Abstract
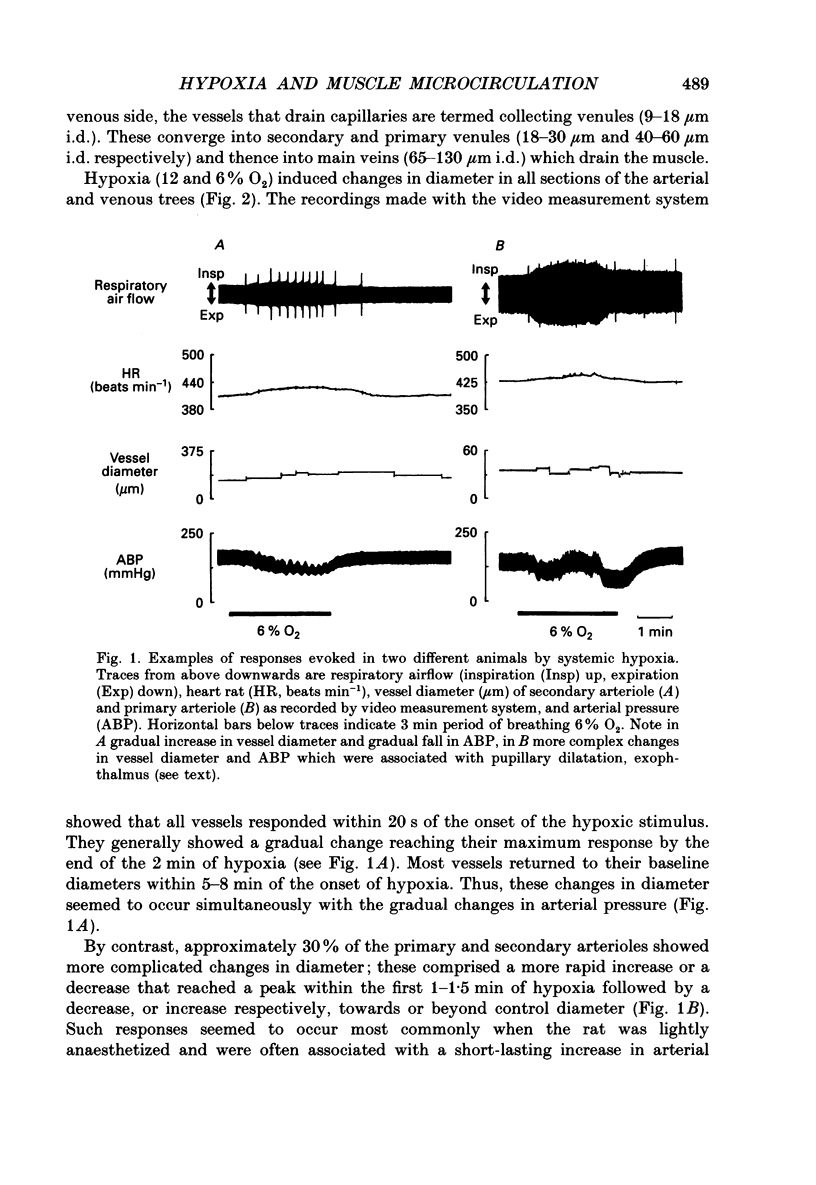
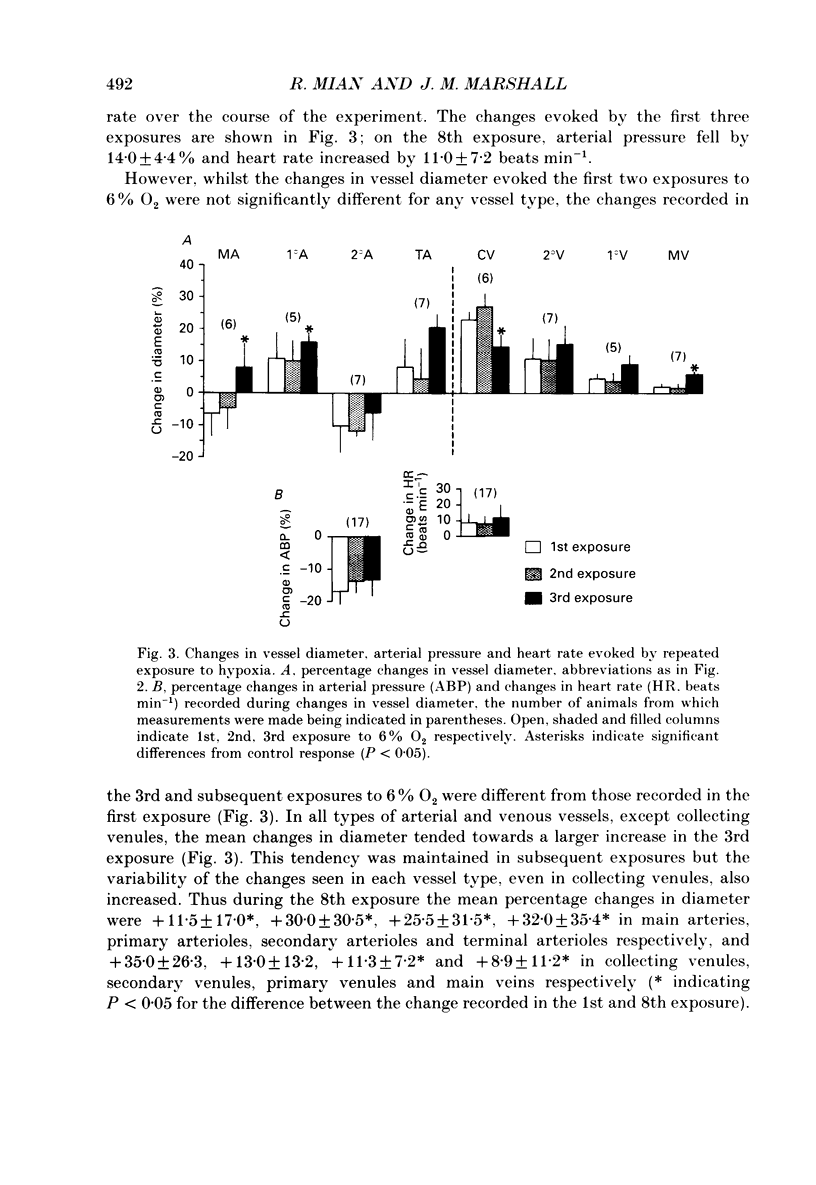
1. In rats anaesthetized with Saffan, responses induced in individual arterial and venous vessels of the spinotrapezius muscle by systemic hypoxia (breathing 12 or 6% O2 for 3 min) were directly observed by in vivo microscopy. 2. Both 12 and 6% O2 induced gradual tachycardia and a fall in arterial pressure. Concommitantly, in each section of the vascular tree, some vessels showed a gradual increase in diameter, others, a gradual decrease. 3. During 12% O2, mean diameter changes were graded from mean increases of approximately 2% in main arteries (resting diameter 40-90 microns) to approximately 20% in terminal arterioles (7-13 microns) and ranged from mean increases of 5-8% in collecting and secondary venules (9-18 microns, 18-30 microns), to a decrease of approximately 2% in main veins (65-130 microns). 4. During 6% O2, constrictor responses were more common in arterial vessels. Thus, mean changes amounted to diameter decreases of less than 5% in main arteries and secondary arterioles (13-18 microns), and increases of approximately 5% in primary arterioles (22-50 microns) and terminal arterioles. By contrast, diameter increases predominated in venous vessels being graded from approximately 20% in collecting venules to approximately 2% in main veins. 5. In seventeen rats, 6% O2 was administered for eight 3 min periods separated by 30 min control periods. The changes evoked in arterial pressure and heart rate were consistent throughout. Diameter changes evoked in individual arterial and venous vessels were consistent in the first two hypoxic periods. However, diameter changes in the third and successive periods were significantly different from those recorded in the first period: increases in diameter became more common and pronounced. 6. These changes in vessel diameter, especially their variability, are considered in relation to recordings made previously of changes in gross blood flow and vascular conductance of limb muscle during systemic hypoxia.
