Abstract
1. Transmitter release at neuromuscular junctions of extensor digitorum longus (EDL) muscle in mice was studied after 2-8 month periods of unforced running in wheels. 2. Intracellular recordings at 10 Hz stimulation revealed that the quantal content of endplate potentials (EPPs) in Mg(2+)-blocked preparations was larger by 30% in trained (mean number of quanta, m = 1.75 +/- 0.19, n = 7) than in untrained control EDL muscles (m = 1.35 +/- 0.35, n = 7). Similarly the amplitudes of the first, maximum and plateau EPPs during tetanic stimulation (100 Hz for 1 s or 400 ms) in curare-blocked preparations were increased by 28% each; muscle fibre diameters did not differ while other postsynaptic effects were not excluded. 3. Training effects became particularly evident in two pairs of monozygotic twins, in which the time courses of facilitation and depression were changed as well: at 100 Hz stimulation the maximum EPP amplitude was reached on average at 2.6 impulses in controls but at 2.0 impulses in runners, and the following decline below the value of the first EPP at 5.0 and 3.8 impulses respectively. 4. Block resistance, as monitored by isometric tension measurements in different presynaptic (Mg2+) and postsynaptic (curare) blocking solutions, was higher in trained than in control EDL muscles. Depression in a train of four nerve-evoked single twitches at 2 Hz was lower. 5. As expected from the unchanged fibre diameters (see above) isometric tetanic force was similar in trained and control EDL muscles. Muscle fatigue resistance was larger in trained animals and succinic dehydrogenase activity was higher in fibres of trained muscles indicating an endurance training of the EDL muscle. 6. It is concluded that besides changes in muscle fibre properties, prolonged elevated activity causes increased transmitter release in EDL muscles. As a consequence, the safety margin of transmission in trained EDL muscles is markedly elevated.
Full text
PDF

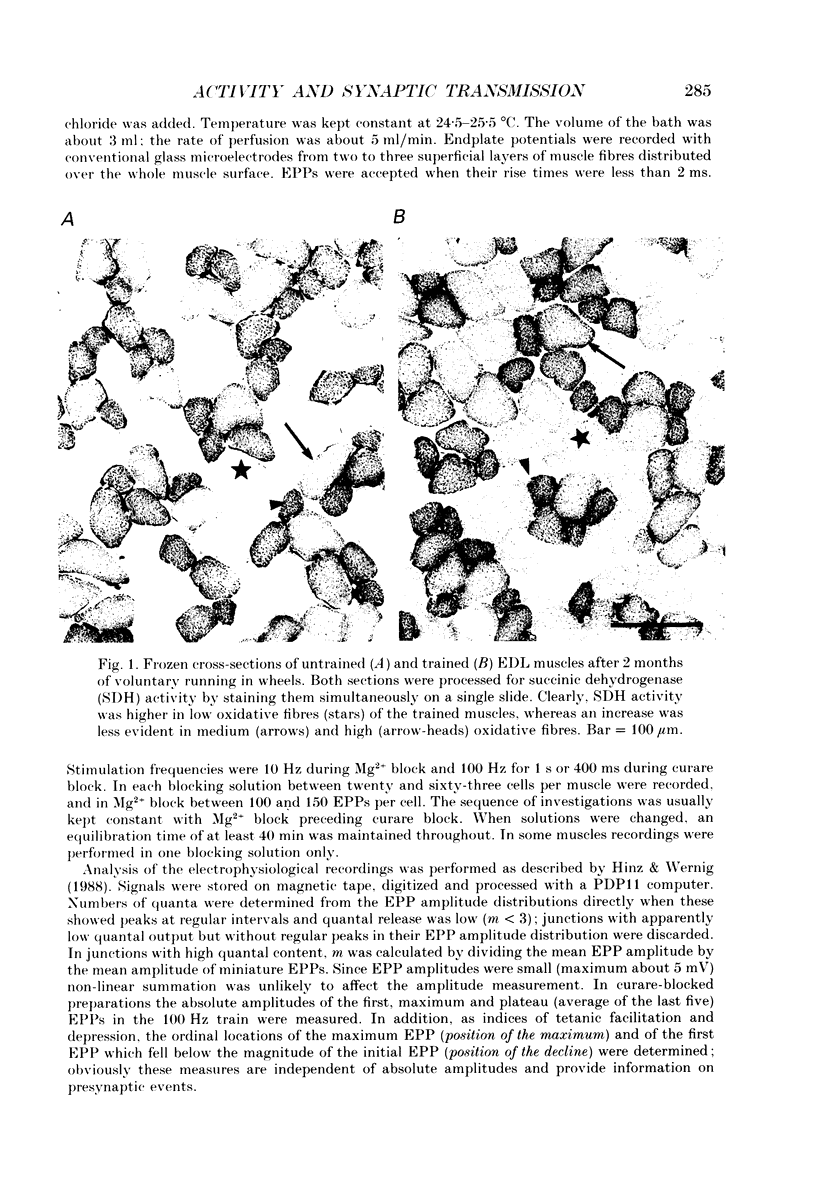

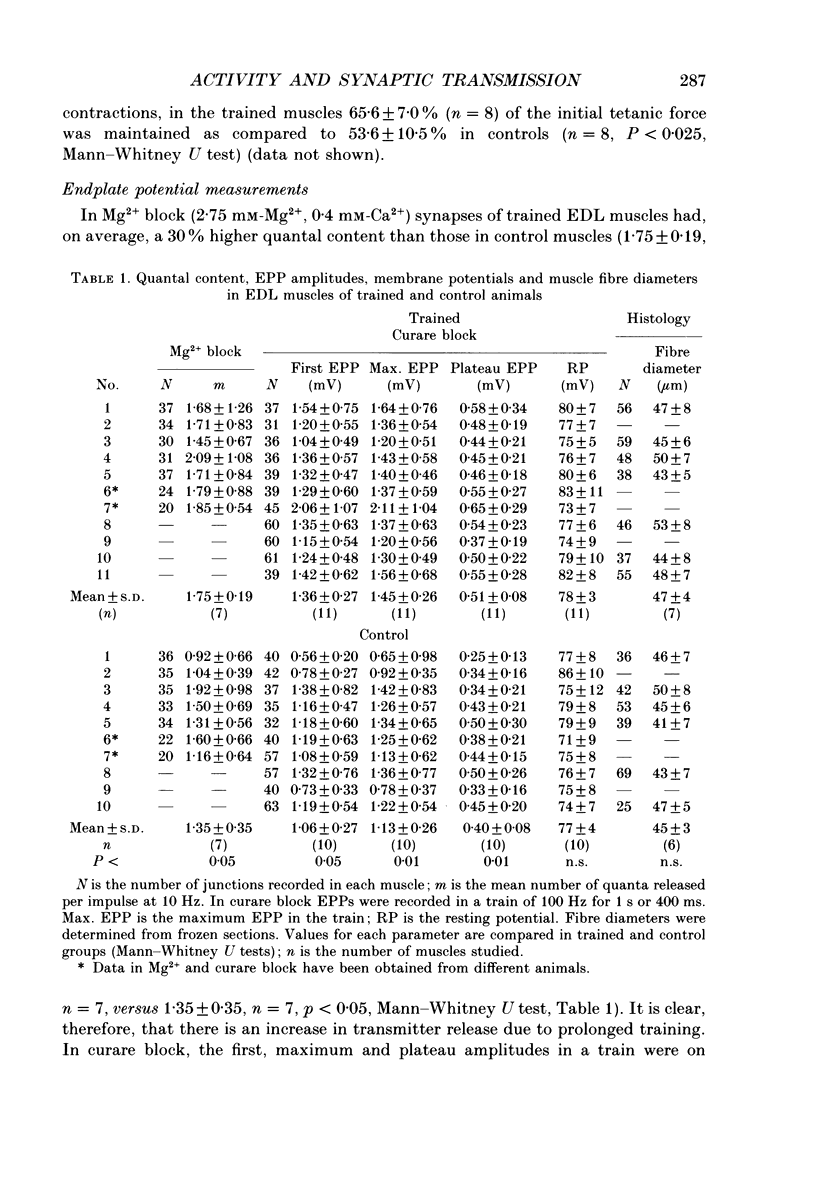
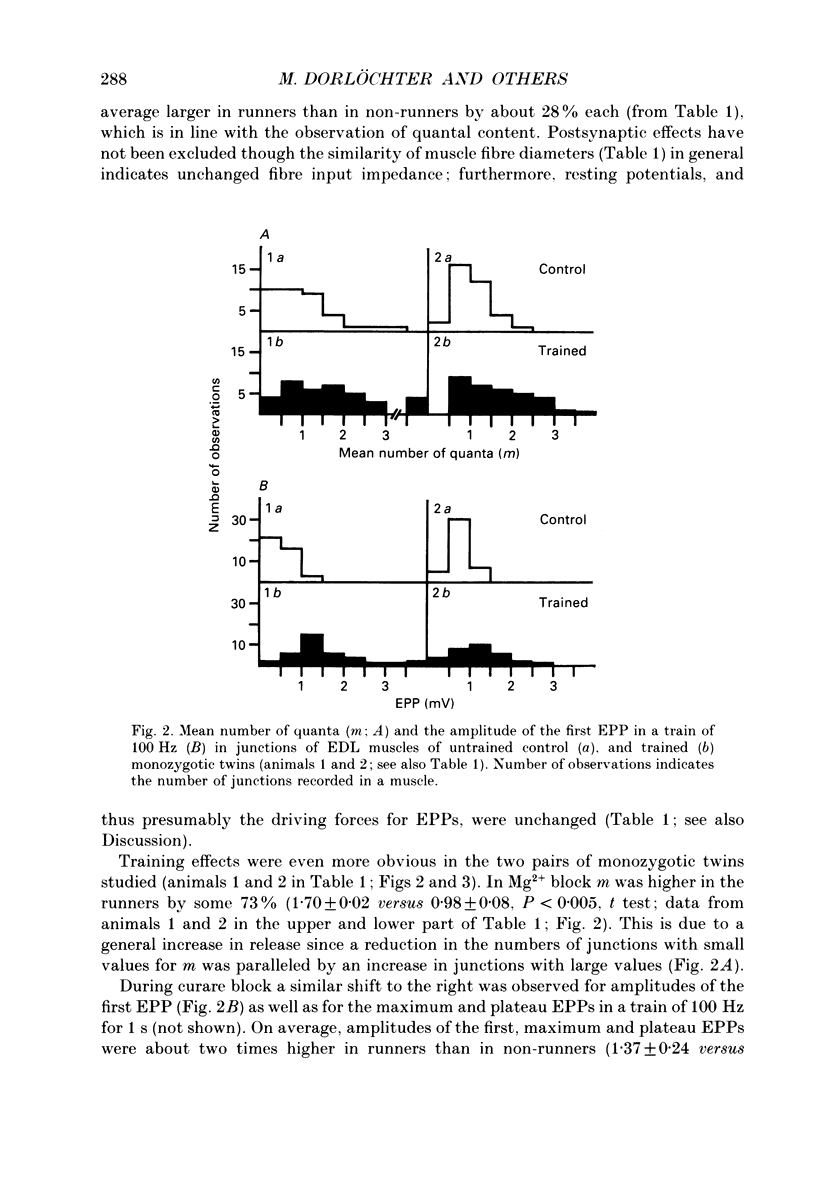
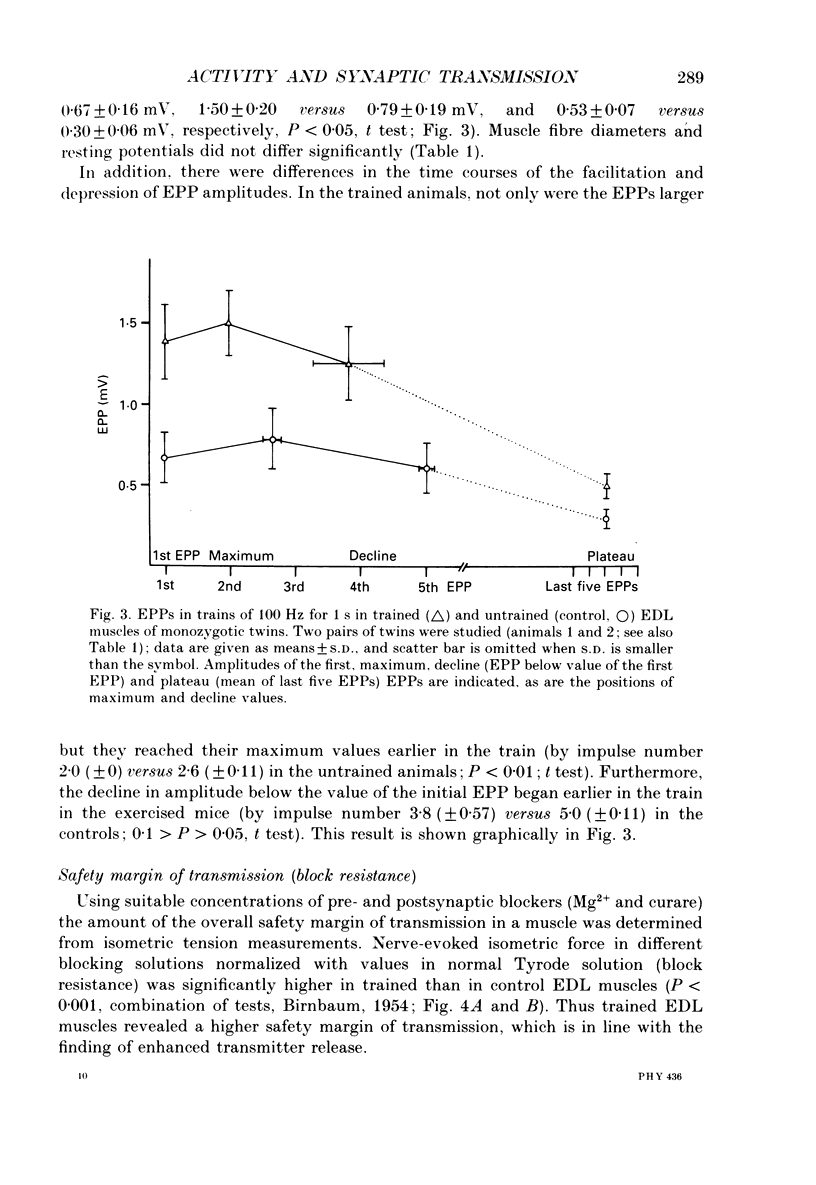


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andonian M. H., Fahim M. A. Endurance exercise alters the morphology of fast- and slow-twitch rat neuromuscular junctions. Int J Sports Med. 1988 Jun;9(3):218–223. doi: 10.1055/s-2007-1025009. [DOI] [PubMed] [Google Scholar]
