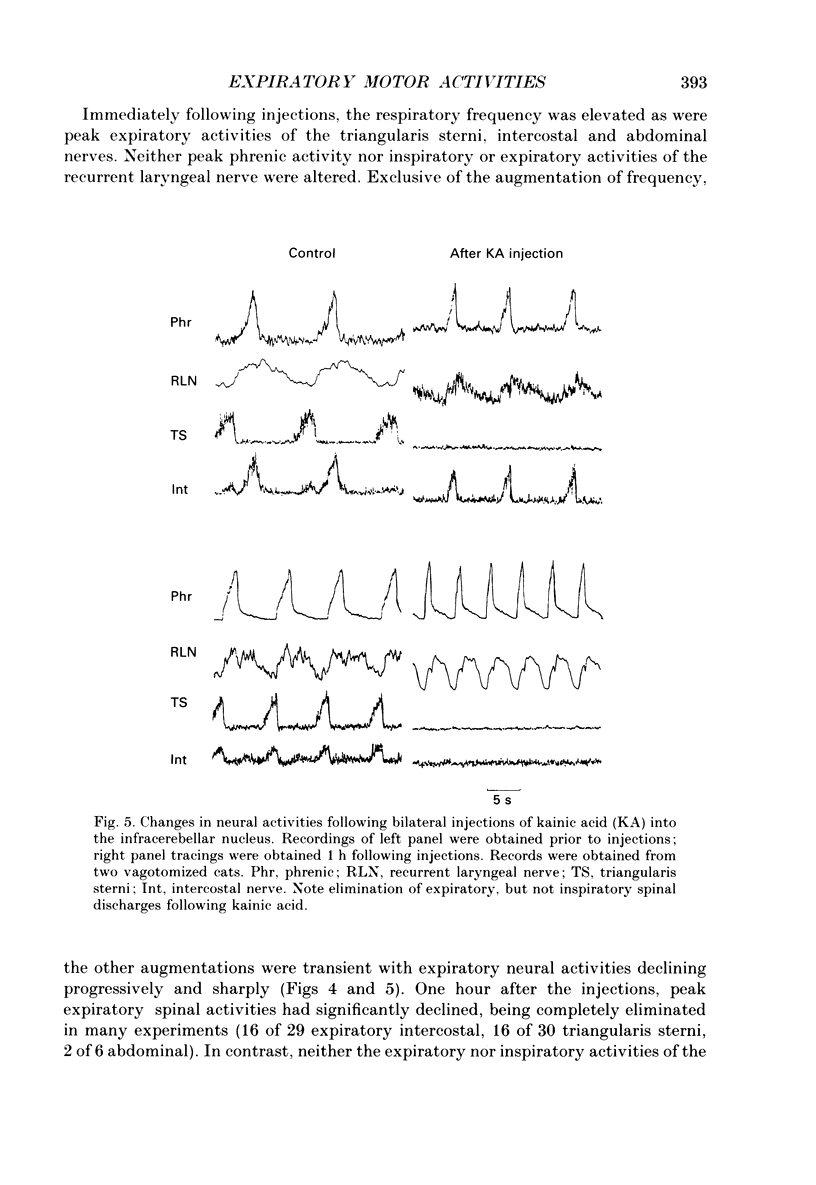
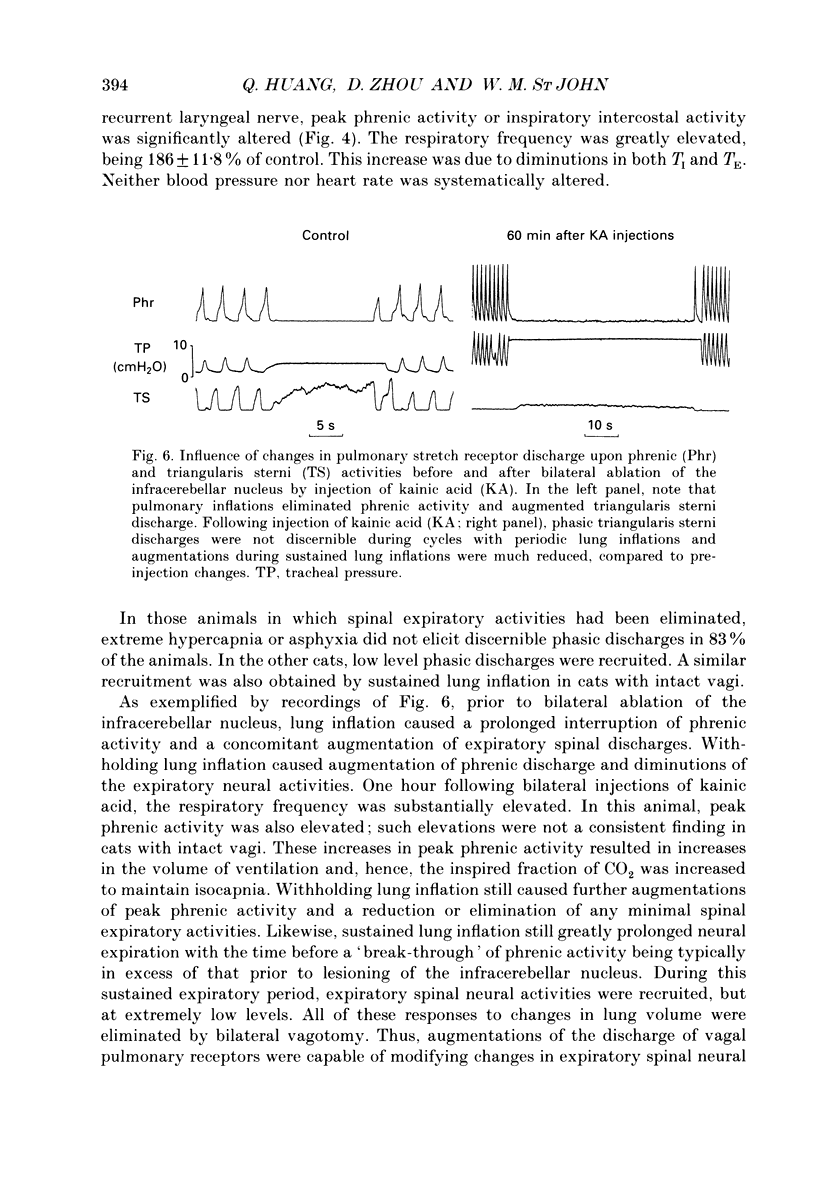
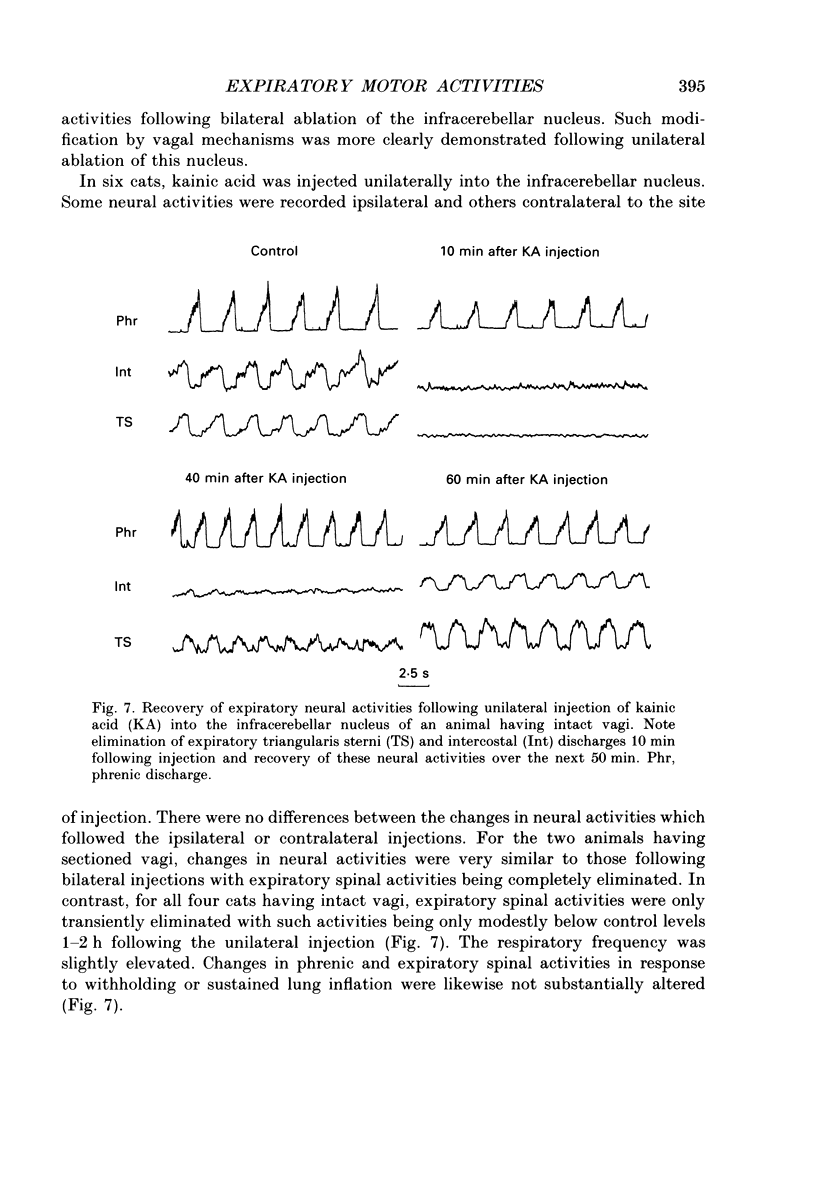
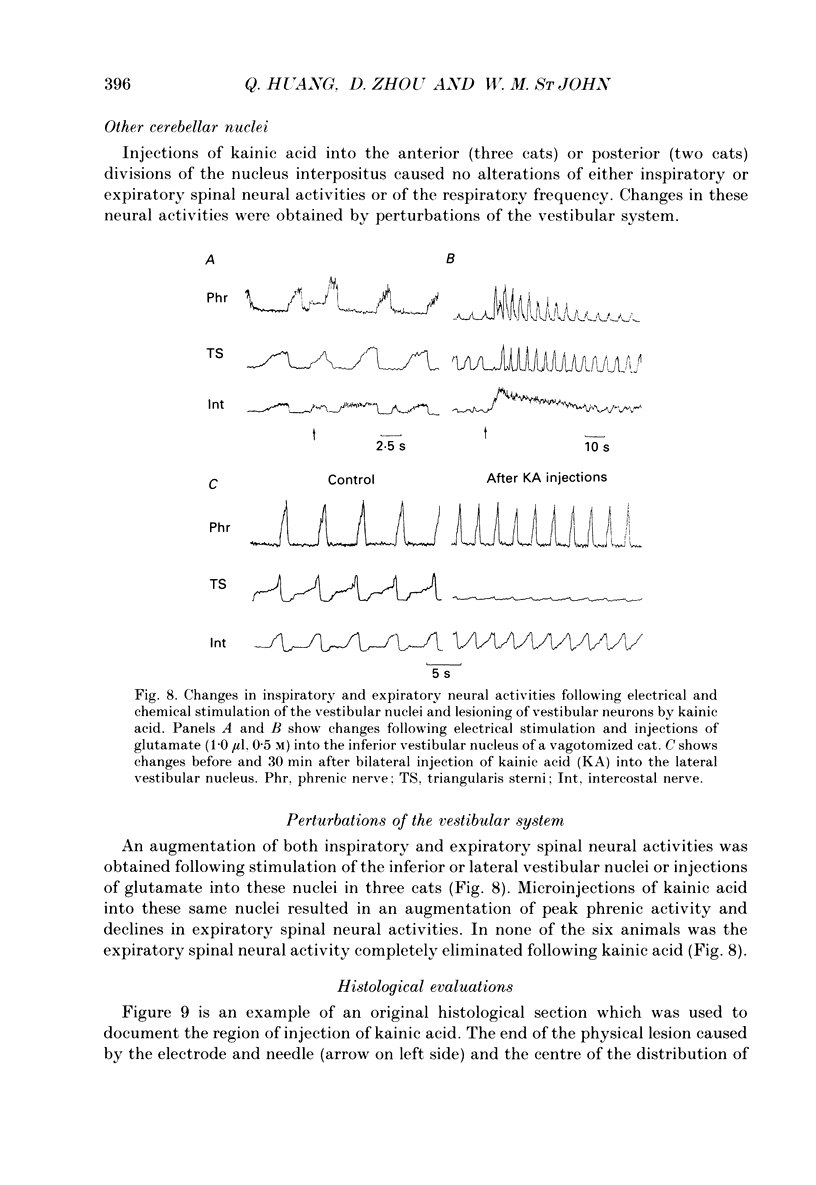
Abstract
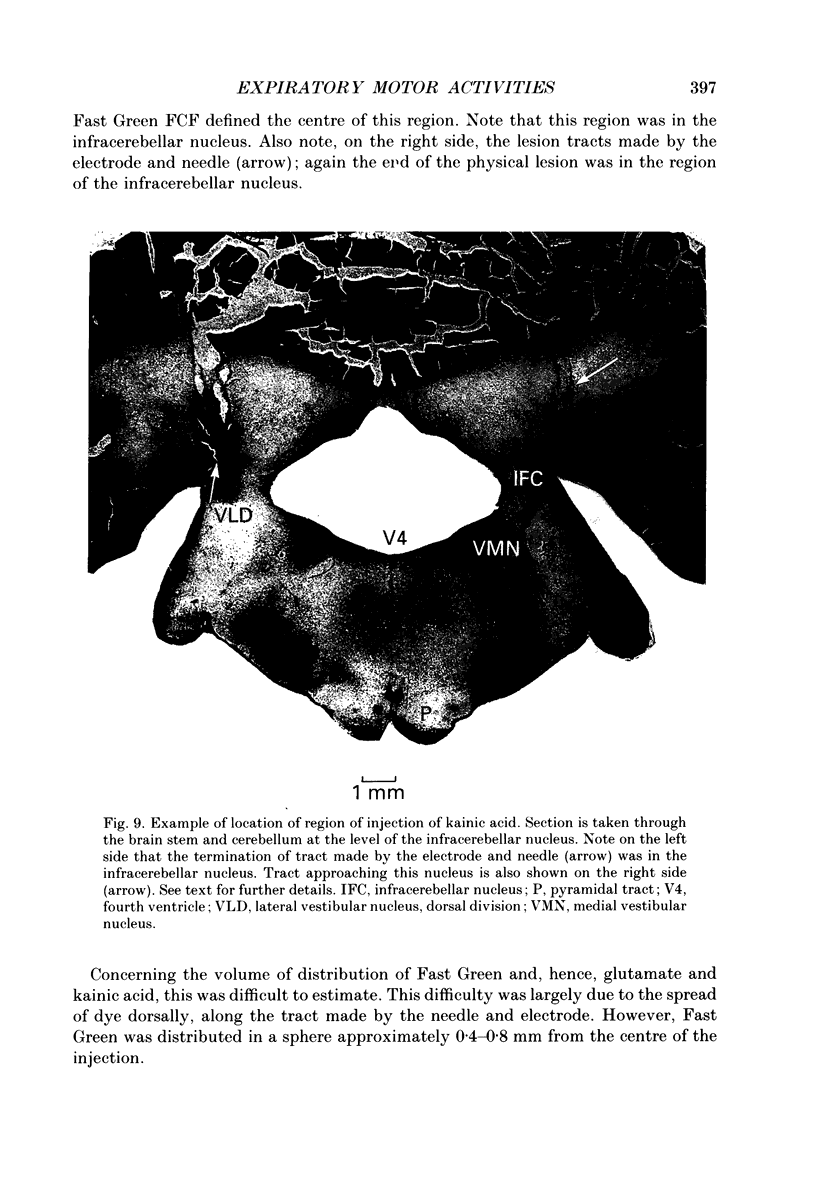
1. The purpose of our investigation was to evaluate the hypothesis that components of the vestibular and cerebellar systems regulate efferent respiratory-modulated activities of cranial and spinal nerves. The hypothesis was based upon the observation that spinal neural activities during expiration are greatly altered subsequent to a change in posture. 2. In decerebrate and paralysed cats, efferent activities were recorded from the central cut ends of the phrenic nerve, intercostal nerve, branch of the intercostal nerve innervating the triangularis sterni, cranial iliohypogastric (abdominal) nerve and recurrent laryngeal nerve. 3. Animals were artificially ventilated. Those with intact vagi were ventilated by a servo-respirator which produced changes in lung volume in parallel with alterations in integrated activity of the phrenic nerve. Animals with bilateral vagotomy were ventilated with a standard respirator. 