Abstract
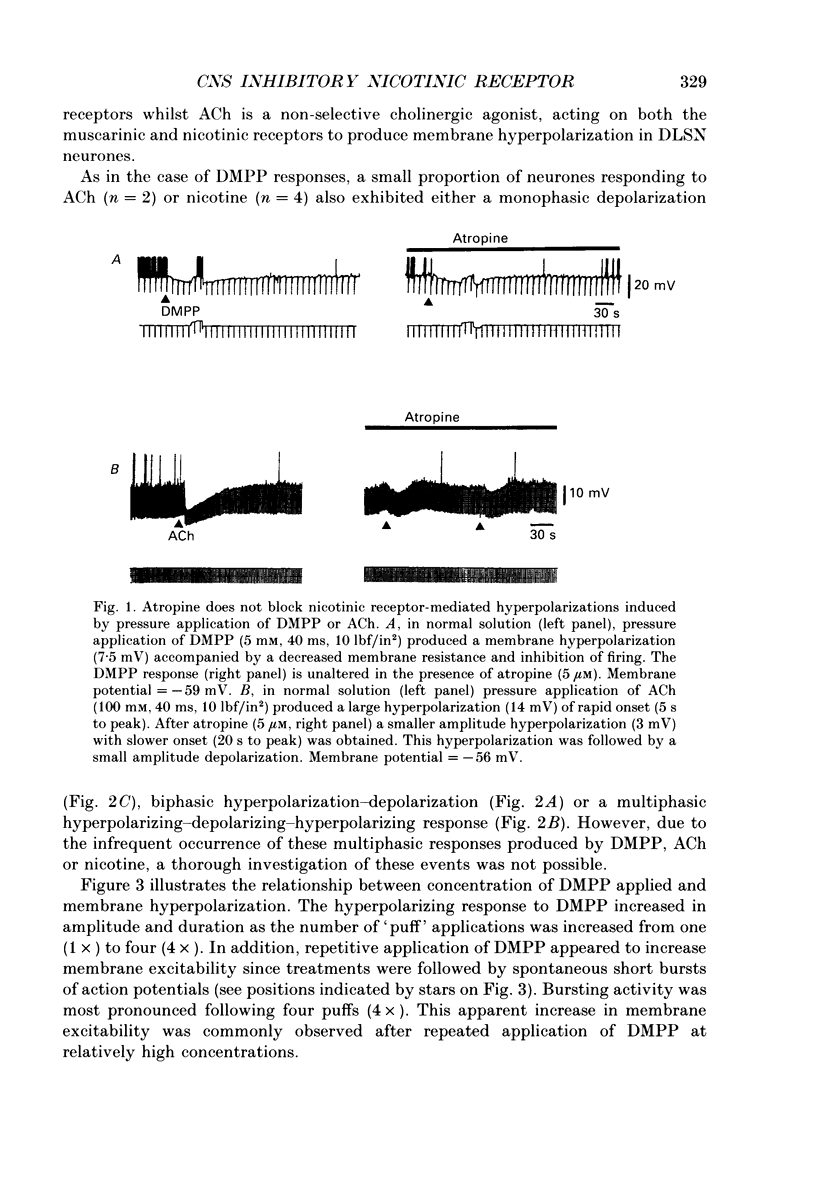
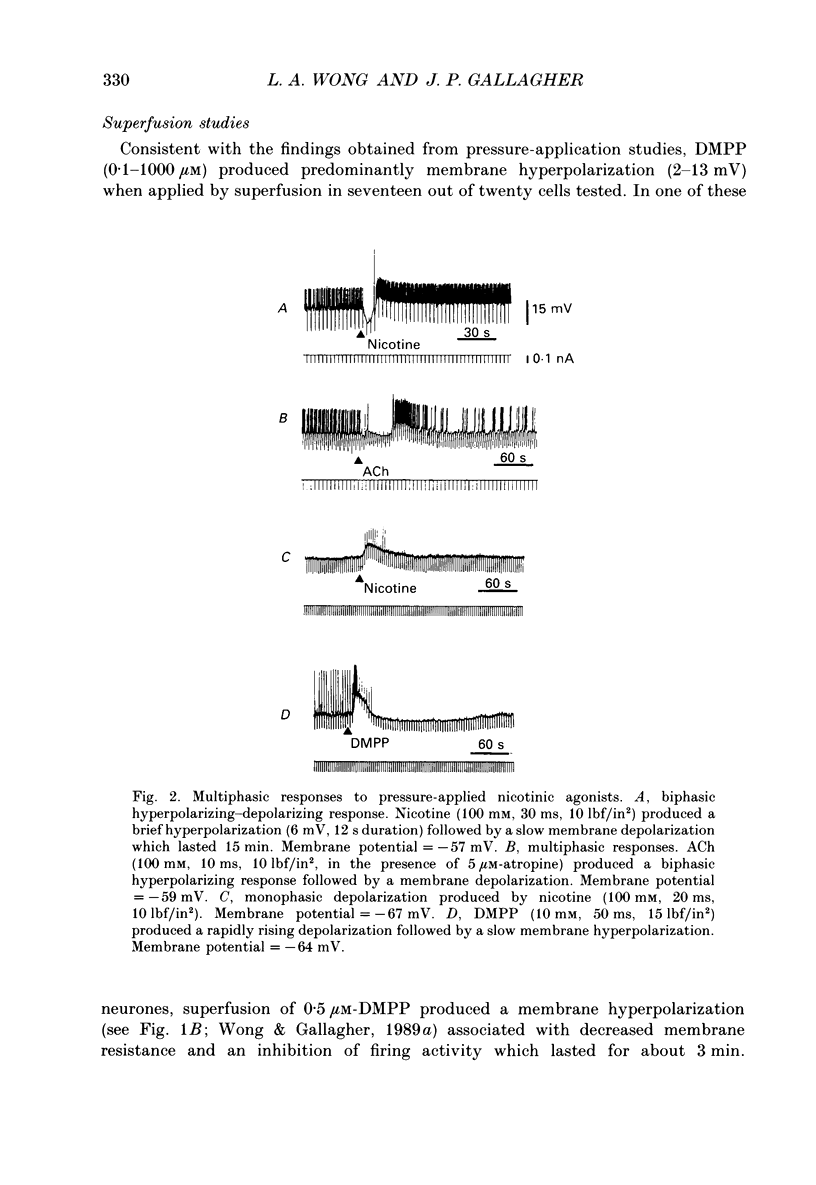
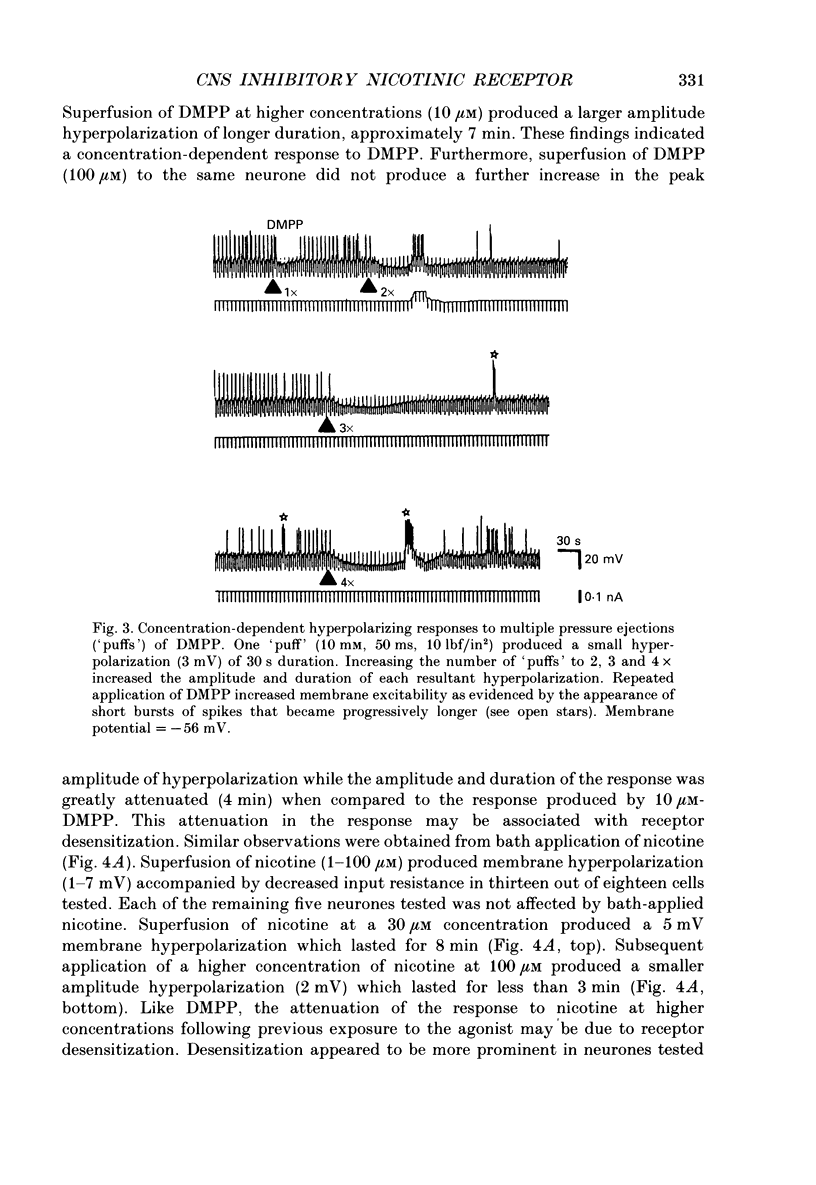
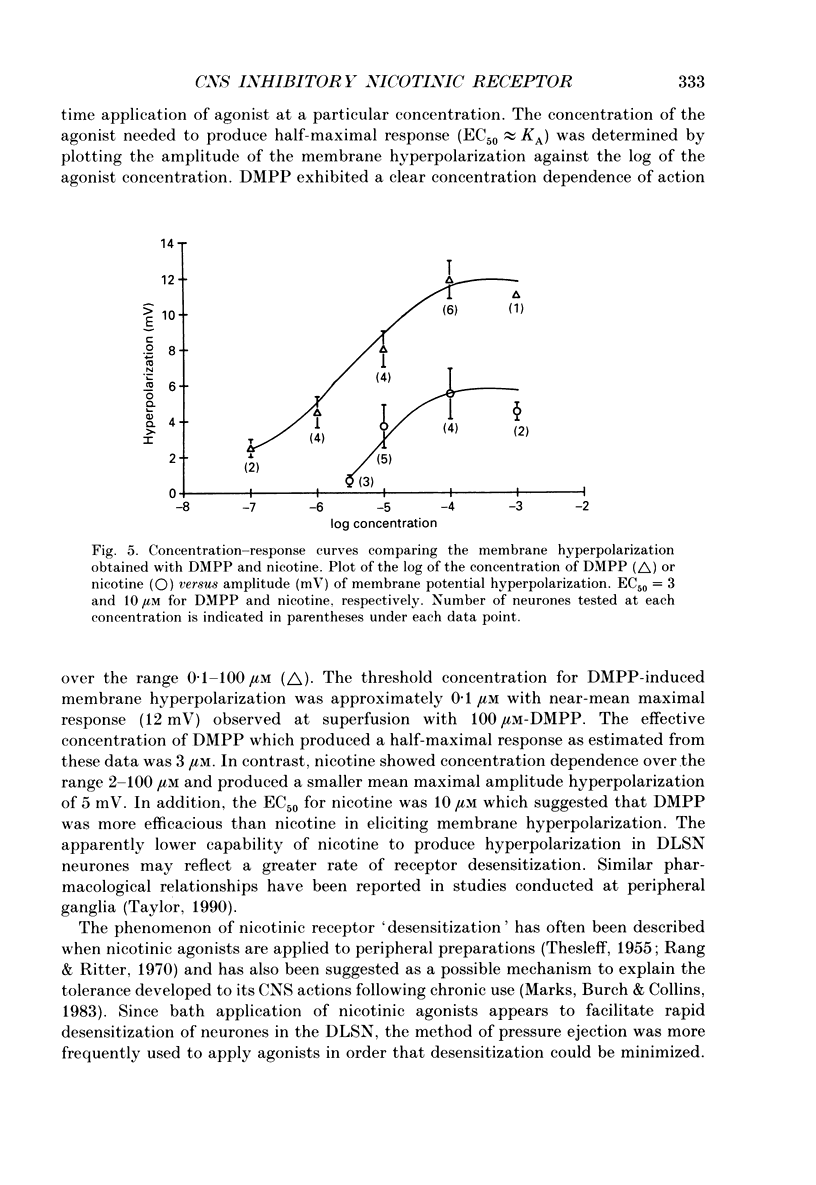
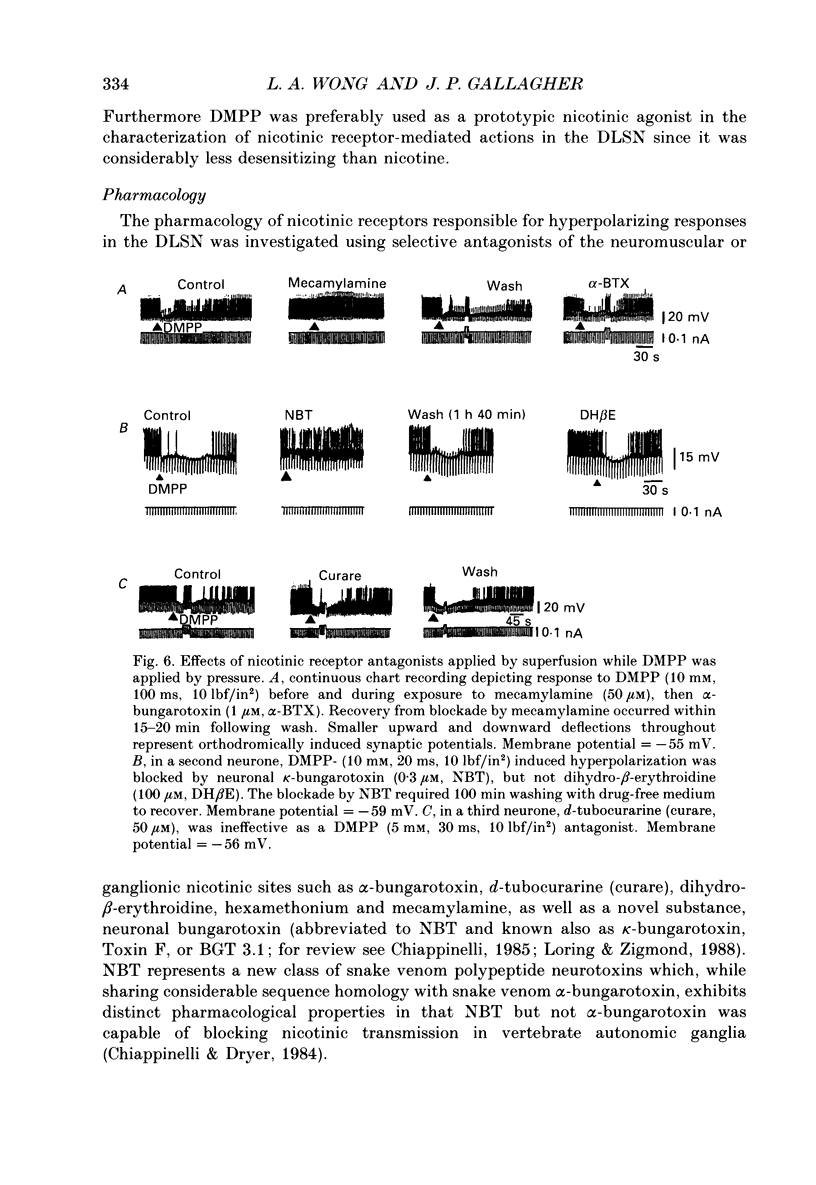
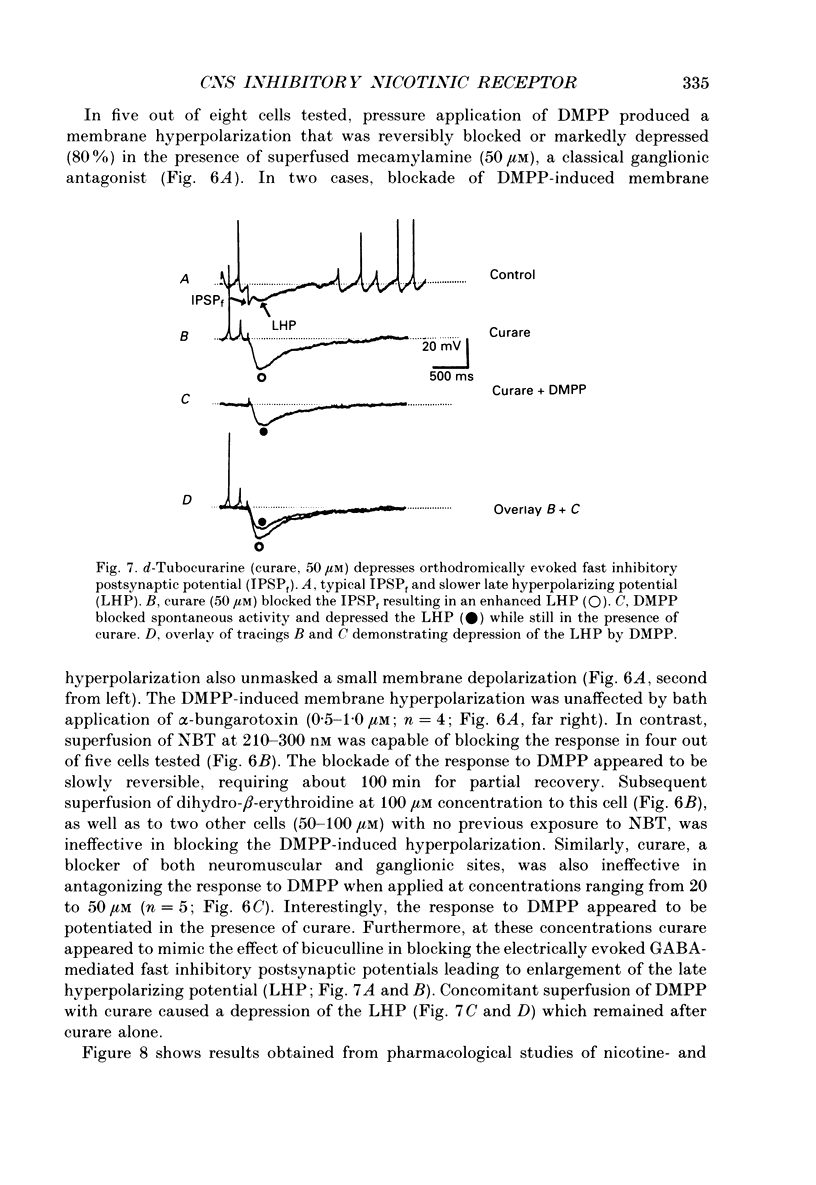
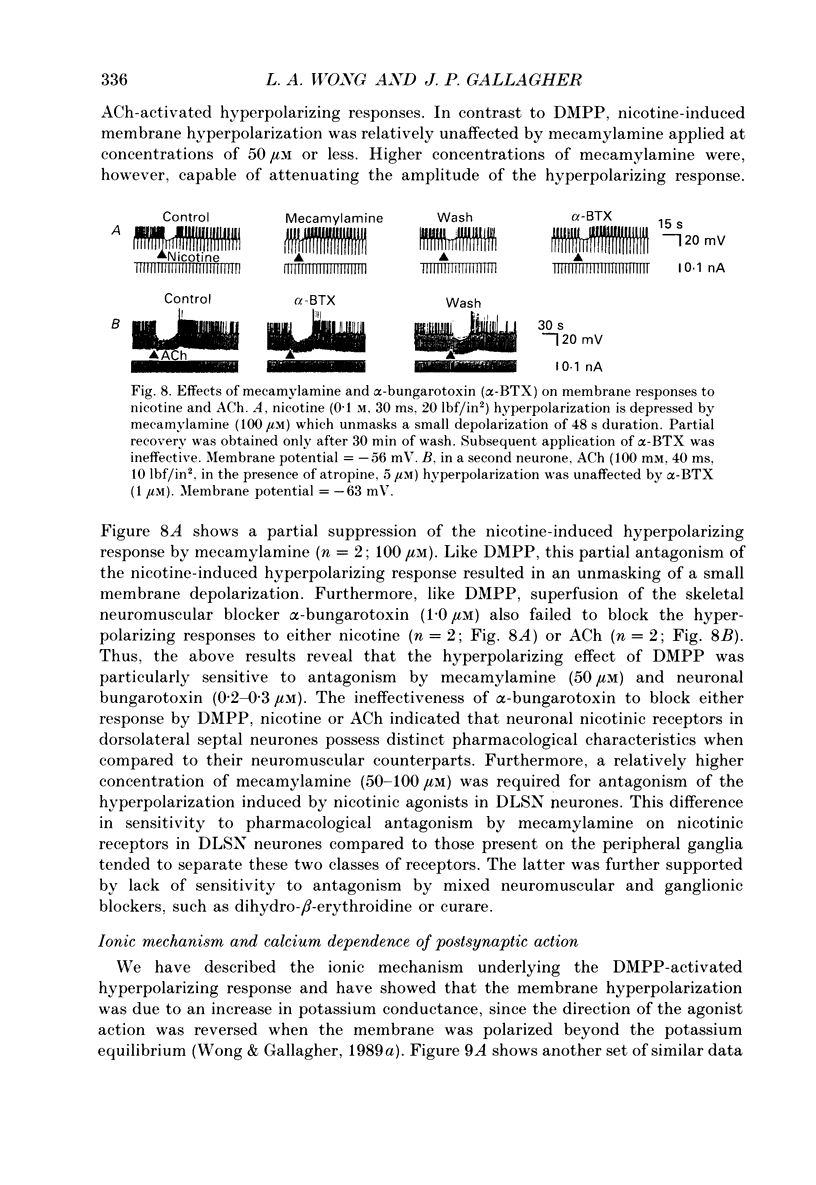
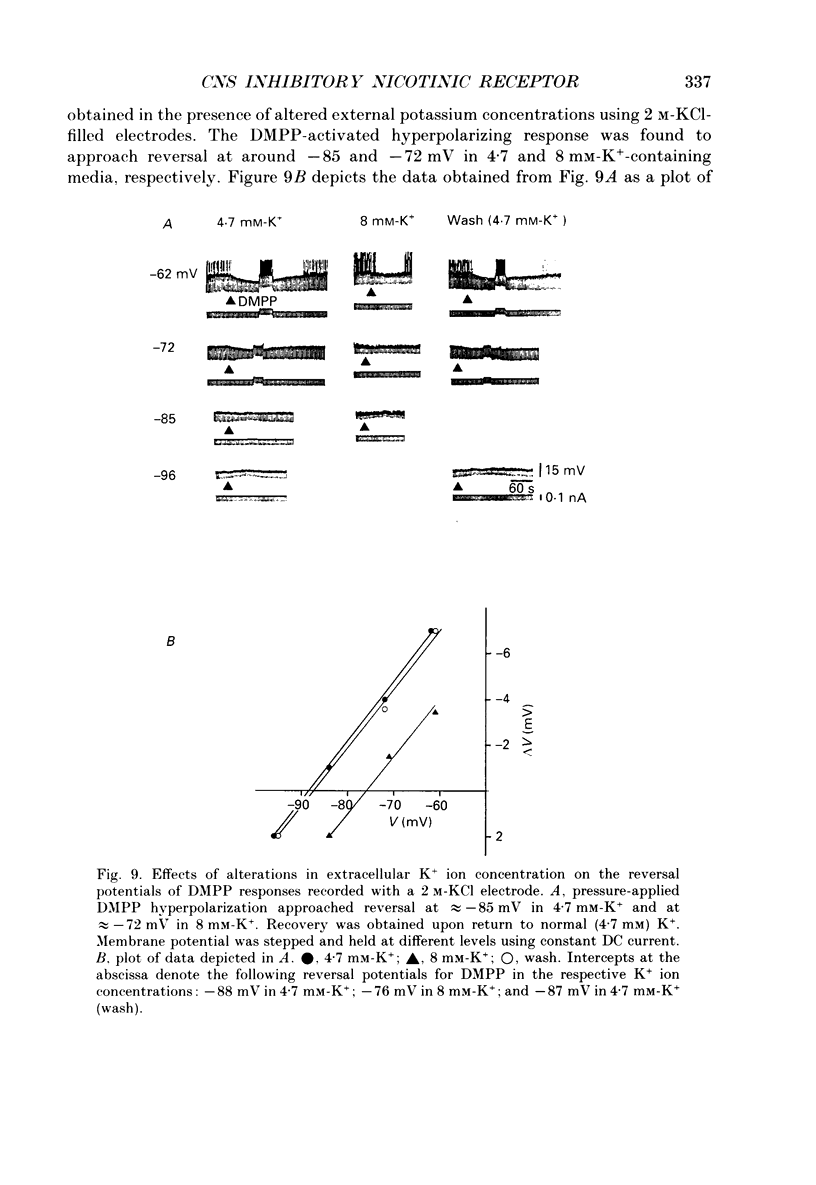
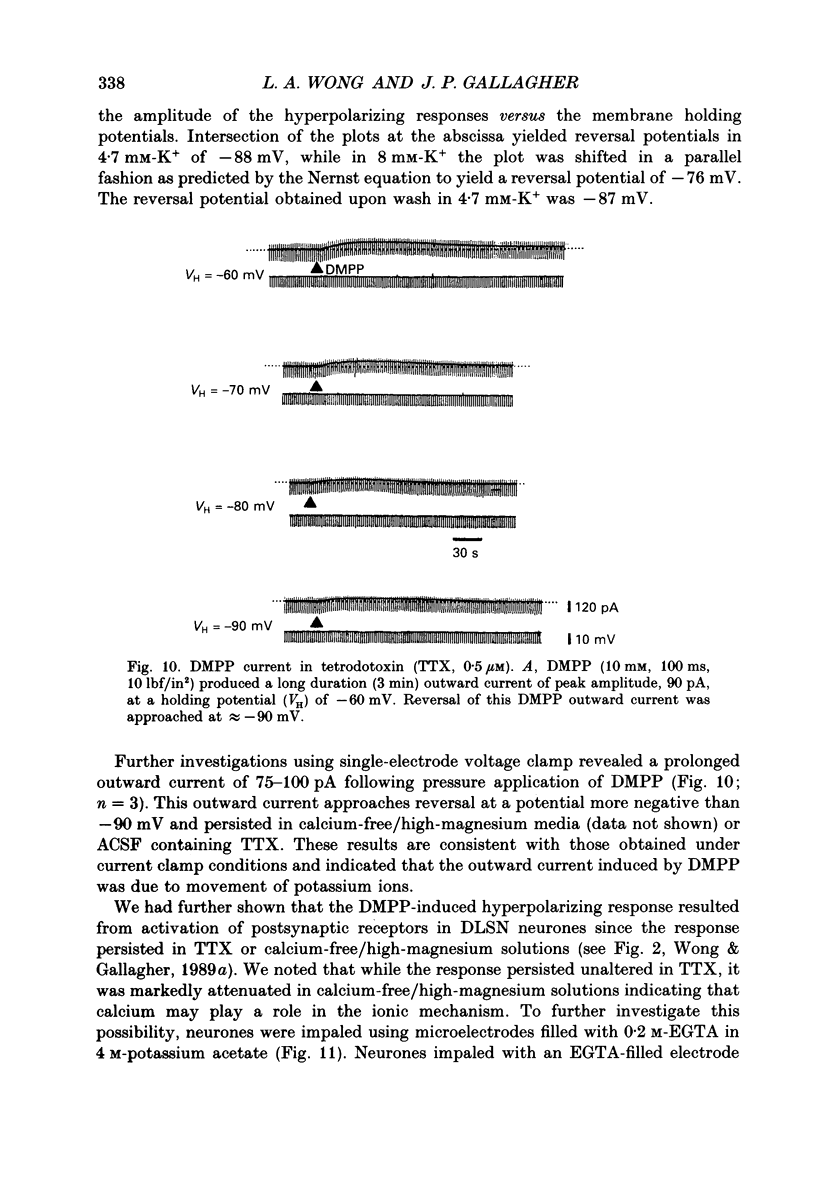
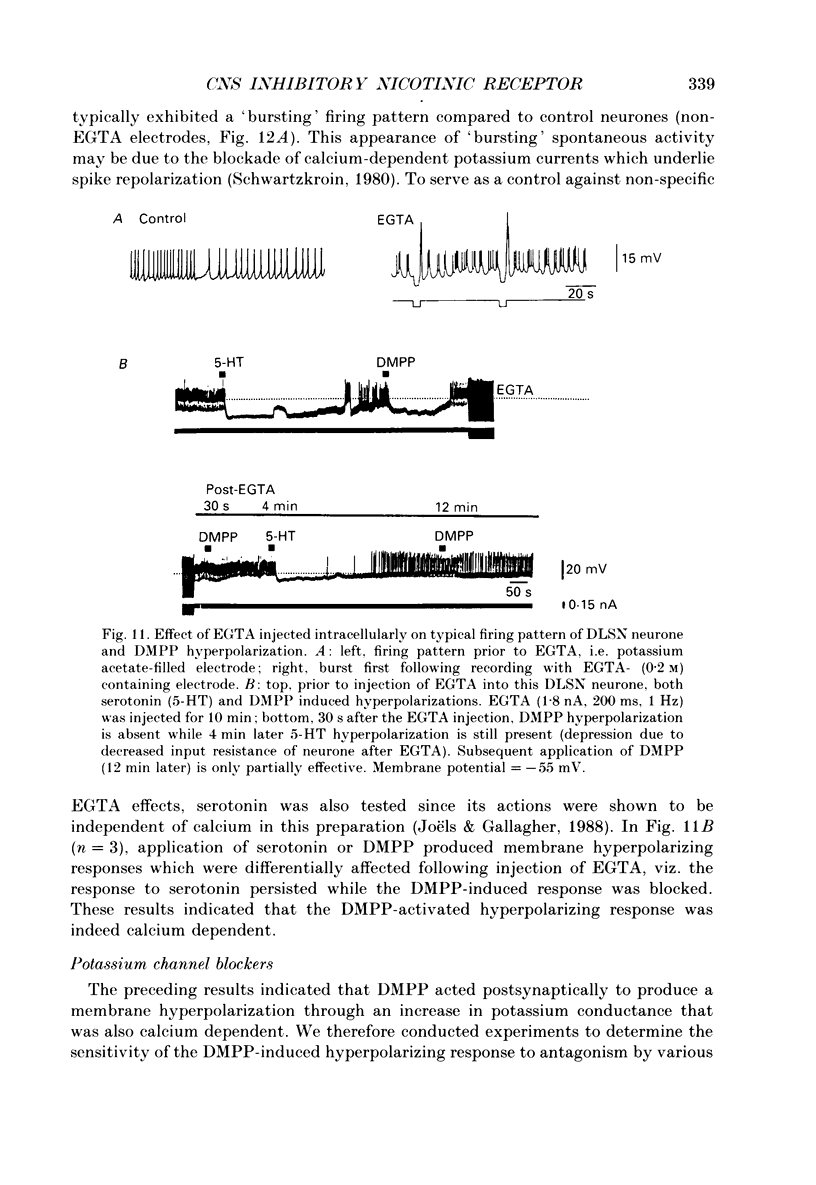
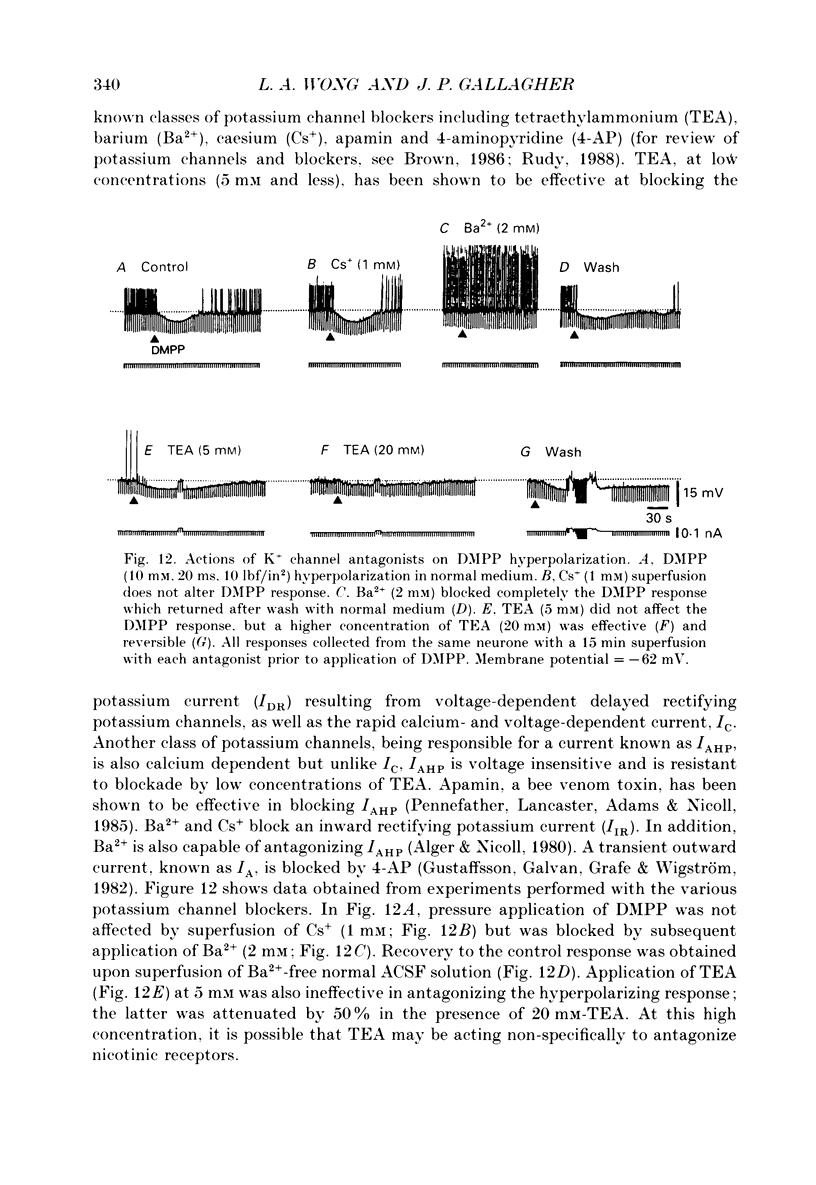
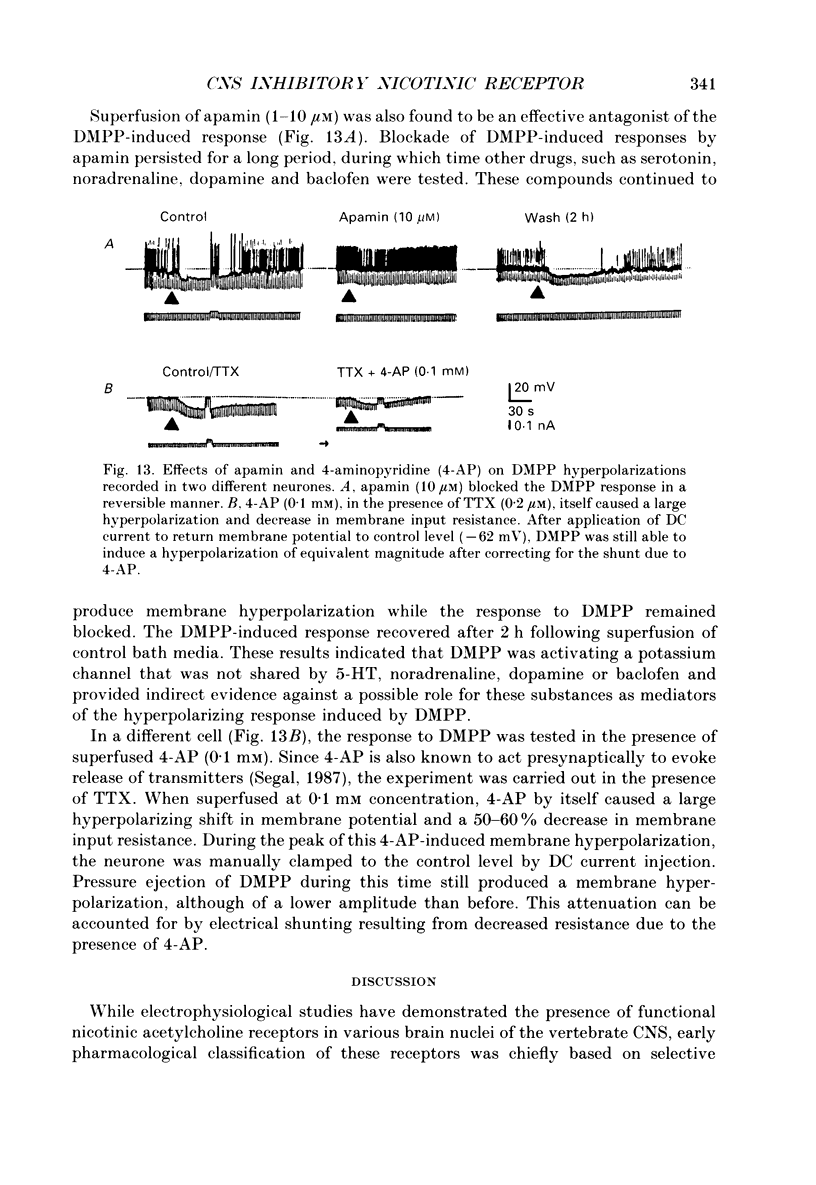
1. Intracellular electrophysiological techniques were employed to investigate the effects of nicotinic receptor stimulation on rat dorsolateral septal nucleus (DLSN) neurones in a submerged rat brain slice preparation. 2. Acetylcholine (in the presence of the muscarinic antagonist, atropine), nicotine or dimethylphenylpiperazinium (DMPP), applied either by pressure ejection or superfusion, produced predominantly a membrane potential hyperpolarization. 3. Following concentration-response comparisons, DMPP appeared to exhibit fewer desensitizing properties and greater efficacy than nicotine with half-maximal hyperpolarizing responses attainable at 3 and 10 microM, respectively. 4. Pharmacological analyses revealed that the agonist-induced membrane hyperpolarization was sensitive to antagonism by mecamylamine (50-100 microM) and neuronal bungarotoxin (0.2-0.3 microM), but not alpha-bungarotoxin (0.5-1.0 microM), curare (10-50 microM) or dihydro-beta-erythroidine (50-100 microM). 5. Hyperpolarizing responses to DMPP were found to reverse near the equilibrium potential for potassium and were sensitive to changes in extracellular potassium concentration as predicted by the Nernst equation. Under single-electrode voltage clamp, application of DMPP produced an outward current (75-100 pA) which approached reversal at around -88 mV. These findings indicated that the hyperpolarizing response to nicotinic receptor stimulation was mediated by changes in membrane permeability to potassium. 