Abstract
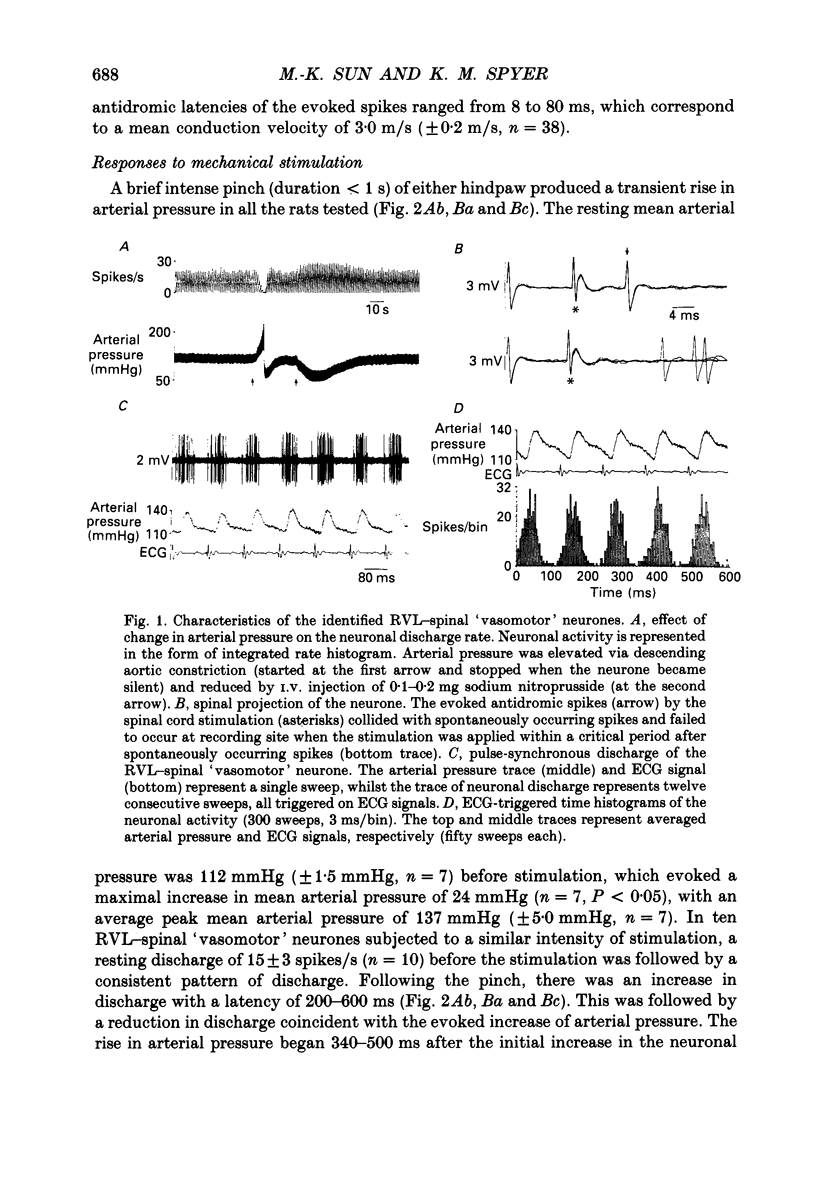
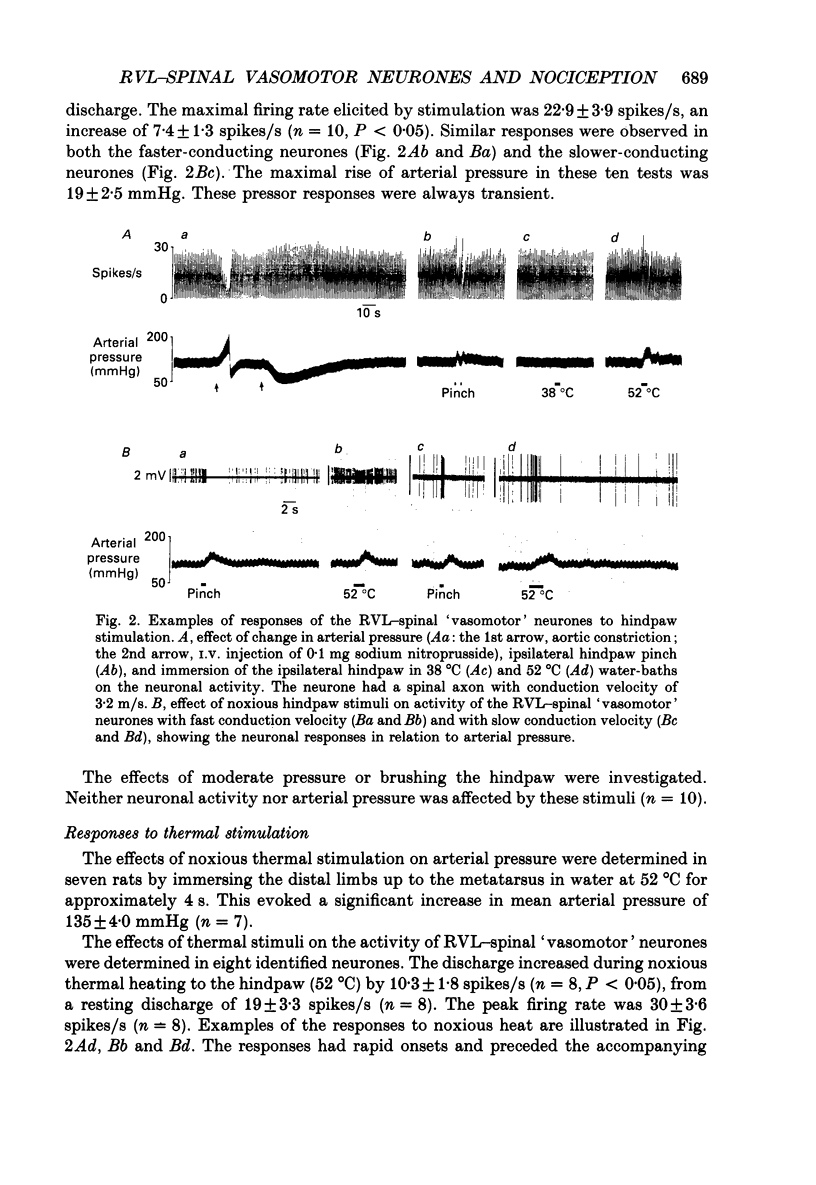
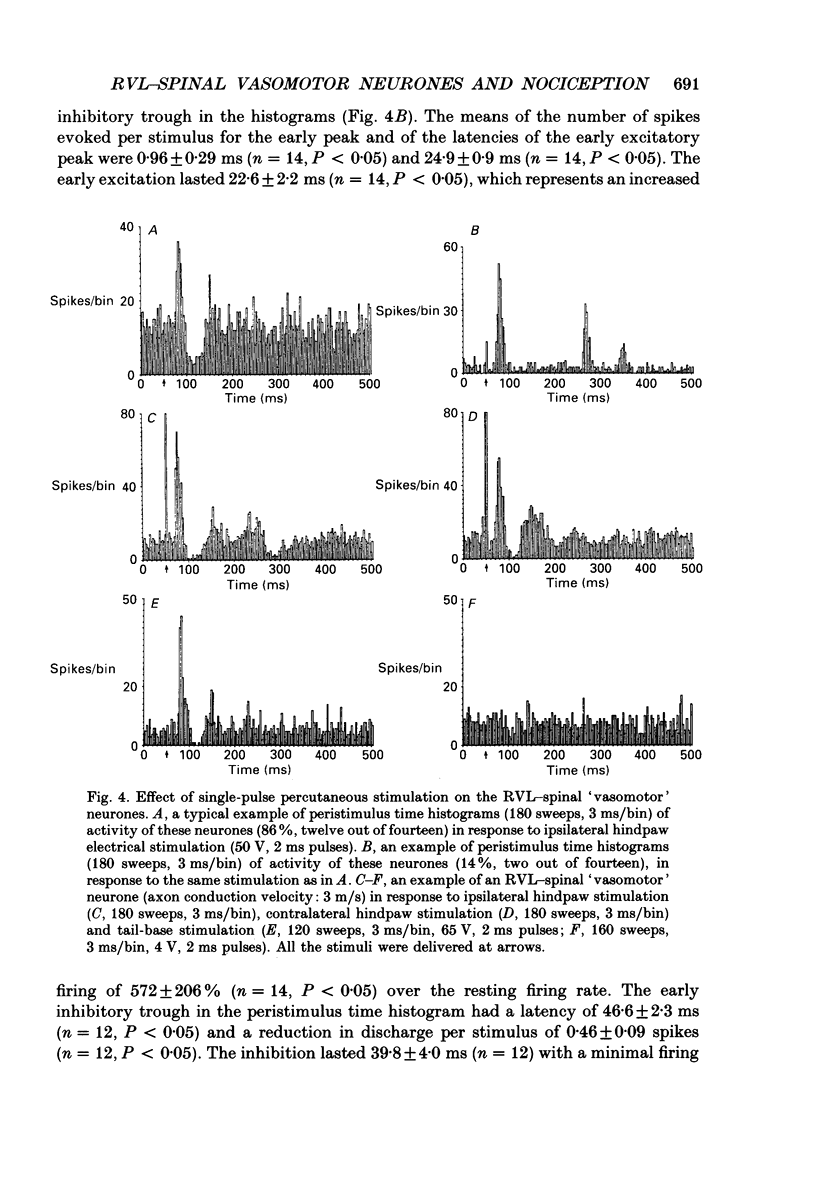
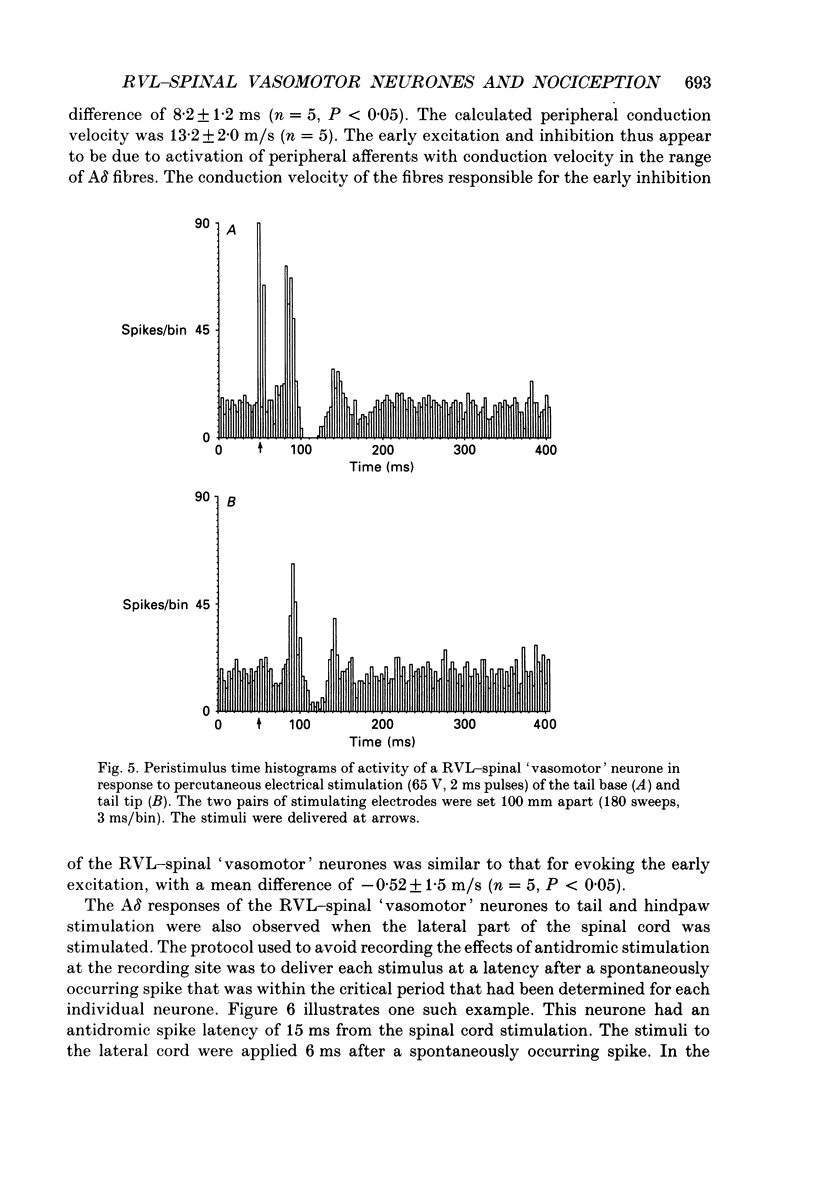
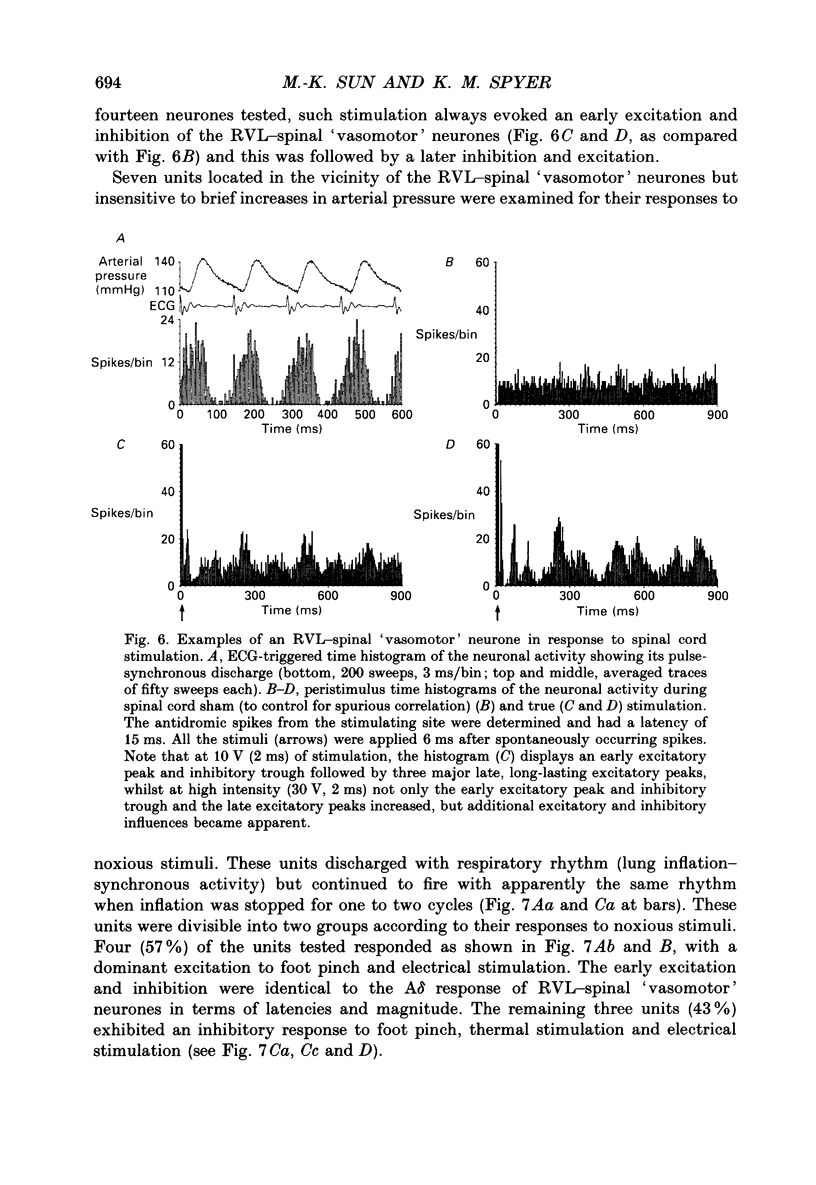
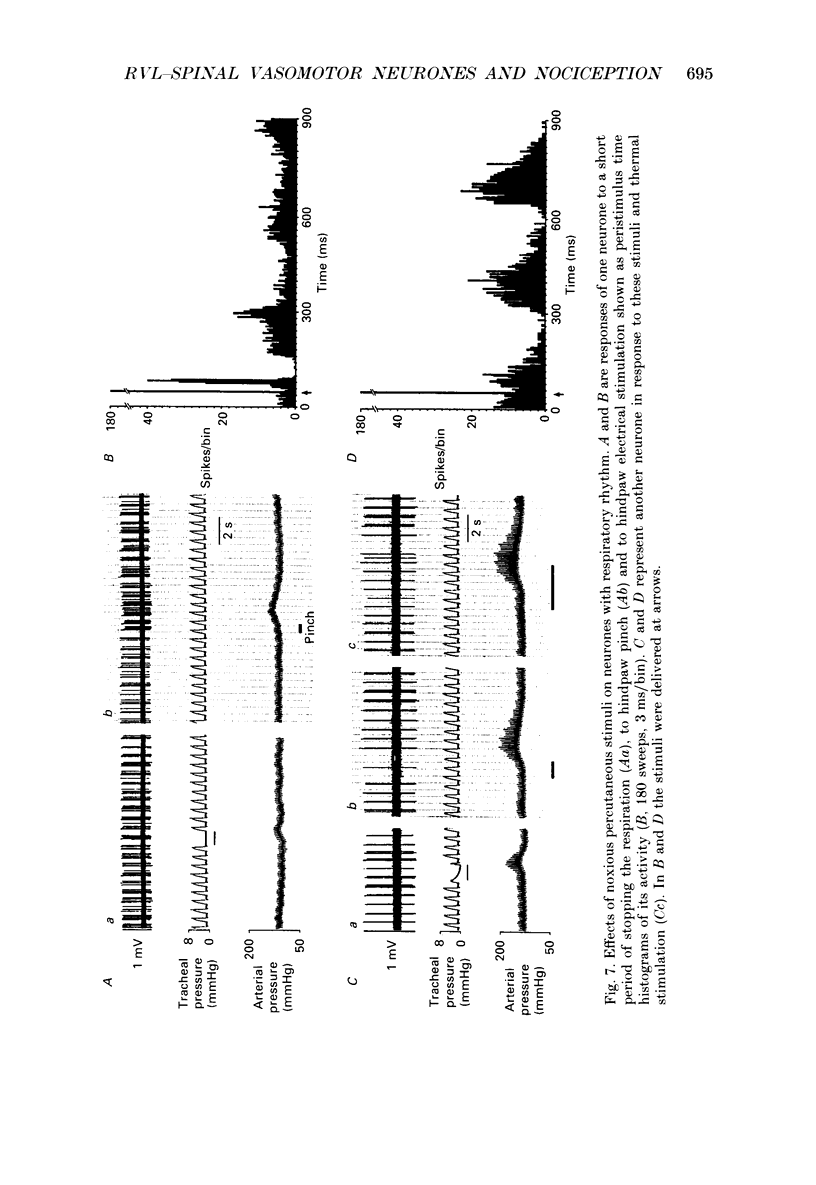
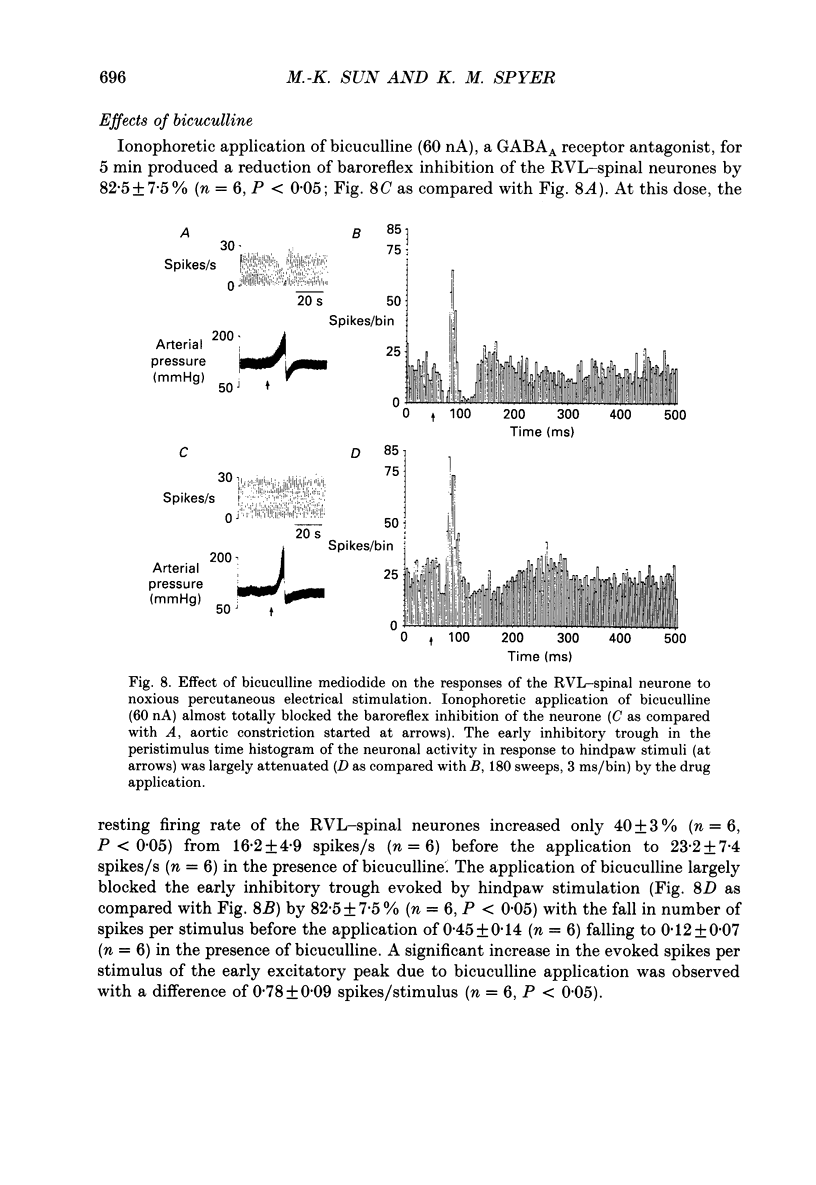