4. Aspiration of the entire cerebellar cortex did not produce alterations in levels of neural activities; the respiratory frequency was increased modestly. Following ablation of the ventrolateral portion of corpus medullare and cerebellar peduncles, expiratory activities of spinal nerves were completely eliminated whereas inspiratory activities were not greatly altered. Results were similar in animals having either intact or sectioned vagi. 5. Electrical stimulation or chemical stimulation by glutamate of regions of the ventrolateral cerebellum produced little change in respiratory neural activities except when these stimulations were within the infracerebellar nucleus. Stimulations in this nucleus caused pronounced increases in expiratory activities of spinal nerves. Neither inspiratory activities of spinal nerves nor inspiratory or expiratory activities of the recurrent laryngeal nerve were altered. Studies in animals having intact or sectioned vagi yielded similar results. 6. Bilateral lesions of neurons in the infracerebellar nucleus by injections of kainic acid in animals having intact or sectioned vagi caused an irreversible loss of expiratory activities of spinal nerves with neither inspiratory spinal activities nor inspiratory and expiratory laryngeal activities being altered. Similar findings were obtained following unilateral ablation of the infracerebellar nucleus in vagotomized cats. However, in cats with intact vagi, unilateral ablation of the infracerebellar nucleus produced only transient changes in either inspiratory or expiratory neural activities.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