6. DMPP-induced membrane hyperpolarization resulted from a direct action on postsynaptic DLSN neurones since the response persisted under conditions of superfusion with calcium-free/high-magnesium media or tetrodotoxin; both conditions blocked orthodromically induced neurotransmission. The hyperpolarizing response remained unaltered in TTX but was diminished in calcium-free/high-magnesium media. Further studies revealed blockade of the DMPP response following intracellular injection of EGTA. This response was also sensitive to antagonism by various calcium-dependent potassium channel blockers including apamin, barium and tetraethylammonium. 7. Our studies reveal a novel class of CNS nicotinic receptor whose action upon stimulation by an agonist results in a membrane hyperpolarization via a calcium-dependent increase in potassium ion conductance.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alger B. E., Nicoll R. A. Epileptiform burst afterhyperolarization: calcium-dependent potassium potential in hippocampal CA1 pyramidal cells. Science. 1980 Dec 5;210(4474):1122–1124. doi: 10.1126/science.7444438. [DOI] [PubMed] [Google Scholar]
- Bradley P. B., Dhawan B. N., Wolstencroft J. H. Pharmacological properties of cholinoceptive neurones in the medulla and pons of the cat. J Physiol. 1966 Apr;183(3):658–674. doi: 10.1113/jphysiol.1966.sp007891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Docherty R. J., Halliwell J. V. Chemical transmission in the rat interpeduncular nucleus in vitro. J Physiol. 