Abstract
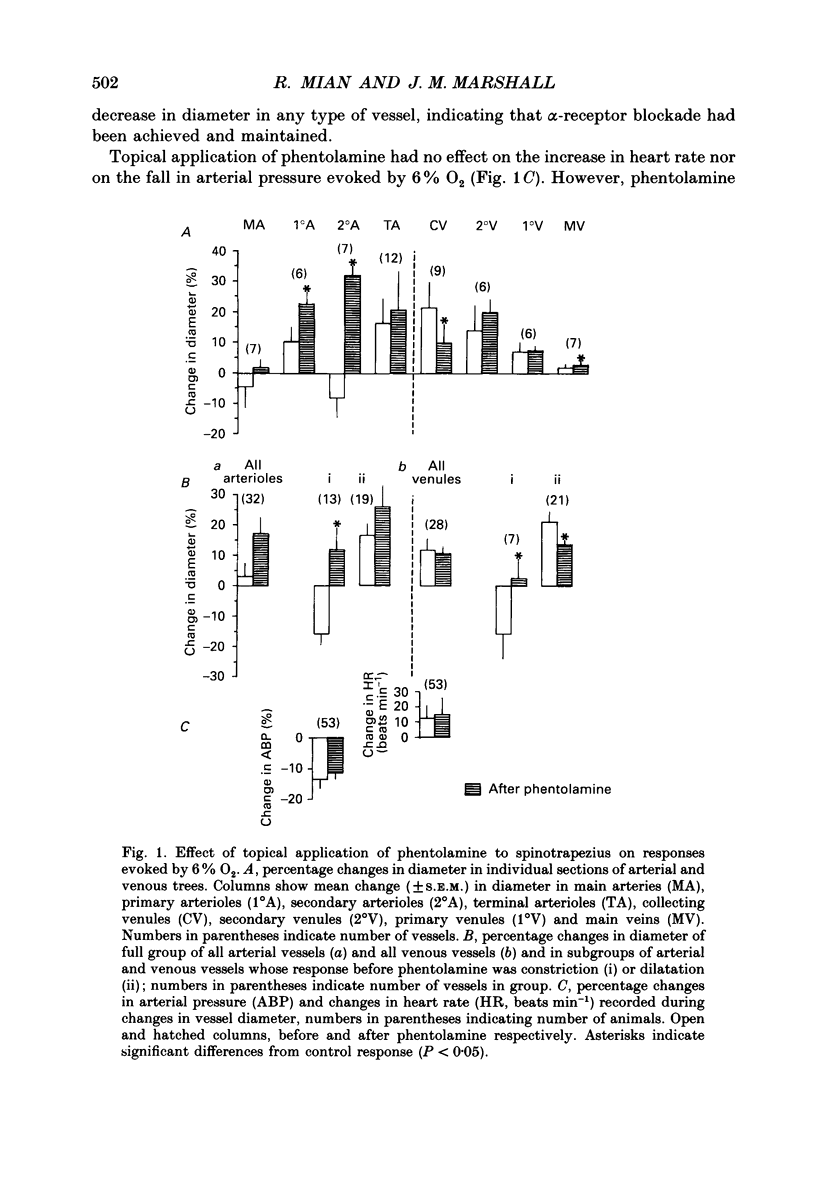
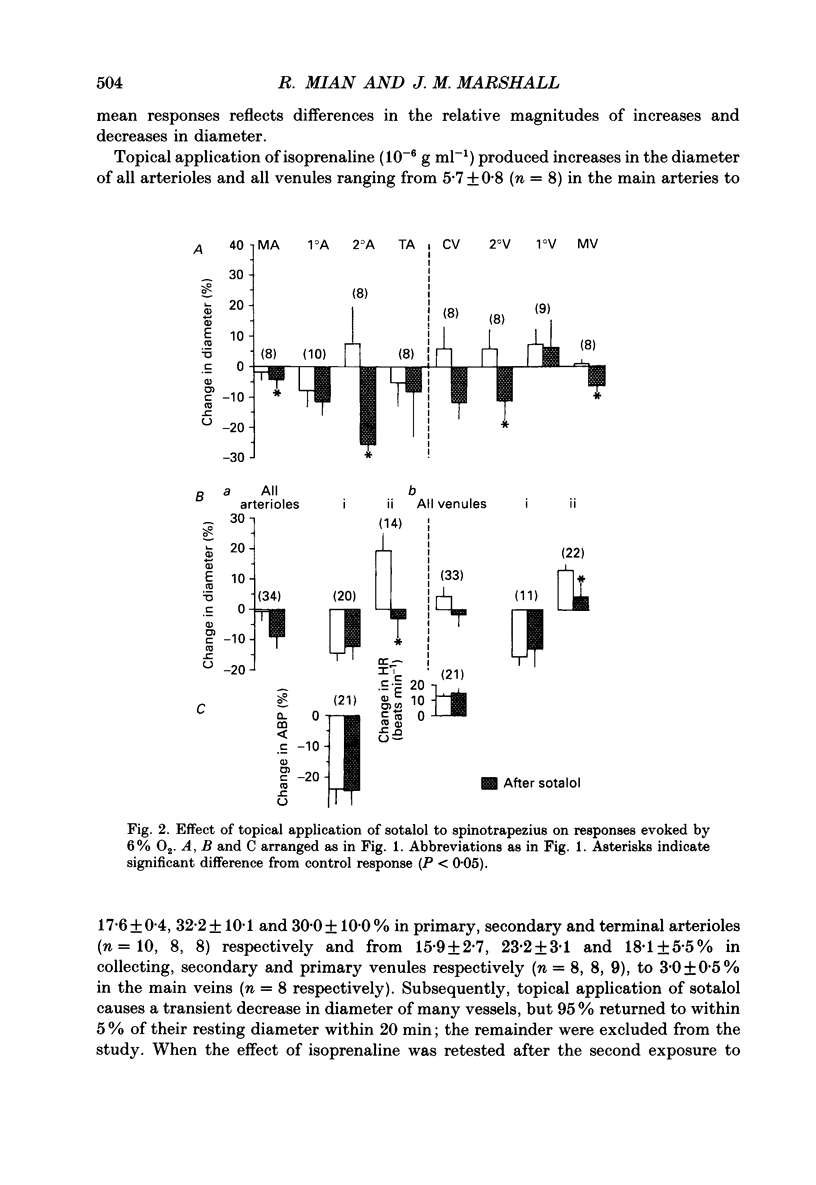
1. Studies have been made in the anaesthetized rat of the roles played by alpha- and beta-adrenoreceptor stimulation in determining diameter changes induced in individual arterioles and venules of the spinotrapezius muscle during systemic hypoxia (breathing 6% O2 for 3 min). 2. Topical application to the spinotrapezius of phentolamine, the alpha-adrenoreceptor antagonist, or sotalol, the beta-adrenoreceptor antagonist, had no effect on the fall in systemic arterial pressure and tachycardia induced by hypoxia. 3. All arterioles and venules showed a decrease in diameter in response to topical application of noradrenaline (10(-6) g ml-1): these responses were abolished by topical application of phentolamine. Moreover, those arterioles and venules that showed a decrease in diameter during hypoxia before phentolamine, showed a significantly smaller decrease, or an increase in diameter after phentolamine. This effect was most marked in primary and secondary arterioles (13-50 microns diameter). 4. All arterioles and venules showed an increase in diameter in response to topical application of isoprenaline (10(-6) g ml-1); these responses were abolished by topical application of sotalol. Moreover, these arterioles and venules that showed an increase in diameter during hypoxia before sotalol, showed a significantly smaller increase or even a decrease in diameter after sotalol. 5. These results suggest that during hypoxia the arterioles of skeletal muscle, especially primary and secondary arterioles, are under the constrictor influence of a reflex increase in sympathetic nerve activity while the venules, which have no sympathetic innervation, are under the constrictor influence of circulating catecholamines. They also suggest that in individual arterioles and venules, these constrictor influences may be overcome by dilatation mediated by the beta-adrenoreceptor influence of circulating catecholamines. 6. Since some arterioles and venules still showed constriction during hypoxia after phentolamine and some still showed dilatation during hypoxia after sotalol, it seems that factors other than catecholamines contribute to the diameter changes. It is suggested that locally released metabolites exert a substantial dilator influence, particularly on terminal arterioles and collecting venules, those vessels nearest to the capillary bed.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borgström P., Lindbom L., Arfors K. E., Intaglietta M. Beta-adrenergic control of resistance in individual vessels in rabbit tenuissimus muscle. Am J Physiol. 1988 Apr;254(4 Pt 2):H631–H635. doi: 10.1152/ajpheart.1988.254.4.H631. [DOI] [PubMed] [Google Scholar]
