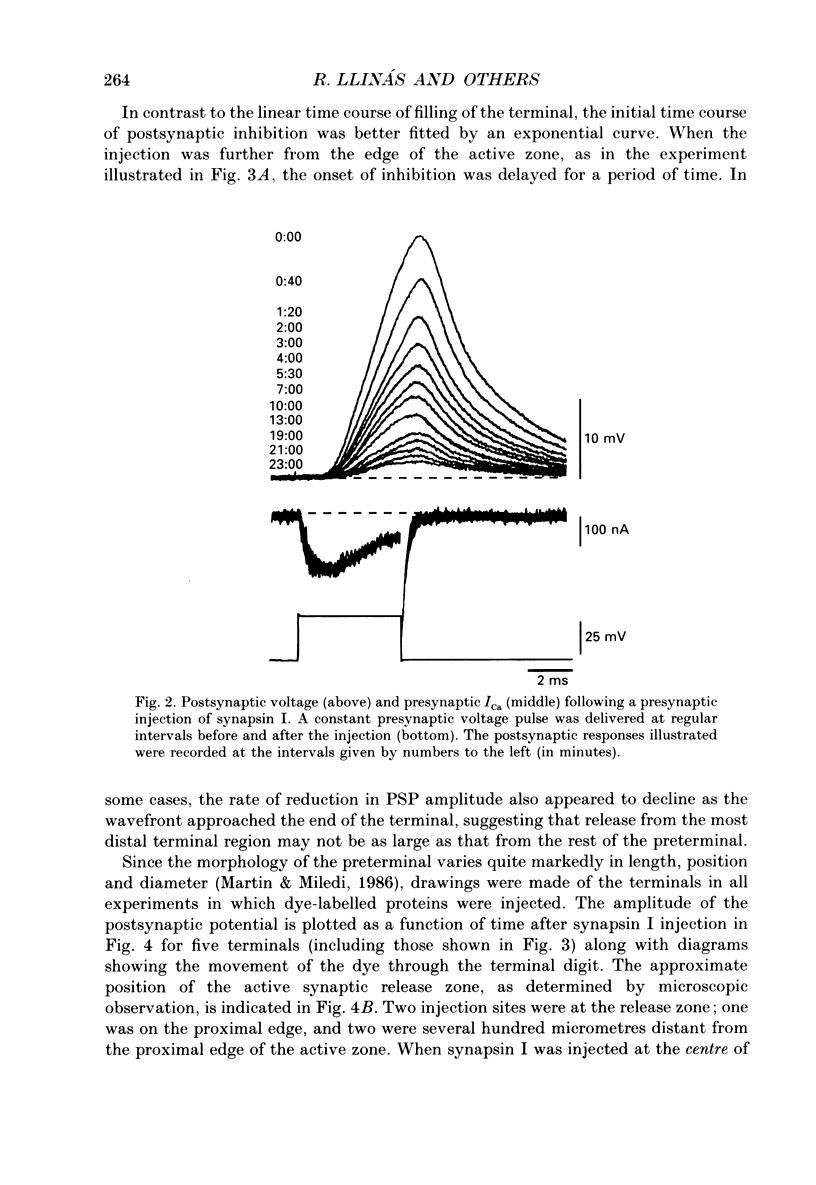
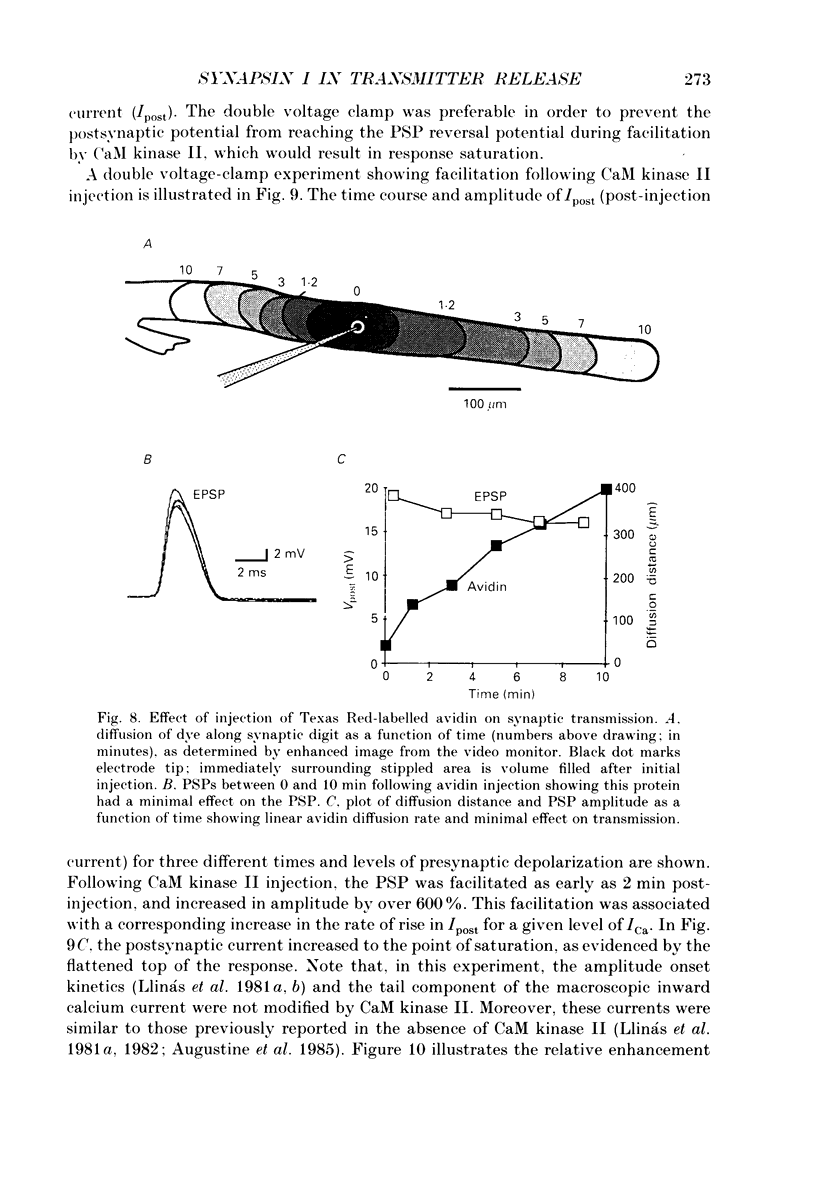
Abstract
1. Presynaptic or simultaneous pre- and postsynaptic voltage-clamp protocols were implemented in the squid giant synapse in order to determine the magnitude and time course of the presynaptic calcium current (ICa) and its relation to transmitter release before and after presynaptic injection of proteins. These included several forms of synapsin I, calcium-calmodulin-dependent protein kinase II (CaM kinase II) and avidin. 2. The quantities and location of these proteins were monitored by fluorescence video-enhanced microscopy during the electrophysiological measurements. 3. Presynaptic injection of dephosphorylated synapsin I inhibited synaptic transmission with a time course consistent with diffusion of the protein through the terminal and action at the active release zone. A mathematical model relating the diffusion of synapsin I into the terminal with transmitter release was developed to aid in the interpretation of these results. 4. Synapsin I inhibition of transmitter release was reversible. 5. The action of synapsin I was highly specific, as phosphorylation of the tail region only or head and tail regions prevented synapsin I from inhibiting release. 6. Injections of heat-treated synapsin I or of avidin, a protein with a size and isoelectric point similar to those of synapsin I, had no effect on transmitter release. 7. CaM kinase II injected presynaptically was found to facilitate transmitter release. This facilitation, which could be as large as 700% of the control response, was related to the level of penetration of the enzyme along the length of the preterminal A mathematical model of this facilitation indicates a reasonable fit between the distribution of CaM kinase II within the terminal and the degree of facilitation. 8. The overall shape of the postsynaptic response was not modified by either synapsin I or CaM kinase II injection. 9. The data suggest that, in addition to releasing transmitter, calcium also penetrates the presynaptic cytosol and activates CaM kinase II. When activated, CaM kinase II phosphorylates synapsin I, which reduces its binding to vesicles and/or cytoskeletal structures, enabling more vesicles to be released during a presynaptic depolarization. The amplitude of the postsynaptic response will then be both directly and indirectly regulated by depolarization induced Ca2+ influx. This model provides a molecular mechanism for synaptic potentiation.