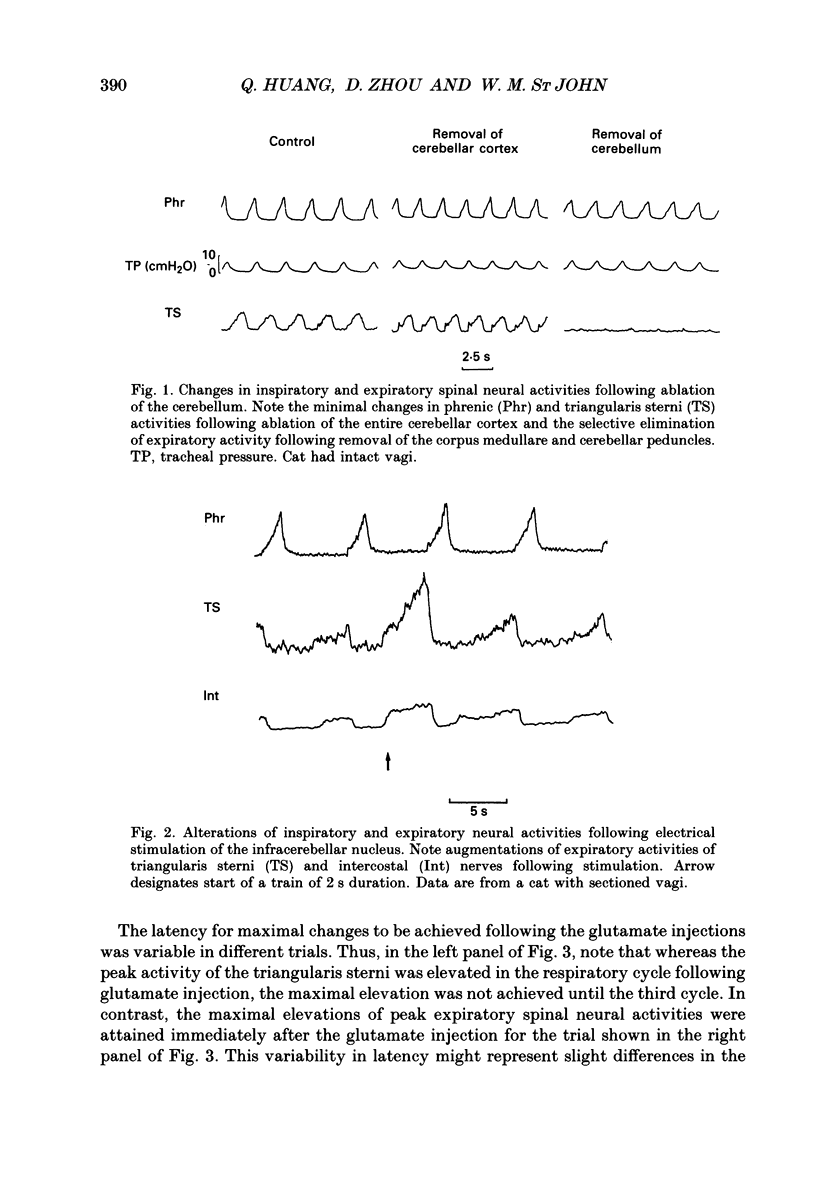
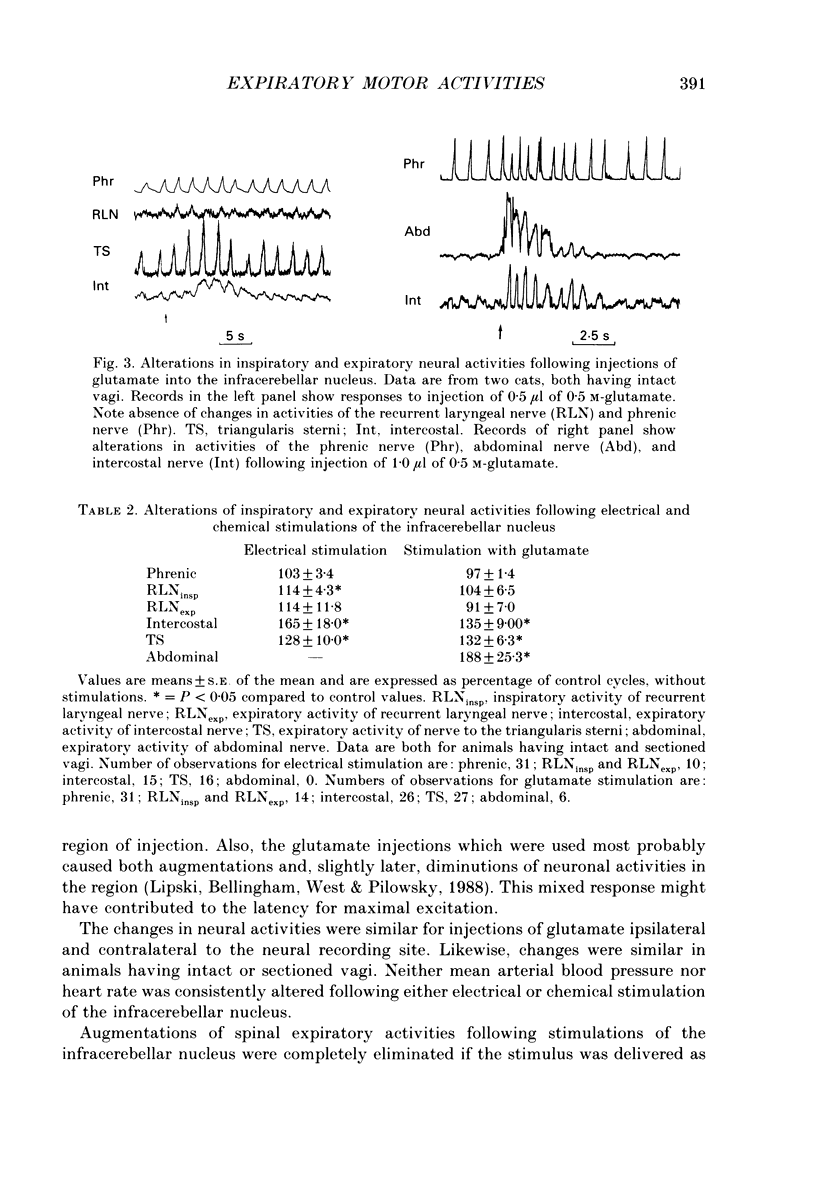
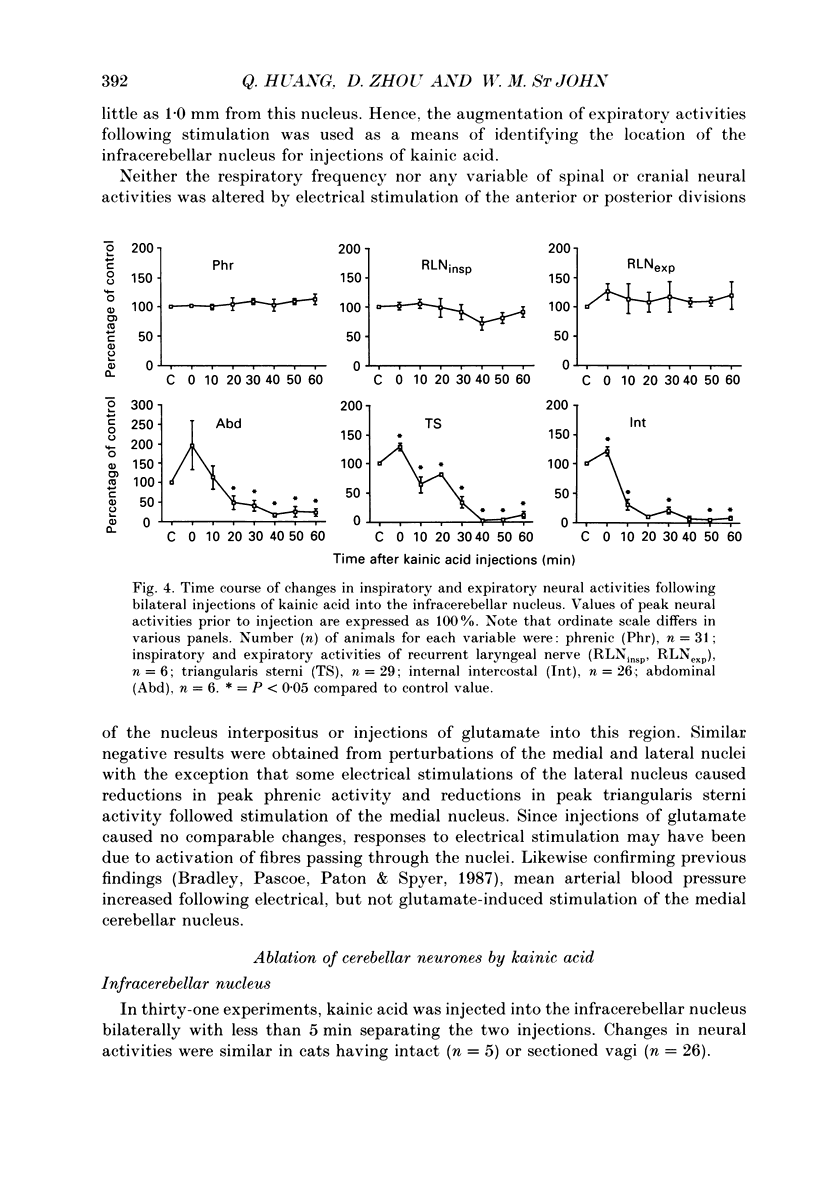







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRODAL A., POMPEIANO O. The vestibular nuclei in cat. J Anat. 1957 Oct;91(4):438–454. [PMC free article] [PubMed] [Google Scholar]
- Berger A. J., Cooney K. A. Ventilatory effects of kainic acid injection of the ventrolateral solitary nucleus. J Appl Physiol Respir Environ Exerc Physiol. 1982 Jan;52(1):131–140. doi: 10.1152/jappl.1982.52.1.131. [DOI] [PubMed] [Google Scholar]
- Bishop B. Diaphragm and abdominal muscle responses to elevated airway pressures in the cat. J Appl Physiol. 1967 May;22(5):959–965. doi: 10.1152/jappl.1967.22.5.959. [DOI] [PubMed] [Google Scholar]
- Bonora M., Bartlett D., Jr, Knuth S. L. Changes in upper airway muscle activity related to head position in awake cats. Respir Physiol. 1985 May;60(2):181–192. doi: 10.1016/0034-5687(85)90102-1. [DOI] [PubMed] [Google Scholar]