1983 Aug;341:655–670. doi: 10.1113/jphysiol.1983.sp014831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappinelli V. A. Actions of snake venom toxins on neuronal nicotinic receptors and other neuronal receptors. Pharmacol Ther. 1985;31(1-2):1–32. doi: 10.1016/0163-7258(85)90035-x. [DOI] [PubMed] [Google Scholar]
- Chiappinelli V. A., Dryer S. E. Nicotinic transmission in sympathetic ganglia: blockade by the snake venom neurotoxin kappa-bungarotoxin. Neurosci Lett. 1984 Sep 7;50(1-3):239–244. doi: 10.1016/0304-3940(84)90492-0. [DOI] [PubMed] [Google Scholar]
- Coyle J. T., Price D. L., DeLong M. R. Alzheimer's disease: a disorder of cortical cholinergic innervation. Science. 1983 Mar 11;219(4589):1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Ryall R. W. The excitation of Renshaw cells by cholinomimetics. Exp Brain Res. 1966;2(1):49–65. doi: 10.1007/BF00234360. [DOI] [PubMed] [Google Scholar]
- Dreifuss J. J., Kelly J. S. Excitation of identified supraoptic neurones by the iontophoretic application of acetylcholine. J Physiol. 1970 Sep;210(2):170P–172P. [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., KOKETSU K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol. 1954 Dec 10;126(3):524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan T. M., North R. A. Acetylcholine hyperpolarizes central neurones by acting on an M2 muscarinic receptor. 1986 Jan 30-Feb 5Nature. 319(6052):405–407. doi: 10.1038/319405a0. [DOI] [PubMed] [Google Scholar]
- Egan T. M., North R. A. Actions of acetylcholine and nicotine on rat locus coeruleus neurons in vitro. Neuroscience. 1986 Oct;19(2):565–571. doi: 10.1016/0306-4522(86)90281-2. [DOI] [PubMed] [Google Scholar]