Full text
PDF



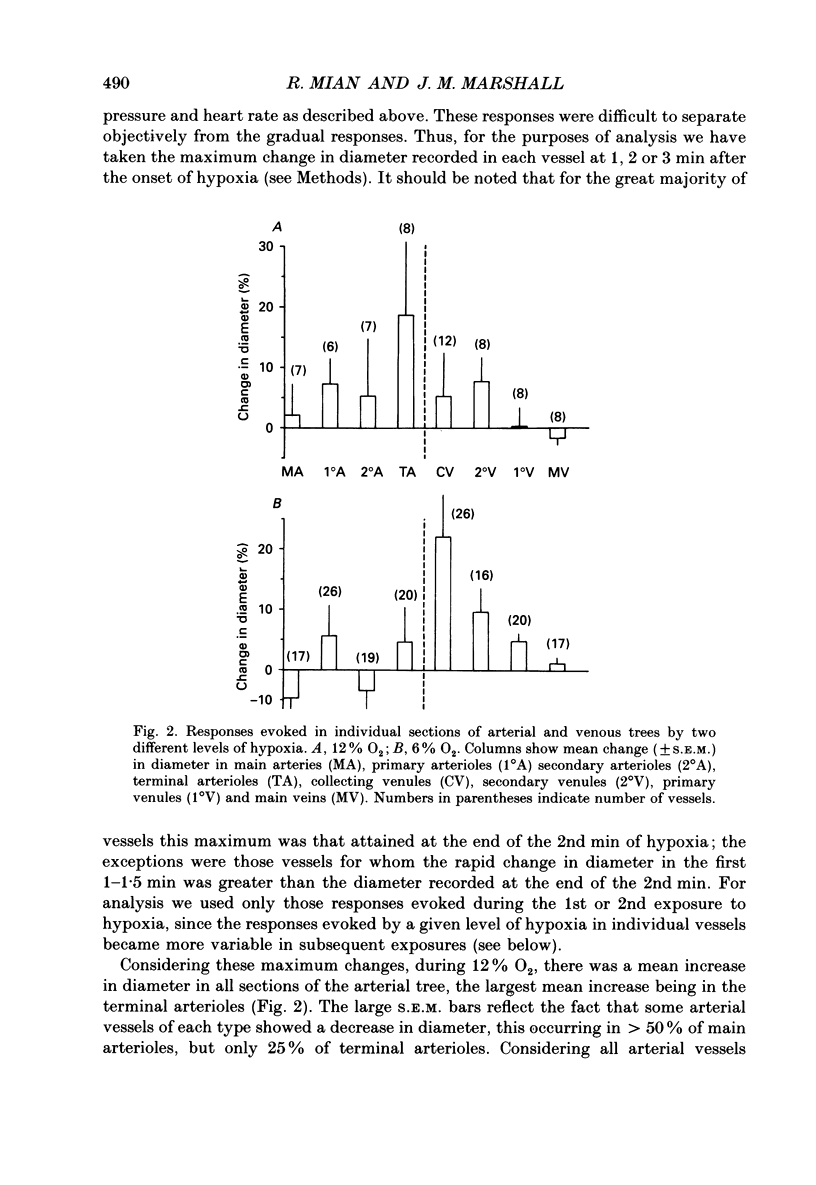






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong R. B., Laughlin M. H. Blood flows within and among rat muscles as a function of time during high speed treadmill exercise. J Physiol. 1983 Nov;344:189–208. doi: 10.1113/jphysiol.1983.sp014933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House S. D., Johnson P. C. Microvascular pressure in venules of skeletal muscle during arterial pressure reduction. Am J Physiol. 1986 May;250(5 Pt 2):H838–H845. doi: 10.1152/ajpheart.1986.250.5.H838. [DOI] [PubMed] [Google Scholar]
- Hutchins P. M., Bond R. F., Green H. D. Participation of oxygen in the local control of skeletal muscle microvasculature. Circ Res. 1974 Jan;34(1):85–93. doi: 10.1161/01.res.40.4.85. [DOI] [PubMed] [Google Scholar]
- Hébert M. T., Marshall J. M. Direct observations of responses of mesenteric microcirculation of the rat to circulating noradrenaline. J Physiol. 1985 Nov;368:393–407. doi: 10.1113/jphysiol.1985.sp015864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert M. T., Marshall J. M. Direct observations of the effects of baroreceptor stimulation on skeletal muscle circulation of the rat. J Physiol. 1988 Jun;400:45–59. doi: 10.1113/jphysiol.1988.sp017109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M. Analysis of cardiovascular responses evoked following changes in peripheral chemoreceptor activity in the rat. J Physiol. 1987 Dec;394:393–414. doi: 10.1113/jphysiol.1987.sp016877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M., Metcalfe J. D. Analysis of the cardiovascular changes induced in the rat by graded levels of systemic hypoxia. J Physiol. 1988 Dec;407:385–403. doi: 10.1113/jphysiol.1988.sp017422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M., Metcalfe J. D. Cardiovascular changes associated with augmented breaths in normoxia and hypoxia in the rat. J Physiol. 1988 Jun;400:15–27. doi: 10.1113/jphysiol.1988.sp017107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M., Metcalfe J. D. Influences on the cardiovascular response to graded levels of systemic hypoxia of the accompanying hypocapnia in the rat. J Physiol. 