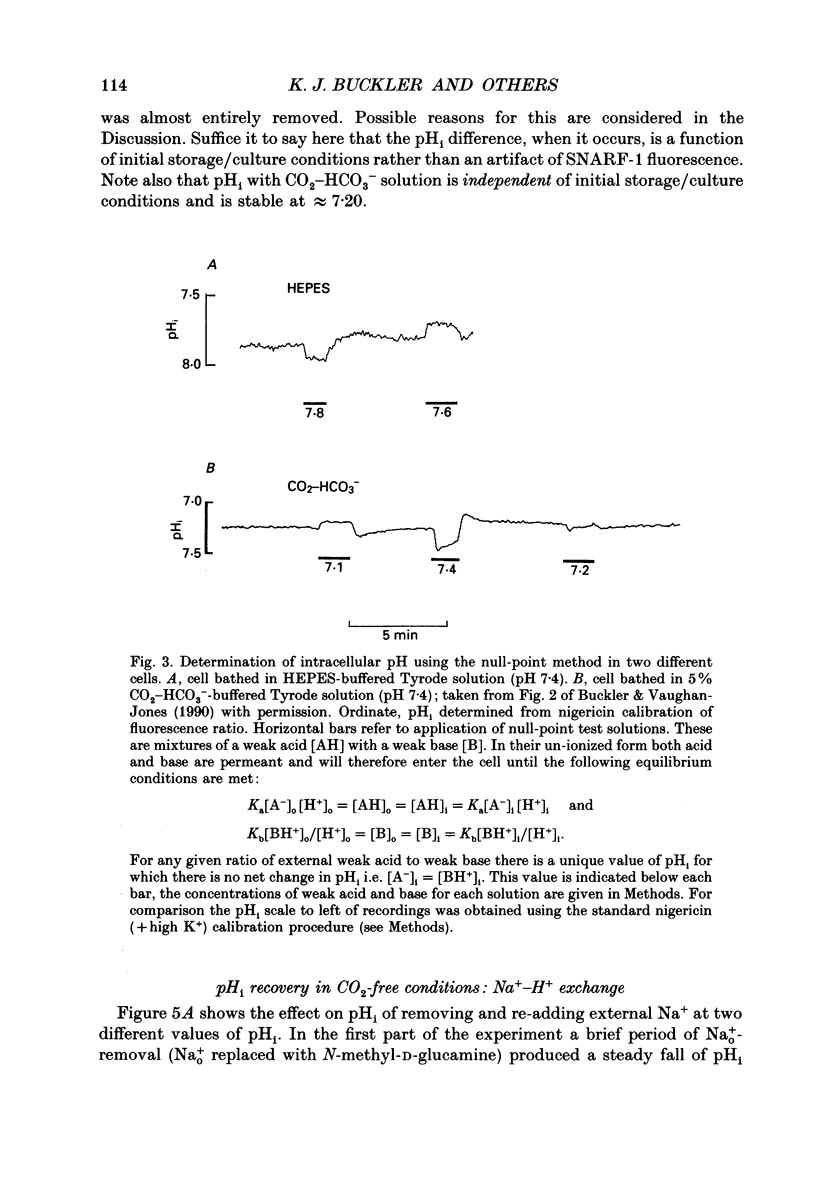
Full text
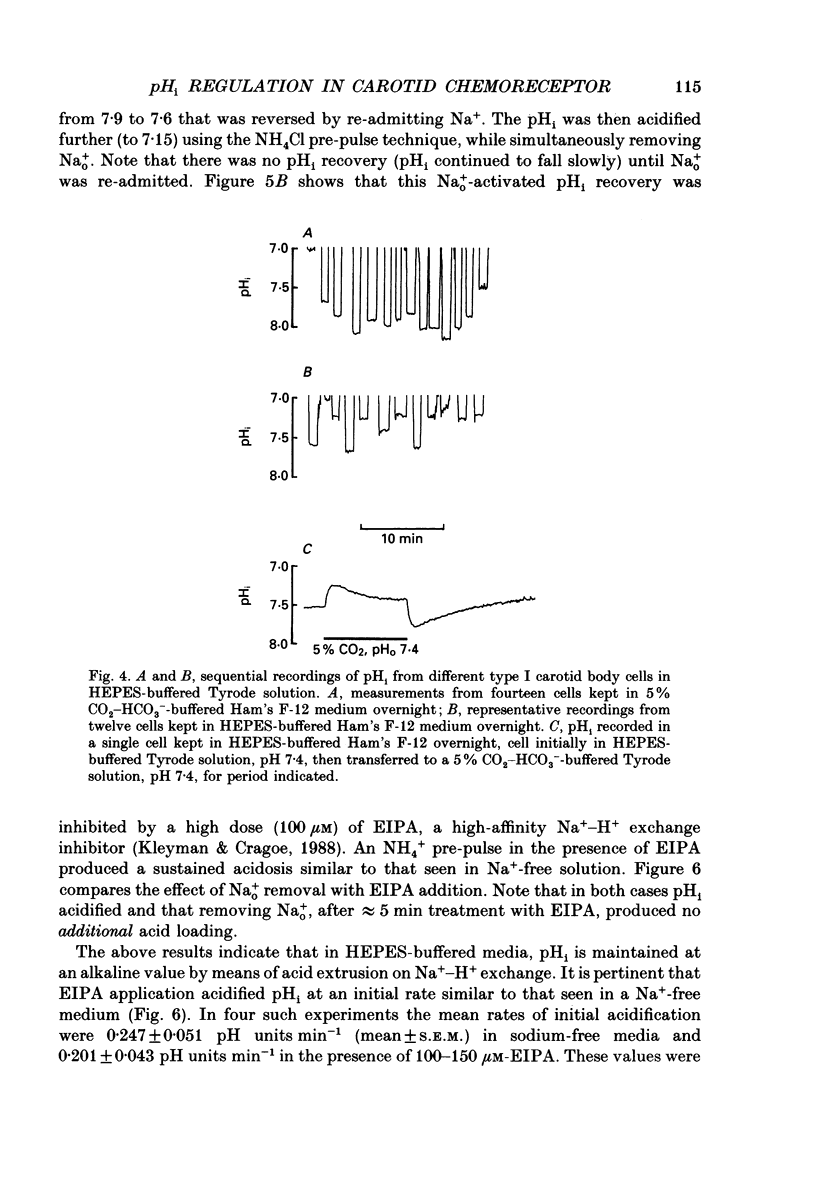