- Evans P. D., Reale V., Villegas J. The role of cyclic nucleotides in modulation of the membrane potential of the Schwann cell of squid giant nerve fibre. J Physiol. 1985 Jun;363:151–167. doi: 10.1113/jphysiol.1985.sp015701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B., Galvan M., Grafe P., Wigström H. A transient outward current in a mammalian central neurone blocked by 4-aminopyridine. Nature. 1982 Sep 16;299(5880):252–254. doi: 10.1038/299252a0. [DOI] [PubMed] [Google Scholar]
- Hasuo H., Gallagher J. P., Shinnick-Gallagher P. Disinhibition in the rat septum mediated by M1 muscarinic receptors. Brain Res. 1988 Jan 12;438(1-2):323–327. doi: 10.1016/0006-8993(88)91356-x. [DOI] [PubMed] [Google Scholar]
- Higashi H., Ueda N., Nishi S., Gallagher J. P., Shinnick-Gallagher P. Chemoreceptors for serotonin (5-HT), acetylcholine (ACh), bradykinin (BK), histamine (H) and gamma-aminobutyric acid (GABA) on rabbit visceral afferent neurons. Brain Res Bull. 1982 Jan;8(1):23–32. doi: 10.1016/0361-9230(82)90023-5. [DOI] [PubMed] [Google Scholar]
- Hill R. G., Simmonds M. A., Straughan D. W. A comparative study of some convulsant substances as gamma-aminobutyric acid antagonists in the feline cerebral cortex. Br J Pharmacol. 1973 Sep;49(1):37–51. doi: 10.1111/j.1476-5381.1973.tb08266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. G., Simmonds M. A., Straughan D. W. Convulsive properties of d-tubocurarine and cortical inhibition. Nature. 1972 Nov 3;240(5375):51–52. doi: 10.1038/240051a0. [DOI] [PubMed] [Google Scholar]
- Joëls M., Gallagher J. P. Actions of serotonin recorded intracellularly in rat dorsal lateral septal neurons. Synapse. 1988;2(1):45–53. doi: 10.1002/syn.890020108. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Pumain R., Renaud L. The mechanism of excitation by acetylcholine in the cerebral cortex. J Physiol. 