- Badke A., Irintchev A. P., Wernig A. Maturation of transmission in reinnervated mouse soleus muscle. Muscle Nerve. 1989 Jul;12(7):580–586. doi: 10.1002/mus.880120709. [DOI] [PubMed] [Google Scholar]
- Cardasis C. A., LaFontaine D. M. Aging rat neuromuscular junctions: a morphometric study of cholinesterase-stained whole mounts and ultrastructure. Muscle Nerve. 1987 Mar-Apr;10(3):200–213. doi: 10.1002/mus.880100303. [DOI] [PubMed] [Google Scholar]
- Grinnell A. D., Herrera A. A. Physiological regulation of synaptic effectiveness at frog neuromuscular junctions. J Physiol. 1980 Oct;307:301–317. doi: 10.1113/jphysiol.1980.sp013436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera A. A., Banner L. R., Nagaya N. Repeated, in vivo observation of frog neuromuscular junctions: remodelling involves concurrent growth and retraction. J Neurocytol. 1990 Feb;19(1):85–99. doi: 10.1007/BF01188441. [DOI] [PubMed] [Google Scholar]
- Hinz I., Wernig A. Prolonged nerve stimulation causes changes in transmitter release at the frog neuromuscular junction. J Physiol. 1988 Jul;401:557–565. doi: 10.1113/jphysiol.1988.sp017179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irintchev A., Wernig A. Muscle damage and repair in voluntarily running mice: strain and muscle differences. Cell Tissue Res. 1987 Sep;249(3):509–521. doi: 10.1007/BF00217322. [DOI] [PubMed] [Google Scholar]