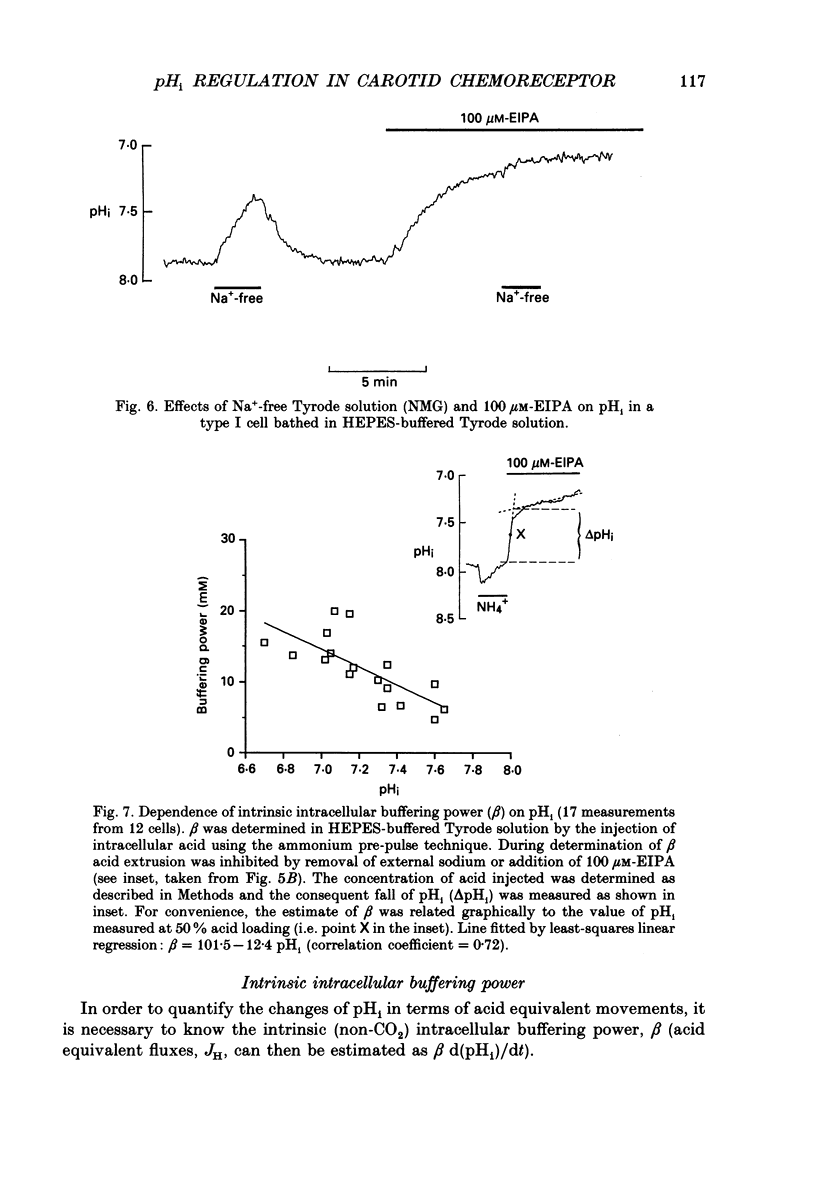
PDF