1971 May;215(1):247–268. doi: 10.1113/jphysiol.1971.sp009467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legge K. F., Randic M., Straughan D. W. The pharmacology of neurones in the pyriform cortex. Br J Pharmacol Chemother. 1966 Jan;26(1):87–107. doi: 10.1111/j.1476-5381.1966.tb01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton S. A., Aizenman E., Loring R. H. Neural nicotinic acetylcholine responses in solitary mammalian retinal ganglion cells. Pflugers Arch. 1987 Sep;410(1-2):37–43. doi: 10.1007/BF00581893. [DOI] [PubMed] [Google Scholar]
- Loring R. H., Chiappinelli V. A., Zigmond R. E., Cohen J. B. Characterization of a snake venom neurotoxin which blocks nicotinic transmission in the avian ciliary ganglion. Neuroscience. 1984 Apr;11(4):989–999. doi: 10.1016/0306-4522(84)90209-4. [DOI] [PubMed] [Google Scholar]
- Loring R. H., Zigmond R. E. Characterization of neuronal nicotinic receptors by snake venom neurotoxins. Trends Neurosci. 1988 Feb;11(2):73–78. doi: 10.1016/0166-2236(88)90168-3. [DOI] [PubMed] [Google Scholar]
- Marks M. J., Burch J. B., Collins A. C. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J Pharmacol Exp Ther. 1983 Sep;226(3):817–825. [PubMed] [Google Scholar]
- McCance I., Phillis J. W. Cholinergic mechanisms in the cerebellar cortex. Int J Neuropharmacol. 1968 Sep;7(5):447–462. doi: 10.1016/0028-3908(68)90044-0. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Prince D. A. Acetylcholine causes rapid nicotinic excitation in the medial habenular nucleus of guinea pig, in vitro. J Neurosci. 1987 Mar;7(3):742–752. doi: 10.1523/JNEUROSCI.07-03-00742.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Prince D. A. Mechanisms of action of acetylcholine in the guinea-pig cerebral cortex in vitro. J Physiol. 