- Busse R., Pohl U., Kellner C., Klemm U. Endothelial cells are involved in the vasodilatory response to hypoxia. Pflugers Arch. 1983 Apr;397(1):78–80. doi: 10.1007/BF00585175. [DOI] [PubMed] [Google Scholar]
- Hillman J., Lundvall J. Hormonal and neurogenic adrenergic control of the fluid transfer from skeletal muscle to blood during hemorrhage. Acta Physiol Scand. 1981 Jul;112(3):271–280. doi: 10.1111/j.1748-1716.1981.tb06816.x. [DOI] [PubMed] [Google Scholar]
- Hutchins P. M., Bond R. F., Green H. D. Participation of oxygen in the local control of skeletal muscle microvasculature. Circ Res. 1974 Jan;34(1):85–93. doi: 10.1161/01.res.40.4.85. [DOI] [PubMed] [Google Scholar]
- Hébert M. T., Marshall J. M. Direct observations of the effects of baroreceptor stimulation on skeletal muscle circulation of the rat. J Physiol. 1988 Jun;400:45–59. doi: 10.1113/jphysiol.1988.sp017109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson W. F. Arteriolar oxygen reactivity: where is the sensor? Am J Physiol. 1987 Nov;253(5 Pt 2):H1120–H1126. doi: 10.1152/ajpheart.1987.253.5.H1120. [DOI] [PubMed] [Google Scholar]
- Lundvall J., Hillman J., Gustafsson D. beta-Adrenergic dilator effects in consecutive vascular sections of skeletal muscle. Am J Physiol. 1982 Nov;243(5):H819–H829. doi: 10.1152/ajpheart.1982.243.5.H819. [DOI] [PubMed] [Google Scholar]
- Marshall J. M. Analysis of cardiovascular responses evoked following changes in peripheral chemoreceptor activity in the rat. J Physiol. 1987 Dec;394:393–414. doi: 10.1113/jphysiol.1987.sp016877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M., Metcalfe J. D. Analysis of the cardiovascular changes induced in the rat by graded levels of systemic hypoxia. J Physiol. 1988 Dec;407:385–403. doi: 10.1113/jphysiol.1988.sp017422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M., Metcalfe J. D. Cardiovascular changes associated with augmented breaths in normoxia and hypoxia in the rat. J Physiol. 1988 Jun;400:15–27. doi: 10.1113/jphysiol.1988.sp017107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M., Metcalfe J. D. Influences on the cardiovascular response to graded levels of systemic hypoxia of the accompanying hypocapnia in the rat. J Physiol. 