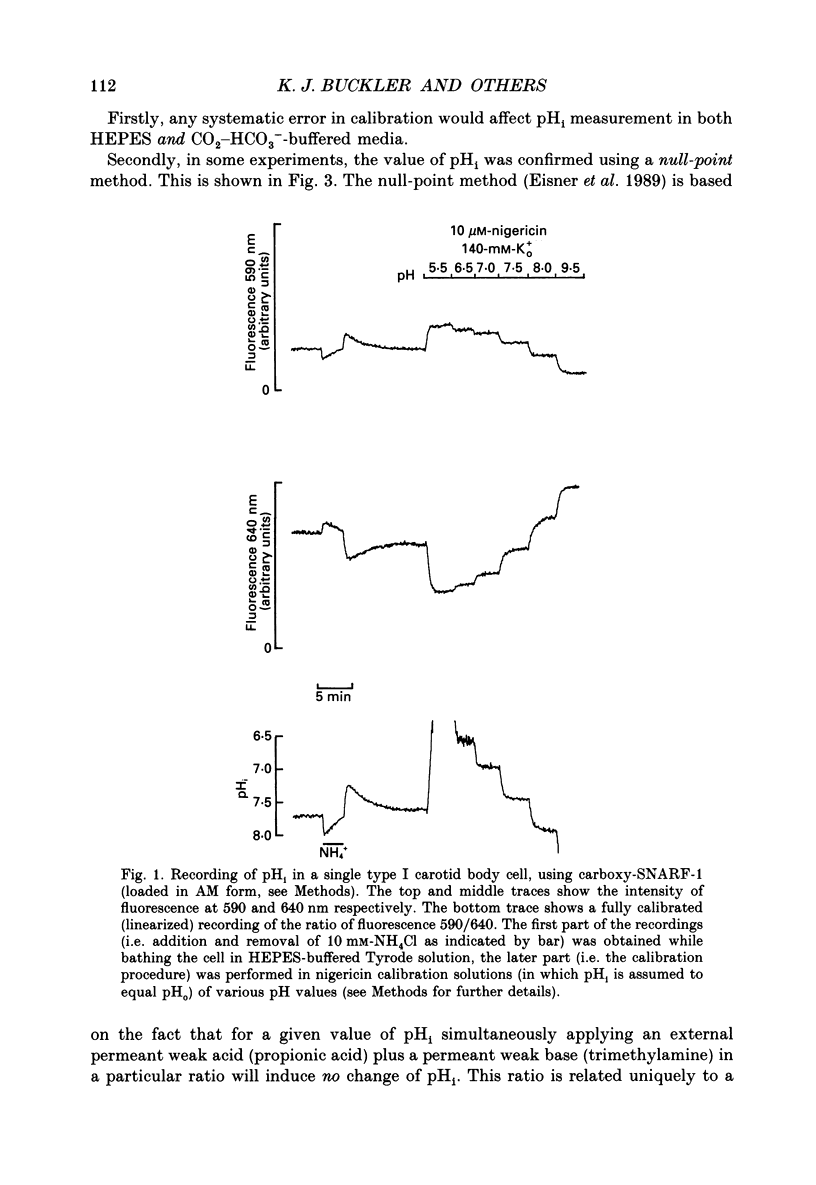
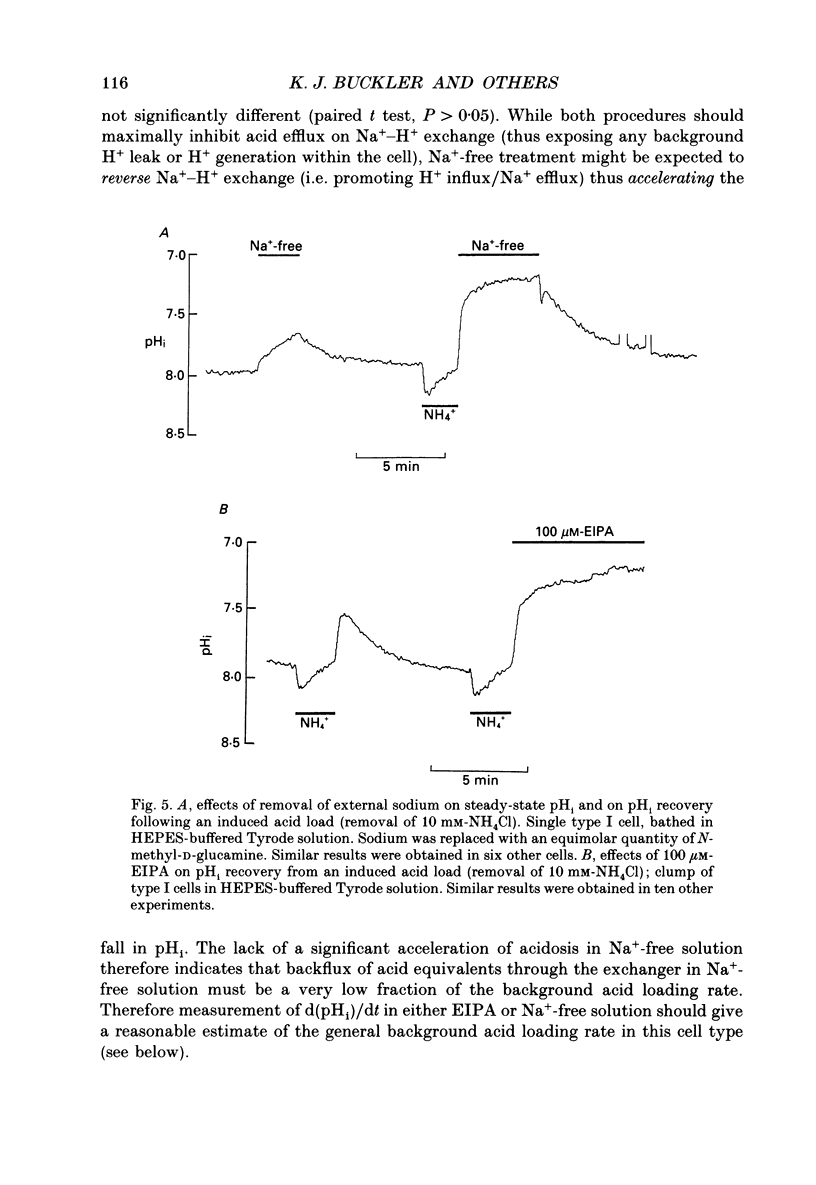