1986 Jun;375:169–194. doi: 10.1113/jphysiol.1986.sp016112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Prince D. A. Two types of muscarinic response to acetylcholine in mammalian cortical neurons. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6344–6348. doi: 10.1073/pnas.82.18.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennefather P., Lancaster B., Adams P. R., Nicoll R. A. Two distinct Ca-dependent K currents in bullfrog sympathetic ganglion cells. Proc Natl Acad Sci U S A. 1985 May;82(9):3040–3044. doi: 10.1073/pnas.82.9.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillis J. W. Acetylcholine release from the cerebral cortex: its role in cortical arousal. Brain Res. 1968 Mar;7(3):378–389. doi: 10.1016/0006-8993(68)90004-8. [DOI] [PubMed] [Google Scholar]
- Phillis J. W., Tebecis A. K., York D. H. A study of cholinoceptive cells in the lateral geniculate nucleus. J Physiol. 1967 Oct;192(3):695–713. doi: 10.1113/jphysiol.1967.sp008326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D. L., Kitt C. A., Struble R. G., Whitehouse P. J., Cork L. C., Walker L. C. Neurobiological studies of transmitter systems in aging and in Alzheimer-type dementia. Ann N Y Acad Sci. 1985;457:35–51. doi: 10.1111/j.1749-6632.1985.tb20798.x. [DOI] [PubMed] [Google Scholar]
- Rang H. P., Ritter J. M. A new kind of drug antagonism: evidence that agonists cause a molecular change in acetylcholine receptors. Mol Pharmacol. 1969 Jul;5(4):394–411. [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Stafstrom C. E. Effects of EGTA on the calcium-activated afterhyperpolarization in hippocampal CA3 pyramidal cells. Science. 1980 Dec 5;210(4474):1125–1126. doi: 10.1126/science.6777871. [DOI] [PubMed] [Google Scholar]