1989 Mar;410:381–394. doi: 10.1113/jphysiol.1989.sp017539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M., Tandon H. C. Direct observations of muscle arterioles and venules following contraction of skeletal muscle fibres in the rat. J Physiol. 1984 May;350:447–459. doi: 10.1113/jphysiol.1984.sp015211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M. The influence of the sympathetic nervous system on individual vessels of the microcirculation of skeletal muscle of the rat. J Physiol. 1982 Nov;332:169–186. doi: 10.1113/jphysiol.1982.sp014408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian R., Marshall J. M., Kumar P. Interactions between K+ and beta 2-adrenoreceptors in determining muscle vasodilatation induced in the rat by systemic hypoxia. Exp Physiol. 1990 May;75(3):407–410. doi: 10.1113/expphysiol.1990.sp003416. [DOI] [PubMed] [Google Scholar]
- Mian R., Marshall J. M. Responses observed in individual arterioles and venules of rat skeletal muscle during systemic hypoxia. J Physiol. 1991 May;436:485–497. doi: 10.1113/jphysiol.1991.sp018562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morff R. J., Harris P. D., Wiegman D. L., Miller F. N. muscle microcirculation: effects of tissue pH, PCO2, and PO2 during systemic hypoxia. Am J Physiol. 1981 May;240(5):H746–H754. doi: 10.1152/ajpheart.1981.240.5.H746. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Hypoxia releases a vasoconstrictor substance from the canine vascular endothelium. J Physiol. 1985 Jul;364:45–56. doi: 10.1113/jphysiol.1985.sp015728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan S. M., Johnson P. C. Effect of oxygen on arteriolar dimensions and blood flow in cat sartorius muscle. Am J Physiol. 1981 Oct;241(4):H547–H556. doi: 10.1152/ajpheart.1981.241.4.H547. [DOI] [PubMed] [Google Scholar]
- Uther J. B., Hunyor S. N., Shaw J., Korner P. I. Bulbar and suprabulbar control of the cardiovascular autonomic effects during arterial hypoxia in the rabbit. Circ Res. 1970 Apr;26(4):491–506. doi: 10.1161/01.res.26.4.491. [DOI] [PubMed] [Google Scholar]
- Yardley C. P., Hilton S. M. Vasodilatation in hind-limb skeletal muscle evoked as part of the defence reaction in the rat. J Auton Nerv Syst. 1987 May;19(2):127–136. doi: 10.1016/0165-1838(87)90006-3. [DOI] [PubMed] [Google Scholar]