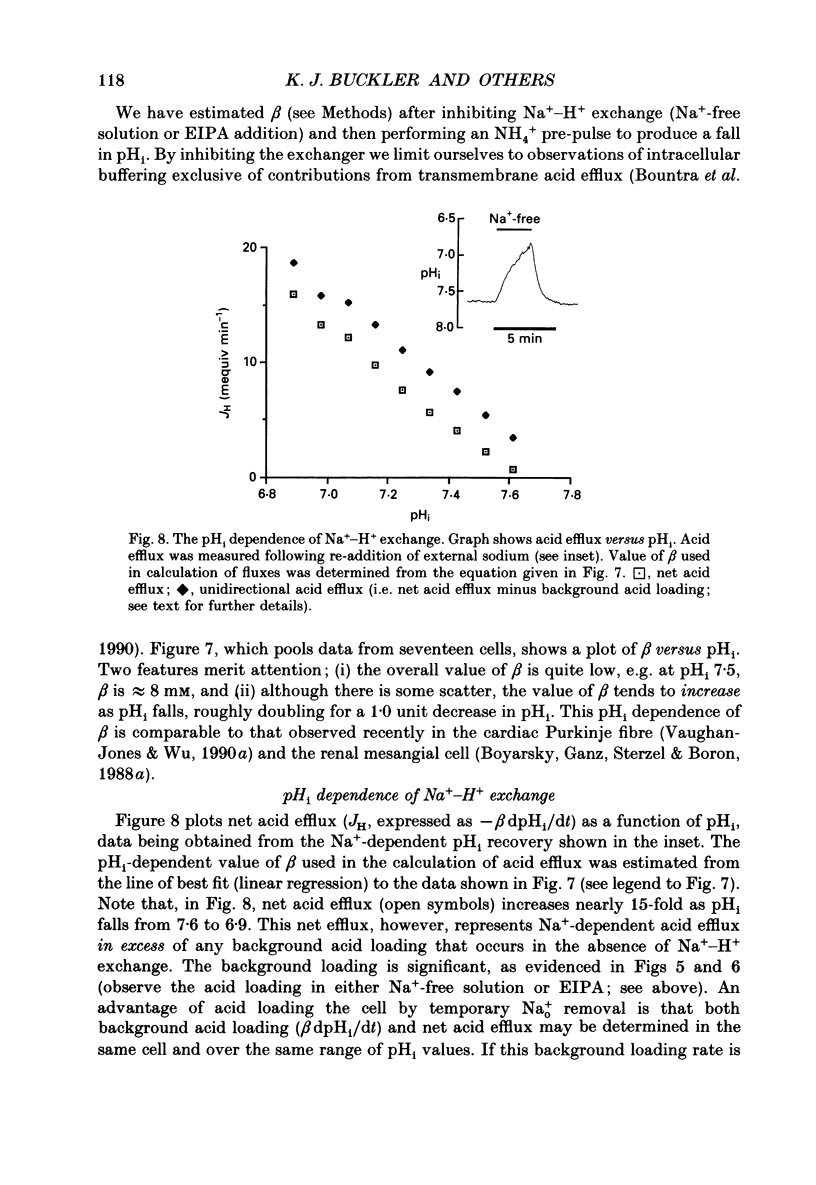





Selected References