- Segal M. Repetitive inhibitory postsynaptic potentials evoked by 4-aminopyridine in hippocampal neurons in vitro. Brain Res. 1987 Jun 30;414(2):285–293. doi: 10.1016/0006-8993(87)90008-4. [DOI] [PubMed] [Google Scholar]
- Siebler M., Köller H., Schmalenbach C., Müller H. W. GABA activated chloride currents in cultured rat hippocampal and septal region neurons can be inhibited by curare and atropine. Neurosci Lett. 1988 Nov 11;93(2-3):220–224. doi: 10.1016/0304-3940(88)90085-7. [DOI] [PubMed] [Google Scholar]
- Stevens D. R., Gallagher J. P., Shinnick-Gallagher P. Intracellular recordings from rat dorsolateral septal neurons, in vitro. Brain Res. 1984 Jul 9;305(2):353–356. doi: 10.1016/0006-8993(84)90441-4. [DOI] [PubMed] [Google Scholar]
- Stone T. W. Cholinergic mechanisms in the rat somatosensory cerebral cortex. J Physiol. 1972 Sep;225(2):485–499. doi: 10.1113/jphysiol.1972.sp009951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm J. F. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol. 1987 Apr;385:733–759. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THESLEFT S. The mode of neuromuscular block caused by acetylcholine, nicotine, decamethonium and succinylcholine. Acta Physiol Scand. 1955 Oct 27;34(2-3):218–231. doi: 10.1111/j.1748-1716.1955.tb01242.x. [DOI] [PubMed] [Google Scholar]
- Takagi M. Actions of cholinergic drugs on cells in the interpeduncular nucleus. Exp Neurol. 1984 May;84(2):358–363. doi: 10.1016/0014-4886(84)90232-2. [DOI] [PubMed] [Google Scholar]
- Thalmann R. H. Pertussis toxin blocks a late inhibitory postsynaptic potential in hippocampal CA3 neurons. Neurosci Lett. 1987 Nov 10;82(1):41–46. doi: 10.1016/0304-3940(87)90168-6. [DOI] [PubMed] [Google Scholar]
- Vidal C., Changeux J. P. Pharmacological profile of nicotinic acetylcholine receptors in the rat prefrontal cortex: an electrophysiological study in a slice preparation. Neuroscience. 1989;29(2):261–270. doi: 10.1016/0306-4522(89)90056-0. [DOI] [PubMed] [Google Scholar]