- Bradley D. J., Pascoe J. P., Paton J. F., Spyer K. M. Cardiovascular and respiratory responses evoked from the posterior cerebellar cortex and fastigial nucleus in the cat. J Physiol. 1987 Dec;393:107–121. doi: 10.1113/jphysiol.1987.sp016813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carleton S. C., Carpenter M. B. Afferent and efferent connections of the medial, inferior and lateral vestibular nuclei in the cat and monkey. Brain Res. 1983 Nov 14;278(1-2):29–51. doi: 10.1016/0006-8993(83)90223-8. [DOI] [PubMed] [Google Scholar]
- Davies A., Sant'Ambrogio F. B., Sant'Ambrogio G. Control of postural changes of end expiratory volume (FRC) by airways slowly adapting mechanoreceptors. Respir Physiol. 1980 Aug;41(2):211–216. doi: 10.1016/0034-5687(80)90053-5. [DOI] [PubMed] [Google Scholar]
- De Troyer A., Gilmartin J. J., Ninane V. Abdominal muscle use during breathing in unanesthetized dogs. J Appl Physiol (1985) 1989 Jan;66(1):20–27. doi: 10.1152/jappl.1989.66.1.20. [DOI] [PubMed] [Google Scholar]
- De Troyer A., Ninane V. Effect of posture on expiratory muscle use during breathing in the dog. Respir Physiol. 1987 Mar;67(3):311–322. doi: 10.1016/0034-5687(87)90061-2. [DOI] [PubMed] [Google Scholar]
- De Troyer A., Ninane V. Triangularis sterni: a primary muscle of breathing in the dog. J Appl Physiol (1985) 1986 Jan;60(1):14–21. doi: 10.1152/jappl.1986.60.1.14. [DOI] [PubMed] [Google Scholar]