Full text
PDF























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustine G. J., Charlton M. P. Calcium dependence of presynaptic calcium current and post-synaptic response at the squid giant synapse. J Physiol. 1986 Dec;381:619–640. doi: 10.1113/jphysiol.1986.sp016347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P., Smith S. J. Calcium action in synaptic transmitter release. Annu Rev Neurosci. 1987;10:633–693. doi: 10.1146/annurev.ne.10.030187.003221. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P., Smith S. J. Calcium entry into voltage-clamped presynaptic terminals of squid. J Physiol. 1985 Oct;367:143–162. doi: 10.1113/jphysiol.1985.sp015818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULLOCK T. H., HAGIWARA S. Intracellular recording from the giant synapse of the squid. J Gen Physiol. 1957 Mar 20;40(4):565–577. doi: 10.1085/jgp.40.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfenati F., Bähler M., Jahn R., Greengard P. Interactions of synapsin I with small synaptic vesicles: distinct sites in synapsin I bind to vesicle phospholipids and vesicle proteins. J Cell Biol. 1989 May;108(5):1863–1872. doi: 10.1083/jcb.108.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähler M., Greengard P. Synapsin I bundles F-actin in a phosphorylation-dependent manner. Nature. 1987 Apr 16;326(6114):704–707. doi: 10.1038/326704a0. [DOI] [PubMed] [Google Scholar]
- Czernik A. J., Pang D. T., Greengard P. Amino acid sequences surrounding the cAMP-dependent and calcium/calmodulin-dependent phosphorylation sites in rat and bovine synapsin I. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7518–7522. doi: 10.1073/pnas.84.21.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P., Benfenati F., Valtorta F., Greengard P. The synapsins. Annu Rev Cell Biol. 1990;6:433–460. doi: 10.1146/annurev.cb.06.110190.002245. [DOI] [PubMed] [Google Scholar]
- De Camilli P., Cameron R., Greengard P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. I. Its general distribution in synapses of the central and peripheral nervous system demonstrated by immunofluorescence in frozen and plastic sections. J Cell Biol. 1983 May;96(5):1337–1354. doi: 10.1083/jcb.96.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grand R. J., Perry S. V., Weeks R. A. Troponin C-like proteins (calmodulins) from mammalian smooth muscle and other tissues. Biochem J. 1979 Feb 1;177(2):521–529. doi: 10.1042/bj1770521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P., Browning M. D., McGuinness T. L., Llinas R. Synapsin I, a phosphoprotein associated with synaptic vesicles: possible role in regulation of neurotransmitter release. Adv Exp Med Biol. 1987;221:135–153. doi: 10.1007/978-1-4684-7618-7_11. [DOI] [PubMed] [Google Scholar]
- HAGIWARA S., TASAKI I. A study on the mechanism of impulse transmission across the giant synapse of the squid. J Physiol. 1958 Aug 29;143(1):114–137. doi: 10.1113/jphysiol.1958.sp006048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett J. T., Cochran S. L., Greenfield L. J., Jr, Brosius D. C., Ueda T. Synapsin I injected presynaptically into goldfish mauthner axons reduces quantal synaptic transmission. J Neurophysiol. 1990 Apr;63(4):701–706. doi: 10.1152/jn.1990.63.4.701. [DOI] [PubMed] [Google Scholar]
- Huttner W. B., DeGennaro L. J., Greengard P. Differential phosphorylation of multiple sites in purified protein I by cyclic AMP-dependent and calcium-dependent protein kinases. J Biol Chem. 1981 Feb 10;256(3):1482–1488. [PubMed] [Google Scholar]
- Huttner W. B., Greengard P. Multiple phosphorylation sites in protein I and their differential regulation by cyclic AMP and calcium. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5402–5406. doi: 10.1073/pnas.76.10.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner W. B., Schiebler W., Greengard P., De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983 May;96(5):1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek L. K., Jennings K. R., Strumwasser F., Nairn A. C., Walter U., Wilson F. D., Greengard P. Microinjection of catalytic subunit of cyclic AMP-dependent protein kinase enhances calcium action potentials of bag cell neurons in cell culture. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7487–7491. doi: 10.1073/pnas.77.12.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. B., Greengard P. Two calcium/calmodulin-dependent protein kinases, which are highly concentrated in brain, phosphorylate protein I at distinct sites. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1293–1297. doi: 10.1073/pnas.78.2.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. B., McGuinness T., Greengard P. A calcium/calmodulin-dependent protein kinase from mammalian brain that phosphorylates Synapsin I: partial purification and characterization. J Neurosci. 1983 Apr;3(4):818–831. doi: 10.1523/JNEUROSCI.03-04-00818.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. W., Sugimori M., Llinás R. R., McGuinness T. L., Greengard P. Effects of synapsin I and calcium/calmodulin-dependent protein kinase II on spontaneous neurotransmitter release in the squid giant synapse. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8257–8261. doi: 10.1073/pnas.87.21.8257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., McGuinness T. L., Leonard C. S., Sugimori M., Greengard P. Intraterminal injection of synapsin I or calcium/calmodulin-dependent protein kinase II alters neurotransmitter release at the squid giant synapse. Proc Natl Acad Sci U S A. 1985 May;82(9):3035–3039. doi: 10.1073/pnas.82.9.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents and their relation to synaptic transmission: voltage clamp study in squid giant synapse and theoretical model for the calcium gate. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2918–2922. doi: 10.1073/pnas.73.8.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents in squid giant synapse. Biophys J. 1981 Mar;33(3):289–321. doi: 10.1016/S0006-3495(81)84898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse. Biophys J. 1981 Mar;33(3):323–351. doi: 10.1016/S0006-3495(81)84899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Simon S. M. Transmission by presynaptic spike-like depolarization in the squid giant synapse. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2415–2419. doi: 10.1073/pnas.79.7.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness T. L., Brady S. T., Gruner J. A., Sugimori M., Llinas R., Greengard P. Phosphorylation-dependent inhibition by synapsin I of organelle movement in squid axoplasm. J Neurosci. 1989 Dec;9(12):4138–4149. doi: 10.1523/JNEUROSCI.09-12-04138.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness T. L., Lai Y., Greengard P. Ca2+/calmodulin-dependent protein kinase II. Isozymic forms from rat forebrain and cerebellum. J Biol Chem. 1985 Feb 10;260(3):1696–1704. [PubMed] [Google Scholar]
- Miledi R., Slater C. R. The action of calcium on neuronal synapses in the squid. J Physiol. 1966 May;184(2):473–498. doi: 10.1113/jphysiol.1966.sp007927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairn A. C., Greengard P. Purification and characterization of Ca2+/calmodulin-dependent protein kinase I from bovine brain. J Biol Chem. 1987 May 25;262(15):7273–7281. [PubMed] [Google Scholar]
- Nichols R. A., Wu W. C., Haycock J. W., Greengard P. Introduction of impermeant molecules into synaptosomes using freeze/thaw permeabilization. J Neurochem. 1989 Feb;52(2):521–529. doi: 10.1111/j.1471-4159.1989.tb09151.x. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Petrucci T. C., Morrow J. S. Synapsin I: an actin-bundling protein under phosphorylation control. J Cell Biol. 1987 Sep;105(3):1355–1363. doi: 10.1083/jcb.105.3.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiebler W., Jahn R., Doucet J. P., Rothlein J., Greengard P. Characterization of synapsin I binding to small synaptic vesicles. J Biol Chem. 1986 Jun 25;261(18):8383–8390. [PubMed] [Google Scholar]
- Sieghart W., Forn J., Greengard P. Ca2+ and cyclic AMP regulate phosphorylation of same two membrane-associated proteins specific to nerve tissue. Proc Natl Acad Sci U S A. 1979 May;76(5):2475–2479. doi: 10.1073/pnas.76.5.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. M., Llinás R. R. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys J. 1985 Sep;48(3):485–498. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus J. A., Haugland R., Sharrow S. O., Segal D. M. Texas Red, a hydrophilic, red-emitting fluorophore for use with fluorescein in dual parameter flow microfluorometric and fluorescence microscopic studies. J Immunol Methods. 1982;50(2):193–204. doi: 10.1016/0022-1759(82)90225-3. [DOI] [PubMed] [Google Scholar]
- Ueda T., Greengard P. Adenosine 3':5'-monophosphate-regulated phosphoprotein system of neuronal membranes. I. Solubilization, purification, and some properties of an endogenous phosphoprotein. J Biol Chem. 1977 Jul 25;252(14):5155–5163. [PubMed] [Google Scholar]