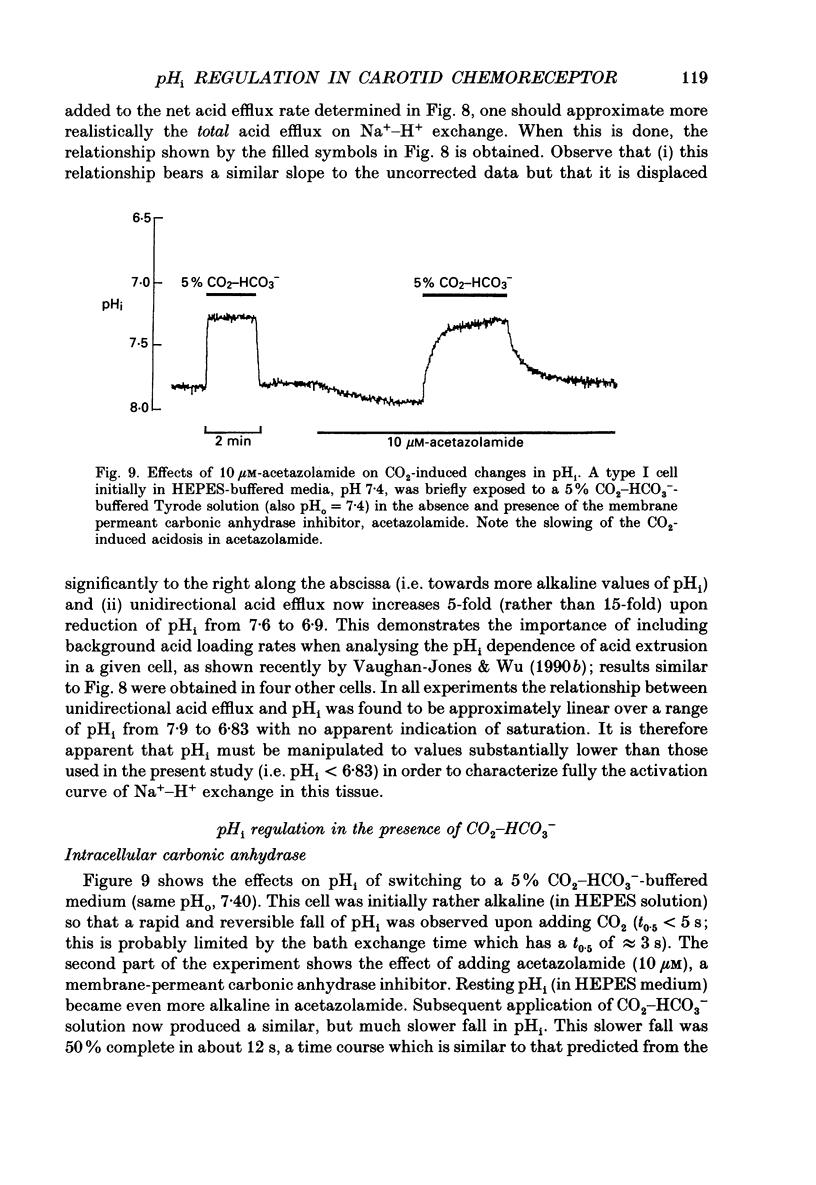
These references are in PubMed. This may not be the complete list of references from this article.