- Duron B., Marlot D. Intercostal and diaphragmatic electrical activity during wakefulness and sleep in normal unrestrained adult cats. Sleep. 1980;3(3-4):269–280. doi: 10.1093/sleep/3.3-4.269. [DOI] [PubMed] [Google Scholar]
- Epema A. H., Gerrits N. M., Voogd J. Commissural and intrinsic connections of the vestibular nuclei in the rabbit: a retrograde labeling study. Exp Brain Res. 1988;71(1):129–146. doi: 10.1007/BF00247528. [DOI] [PubMed] [Google Scholar]
- Estenne M., Ninane V., De Troyer A. Triangularis sterni muscle use during eupnea in humans: effect of posture. Respir Physiol. 1988 Nov;74(2):151–162. doi: 10.1016/0034-5687(88)90101-6. [DOI] [PubMed] [Google Scholar]
- Fregosi R. F., Bartlett D., Jr Internal intercostal nerve discharges in the cat: influence of chemical stimuli. J Appl Physiol (1985) 1989 Feb;66(2):687–694. doi: 10.1152/jappl.1989.66.2.687. [DOI] [PubMed] [Google Scholar]
- Fregosi R. F., Knuth S. L., Ward D. K., Bartlett D., Jr Hypoxia inhibits abdominal expiratory nerve activity. J Appl Physiol (1985) 1987 Jul;63(1):211–220. doi: 10.1152/jappl.1987.63.1.211. [DOI] [PubMed] [Google Scholar]
- Gacek R. R. Location of brain stem neurons projecting to the oculomotor nucleus in the cat. Exp Neurol. 1977 Dec;57(3):725–749. doi: 10.1016/0014-4886(77)90105-4. [DOI] [PubMed] [Google Scholar]