- Villegas J. Characterization of acetylcholine receptors in the Schwann cell membrane of the squid nerve fibre. J Physiol. 1975 Aug;249(3):679–689. doi: 10.1113/jphysiol.1975.sp011037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas J. Effects of acetylcholine and carbamylcholine on the axon and Schwann cell electrical potentials in the squid nerve fibre. J Physiol. 1974 Nov;242(3):647–659. doi: 10.1113/jphysiol.1974.sp010728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada E., Wada K., Boulter J., Deneris E., Heinemann S., Patrick J., Swanson L. W. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989 Jun 8;284(2):314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Wong L. A., Gallagher J. P. A direct nicotinic receptor-mediated inhibition recorded intracellularly in vitro. Nature. 1989 Oct 5;341(6241):439–442. doi: 10.1038/341439a0. [DOI] [PubMed] [Google Scholar]
- de la Garza R., Freedman R., Hoffer B. J. Kappa-bungarotoxin blockade of nicotine electrophysiological actions in cerebellar Purkinje neurons. Neurosci Lett. 1989 Apr 24;99(1-2):95–100. doi: 10.1016/0304-3940(89)90271-1. [DOI] [PubMed] [Google Scholar]
- de la Garza R., McGuire T. J., Freedman R., Hoffer B. J. Selective antagonism of nicotine actions in the rat cerebellum with alpha-bungarotoxin. Neuroscience. 1987 Dec;23(3):887–891. doi: 10.1016/0306-4522(87)90165-5. [DOI] [PubMed] [Google Scholar]
- de la Garza R., McGuire T. J., Freedman R., Hoffer B. J. The electrophysiological effects of nicotine in the rat cerebellum: evidence for direct postsynaptic actions. Neurosci Lett. 1987 Oct 5;80(3):303–308. doi: 10.1016/0304-3940(87)90472-1. [DOI] [PubMed] [Google Scholar]