1989 Mar;410:381–394. doi: 10.1113/jphysiol.1989.sp017539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M., Tandon H. C. Direct observations of muscle arterioles and venules following contraction of skeletal muscle fibres in the rat. J Physiol. 1984 May;350:447–459. doi: 10.1113/jphysiol.1984.sp015211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M. The influence of the sympathetic nervous system on individual vessels of the microcirculation of skeletal muscle of the rat. J Physiol. 1982 Nov;332:169–186. doi: 10.1113/jphysiol.1982.sp014408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian R., Marshall J. M., Kumar P. Interactions between K+ and beta 2-adrenoreceptors in determining muscle vasodilatation induced in the rat by systemic hypoxia. Exp Physiol. 1990 May;75(3):407–410. doi: 10.1113/expphysiol.1990.sp003416. [DOI] [PubMed] [Google Scholar]
- Mian R., Marshall J. M. The roles of catecholamines in responses evoked in arterioles and venules of rat skeletal muscle by systemic hypoxia. J Physiol. 1991 May;436:499–510. doi: 10.1113/jphysiol.1991.sp018563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morff R. J., Harris P. D., Wiegman D. L., Miller F. N. muscle microcirculation: effects of tissue pH, PCO2, and PO2 during systemic hypoxia. Am J Physiol. 1981 May;240(5):H746–H754. doi: 10.1152/ajpheart.1981.240.5.H746. [DOI] [PubMed] [Google Scholar]
- Sullivan S. M., Johnson P. C. Effect of oxygen on arteriolar dimensions and blood flow in cat sartorius muscle. Am J Physiol. 1981 Oct;241(4):H547–H556. doi: 10.1152/ajpheart.1981.241.4.H547. [DOI] [PubMed] [Google Scholar]
- Sullivan S. M., Johnson P. C. Effect of oxygen on blood flow autoregulation in cat sartorius muscle. Am J Physiol. 1981 Dec;241(6):H807–H815. doi: 10.1152/ajpheart.1981.241.6.H807. [DOI] [PubMed] [Google Scholar]
- Taylor K., Calvey T. N. Histochemical characteristics and contractile properties of the spinotrapezius muscle in the rat and the mouse. J Anat. 1977 Feb;123(Pt 1):67–76. [PMC free article] [PubMed] [Google Scholar]
- Yardley C. P., Hilton S. M. Vasodilatation in hind-limb skeletal muscle evoked as part of the defence reaction in the rat. J Auton Nerv Syst. 1987 May;19(2):127–136. doi: 10.1016/0165-1838(87)90006-3. [DOI] [PubMed] [Google Scholar]