- Goodchild A. K., Dampney R. A., Bandler R. A method for evoking physiological responses by stimulation of cell bodies, but not axons of passage, within localized regions of the central nervous system. J Neurosci Methods. 1982 Nov;6(4):351–363. doi: 10.1016/0165-0270(82)90036-x. [DOI] [PubMed] [Google Scholar]
- Hwang J. C., St John W. M., Bartlett D., Jr Respiratory-related hypoglossal nerve activity: influence of anesthetics. J Appl Physiol Respir Environ Exerc Physiol. 1983 Sep;55(3):785–792. doi: 10.1152/jappl.1983.55.3.785. [DOI] [PubMed] [Google Scholar]
- Jodkowski J. S., Berger A. J. Influences from laryngeal afferents on expiratory bulbospinal neurons and motoneurons. J Appl Physiol (1985) 1988 Apr;64(4):1337–1345. doi: 10.1152/jappl.1988.64.4.1337. [DOI] [PubMed] [Google Scholar]
- Lipski J., Bellingham M. C., West M. J., Pilowsky P. Limitations of the technique of pressure microinjection of excitatory amino acids for evoking responses from localized regions of the CNS. J Neurosci Methods. 1988 Dec;26(2):169–179. doi: 10.1016/0165-0270(88)90166-5. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Nonaka S., Lakos S. F., Tan L. K. Diaphragmatic and external intercostal muscle control during vomiting: behavior of inspiratory bulbospinal neurons. J Neurophysiol. 