- Aickin C. C., Thomas R. C. Micro-electrode measurement of the internal pH of crab muscle fibres. J Physiol. 1975 Nov;252(3):803–815. doi: 10.1113/jphysiol.1975.sp011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Duchen M. R., Eisner D. A., O'Neill S. C., Valdeolmillos M. Measurements of intracellular Ca2+ in dissociated type I cells of the rabbit carotid body. J Physiol. 1989 Sep;416:421–434. doi: 10.1113/jphysiol.1989.sp017769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F. Intracellular pH regulation in epithelial cells. Annu Rev Physiol. 1986;48:377–388. doi: 10.1146/annurev.ph.48.030186.002113. [DOI] [PubMed] [Google Scholar]
- Boron W. F., Russell J. M. Stoichiometry and ion dependencies of the intracellular-pH-regulating mechanism in squid giant axons. J Gen Physiol. 1983 Mar;81(3):373–399. doi: 10.1085/jgp.81.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bountra C., Powell T., Vaughan-Jones R. D. Comparison of intracellular pH transients in single ventricular myocytes and isolated ventricular muscle of guinea-pig. J Physiol. 1990 May;424:343–365. doi: 10.1113/jphysiol.1990.sp018071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyarsky G., Ganz M. B., Sterzel R. B., Boron W. F. pH regulation in single glomerular mesangial cells. I. Acid extrusion in absence and presence of HCO3-. Am J Physiol. 1988 Dec;255(6 Pt 1):C844–C856. doi: 10.1152/ajpcell.1988.255.6.C844. [DOI] [PubMed] [Google Scholar]
- Boyarsky G., Ganz M. B., Sterzel R. B., Boron W. F. pH regulation in single glomerular mesangial cells. II. Na+-dependent and -independent Cl(-)-HCO3- exchangers. Am J Physiol. 1988 Dec;255(6 Pt 1):C857–C869. doi: 10.1152/ajpcell.1988.255.6.C857. [DOI] [PubMed] [Google Scholar]
- Buckler K. J., Vaughan-Jones R. D. Application of a new pH-sensitive fluoroprobe (carboxy-SNARF-1) for intracellular pH measurement in small, isolated cells. Pflugers Arch. 1990 Oct;417(2):234–239. doi: 10.1007/BF00370705. [DOI] [PubMed] [Google Scholar]
- Deitmer J. W., Schlue W. R. An inwardly directed electrogenic sodium-bicarbonate co-transport in leech glial cells. J Physiol. 1989 Apr;411:179–194. doi: 10.1113/jphysiol.1989.sp017567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Kenning N. A., O'Neill S. C., Pocock G., Richards C. D., Valdeolmillos M. A novel method for absolute calibration of intracellular pH indicators. Pflugers Arch. 1989 Mar;413(5):553–558. doi: 10.1007/BF00594188. [DOI] [PubMed] [Google Scholar]
- Green R. D., Frelin C., Vigne P., Lazdunski M. The activity of the Na+/H+ antiporter in cultured cardiac cells is dependent on the culture conditions used. FEBS Lett. 1986 Feb 3;196(1):163–166. doi: 10.1016/0014-5793(86)80234-4. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hescheler J., Delpiano M. A., Acker H., Pietruschka F. Ionic currents on type-I cells of the rabbit carotid body measured by voltage-clamp experiments and the effect of hypoxia. Brain Res. 1989 May 1;486(1):79–88. doi: 10.1016/0006-8993(89)91280-8. [DOI] [PubMed] [Google Scholar]
- Kleyman T. R., Cragoe E. J., Jr Amiloride and its analogs as tools in the study of ion transport. J Membr Biol. 1988 Oct;105(1):1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- López-López J., González C., Ureña J., López-Barneo J. Low pO2 selectively inhibits K channel activity in chemoreceptor cells of the mammalian carotid body. J Gen Physiol. 1989 May;93(5):1001–1015. doi: 10.1085/jgp.93.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H. Effects of growth factors on intracellular pH regulation. Annu Rev Physiol. 1986;48:363–376. doi: 10.1146/annurev.ph.48.030186.002051. [DOI] [PubMed] [Google Scholar]
- Peers C. Effect of lowered extracellular pH on Ca2(+)-dependent K+ currents in type I cells from the neonatal rat carotid body. J Physiol. 1990 Mar;422:381–395. doi: 10.1113/jphysiol.1990.sp017990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers C., O'Donnell J. Potassium currents recorded in type I carotid body cells from the neonatal rat and their modulation by chemoexcitatory agents. Brain Res. 1990 Jul 9;522(2):259–266. doi: 10.1016/0006-8993(90)91470-2. [DOI] [PubMed] [Google Scholar]