- Kelly S. S., Robbins N. Progression of age changes in synaptic transmission at mouse neuromuscular junctions. J Physiol. 1983 Oct;343:375–383. doi: 10.1113/jphysiol.1983.sp014898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W., Magrassi L., Purves D. Visualization of neuromuscular junctions over periods of several months in living mice. J Neurosci. 1987 Apr;7(4):1215–1222. doi: 10.1523/JNEUROSCI.07-04-01215.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lømo T., Waerhaug O. Motor endplates in fast and slow muscles of the rat: what determines their difference? J Physiol (Paris) 1985;80(4):290–297. [PubMed] [Google Scholar]
- Mallart A., Martin A. R. The relation between quantum content and facilitation at the neuromuscular junction of the frog. J Physiol. 1968 Jun;196(3):593–604. doi: 10.1113/jphysiol.1968.sp008525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NACHLAS M. M., TSOU K. C., DE SOUZA E., CHENG C. S., SELIGMAN A. M. Cytochemical demonstration of succinic dehydrogenase by the use of a new p-nitrophenyl substituted ditetrazole. J Histochem Cytochem. 1957 Jul;5(4):420–436. doi: 10.1177/5.4.420. [DOI] [PubMed] [Google Scholar]
- Rahamimoff R. A dual effect of calcium ions on neuromuscular facilitation. J Physiol. 1968 Mar;195(2):471–480. doi: 10.1113/jphysiol.1968.sp008468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins N., Fahim M. A. Progression of age changes in mature mouse motor nerve terminals and its relation to locomotor activity. J Neurocytol. 1985 Dec;14(6):1019–1036. doi: 10.1007/BF01224810. [DOI] [PubMed] [Google Scholar]
- SONG S. K., SHIMADA N., ANDERSON P. J. ORTHOGONAL DIAMETERS IN THE ANALYSIS OF MUSCLE FIBRE SIZE AND FORM. Nature. 1963 Dec 21;200:1220–1221. doi: 10.1038/2001220a0. [DOI] [PubMed] [Google Scholar]
- Schmitt H. P. Measurement of voluntary muscle fiber cross sections: a comparative study of different possible methods. Microsc Acta. 1976 Jan;77(5):427–440. [PubMed] [Google Scholar]
- Wernig A., Herrera A. A. Sprouting and remodelling at the nerve-muscle junction. Prog Neurobiol. 1986;27(3):251–291. doi: 10.1016/0301-0082(86)90023-7. [DOI] [PubMed] [Google Scholar]
- Wernig A., Irintchev A., Weisshaupt P. Muscle injury, cross-sectional area and fibre type distribution in mouse soleus after intermittent wheel-running. J Physiol. 1990 Sep;428:639–652. doi: 10.1113/jphysiol.1990.sp018232. [DOI] [PMC free article] [PubMed] [Google Scholar]