1990 Jan;63(1):31–36. doi: 10.1152/jn.1990.63.1.31. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Tan L. K., Lakos S. F. Brainstem projections to cats' upper lumbar spinal cord: implications for abdominal muscle control. Brain Res. 1989 Jul 31;493(2):348–356. doi: 10.1016/0006-8993(89)91169-4. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Tan L. K., Suzuki I. Control of abdominal and expiratory intercostal muscle activity during vomiting: role of ventral respiratory group expiratory neurons. J Neurophysiol. 1987 Jun;57(6):1854–1866. doi: 10.1152/jn.1987.57.6.1854. [DOI] [PubMed] [Google Scholar]
- Nattie E. E., Mills J. W., Ou L. C., St John W. M. Kainic acid on the rostral ventrolateral medulla inhibits phrenic output and CO2 sensitivity. J Appl Physiol (1985) 1988 Oct;65(4):1525–1534. doi: 10.1152/jappl.1988.65.4.1525. [DOI] [PubMed] [Google Scholar]
- Oliven A., Kelsen S. G. Effect of hypercapnia and PEEP on expiratory muscle EMG and shortening. J Appl Physiol (1985) 1989 Mar;66(3):1408–1413. doi: 10.1152/jappl.1989.66.3.1408. [DOI] [PubMed] [Google Scholar]
- Richter D. W. Generation and maintenance of the respiratory rhythm. J Exp Biol. 1982 Oct;100:93–107. doi: 10.1242/jeb.100.1.93. [DOI] [PubMed] [Google Scholar]