- Putnam R. W., Grubbs R. D. Steady-state pHi, buffering power, and effect of CO2 in a smooth muscle-like cell line. Am J Physiol. 1990 Mar;258(3 Pt 1):C461–C469. doi: 10.1152/ajpcell.1990.258.3.C461. [DOI] [PubMed] [Google Scholar]
- Putnam R. W. pH regulatory transport systems in a smooth muscle-like cell line. Am J Physiol. 1990 Mar;258(3 Pt 1):C470–C479. doi: 10.1152/ajpcell.1990.258.3.C470. [DOI] [PubMed] [Google Scholar]
- Ridderstråle Y., Hanson M. A. Histochemical localization of carbonic anhydrase in the cat carotid body. Ann N Y Acad Sci. 1984;429:398–400. doi: 10.1111/j.1749-6632.1984.tb12363.x. [DOI] [PubMed] [Google Scholar]
- Rigual R., Iñiguez C., Carreres J., Gonzalez C. Carbonic anhydrase in the carotid body and the carotid sinus nerve. Histochemistry. 1985;82(6):577–580. doi: 10.1007/BF00489979. [DOI] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Sardet C., Franchi A., Pouysségur J. Molecular cloning, primary structure, and expression of the human growth factor-activatable Na+/H+ antiporter. Cell. 1989 Jan 27;56(2):271–280. doi: 10.1016/0092-8674(89)90901-x. [DOI] [PubMed] [Google Scholar]
- Stea A., Nurse C. A. Chloride channels in cultured glomus cells of the rat carotid body. Am J Physiol. 1989 Aug;257(2 Pt 1):C174–C181. doi: 10.1152/ajpcell.1989.257.2.C174. [DOI] [PubMed] [Google Scholar]
- Szatkowski M. S., Thomas R. C. The intrinsic intracellular H+ buffering power of snail neurones. J Physiol. 1989 Feb;409:89–101. doi: 10.1113/jphysiol.1989.sp017486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Experimental displacement of intracellular pH and the mechanism of its subsequent recovery. J Physiol. 1984 Sep;354:3P–22P. doi: 10.1113/jphysiol.1984.sp015397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. The role of bicarbonate, chloride and sodium ions in the regulation of intracellular pH in snail neurones. J Physiol. 1977 Dec;273(1):317–338. doi: 10.1113/jphysiol.1977.sp012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tønnessen T. I., Ludt J., Sandvig K., Olsnes S. Bicarbonate/chloride antiport in Vero cells: I. Evidence for both sodium-linked and sodium-independent exchange. J Cell Physiol. 1987 Aug;132(2):183–191. doi: 10.1002/jcp.1041320202. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D. An investigation of chloride-bicarbonate exchange in the sheep cardiac Purkinje fibre. J Physiol. 1986 Oct;379:377–406. doi: 10.1113/jphysiol.1986.sp016259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R. D. Non-passive chloride distribution in mammalian heart muscle: micro-electrode measurement of the intracellular chloride activity. J Physiol. 1979 Oct;295:83–109. doi: 10.1113/jphysiol.1979.sp012956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R. D. Regulation of chloride in quiescent sheep-heart Purkinje fibres studied using intracellular chloride and pH-sensitive micro-electrodes. J Physiol. 1979 Oct;295:111–137. doi: 10.1113/jphysiol.1979.sp012957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Wu M. L. Extracellular H+ inactivation of Na(+)-H+ exchange in the sheep cardiac Purkinje fibre. J Physiol. 1990 Sep;428:441–466. doi: 10.1113/jphysiol.1990.sp018221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Wu M. L. pH dependence of intrinsic H+ buffering power in the sheep cardiac Purkinje fibre. J Physiol. 1990 Jun;425:429–448. doi: 10.1113/jphysiol.1990.sp018112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzl E., Sjaastad M. D., Weintraub W. H., Machen T. E. Intracellular pH regulation in IEC-6 cells, a cryptlike intestinal cell line. Am J Physiol. 1989 Nov;257(5 Pt 1):G732–G740. doi: 10.1152/ajpgi.1989.257.5.G732. [DOI] [PubMed] [Google Scholar]