- Sato Y., Kawasaki T. Target neurons of floccular caudal zone inhibition in Y-group nucleus of vestibular nuclear complex. J Neurophysiol. 1987 Feb;57(2):460–480. doi: 10.1152/jn.1987.57.2.460. [DOI] [PubMed] [Google Scholar]
- St John W. M., Bledsoe T. A. Comparison of respiratory-related trigeminal, hypoglossal and phrenic activities. Respir Physiol. 1985 Oct;62(1):61–78. doi: 10.1016/0034-5687(85)90050-7. [DOI] [PubMed] [Google Scholar]
- St John W. M., Zhou D. Differing control of neural activities during various portions of expiration in the cat. J Physiol. 1989 Nov;418:189–204. doi: 10.1113/jphysiol.1989.sp017834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John W. M., Zhou D. Discharge of vagal pulmonary receptors differentially alters neural activities during various stages of expiration in the cat. J Physiol. 1990 May;424:1–12. doi: 10.1113/jphysiol.1990.sp018051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John W. M., Zhou D., Fregosi R. F. Expiratory neural activities in gasping. J Appl Physiol (1985) 1989 Jan;66(1):223–231. doi: 10.1152/jappl.1989.66.1.223. [DOI] [PubMed] [Google Scholar]
- Wilson V. J., Yoshida M., Schor R. H. Supraspinal monosynaptic excitation and inhibition of thoracic back montoneurons. Exp Brain Res. 1970;11(3):282–295. doi: 10.1007/BF01474387. [DOI] [PubMed] [Google Scholar]