1. In anaesthetized rats recordings were made from thirty-eight neurones in the rostral ventrolateral medulla (RVL) with spinal-projecting axons. Their responses to mechanical, thermal and/or electrical stimulation were examined as were the accompanying changes in arterial pressure. 2. Mechanical, thermal and electrical stimulation of either hindpaw at a strength that can be regarded as noxious produced a consistent rise in arterial pressure. RVL-spinal-projecting 'vasomotor' neurones were excited by the noxious mechanical and thermal (52 degrees C) stimulation at a latency that was shorter than that of the evoked pressor response. 3. Percutaneous electrical stimulation of either hindlimb extremity resulted in an early peak of excitation (fourteen out of fourteen), an early trough of inhibition (twelve out fourteen), and a later peak of excitation (two out of fourteen). This response pattern to stimulation of either limb was independent of which limb was activated, but contralateral hindpaw stimulation elicited excitation at a shorter latency. The differences in latency of responses to stimulating two locations along the tail suggested that the early excitation and inhibition of RVL-spinal 'vasomotor' neurones were evoked by activation of peripheral fibres with a mean conduction velocity in the A delta range. 4. Short-latency excitatory and inhibitory responses in RVL-spinal 'vasomotor' neurones were observed also when single-pulse stimuli were delivered within the lateral part of the spinal cord. 5. Ionophoretic application of bicuculline, a GABAA receptor antagonist, blocked the evoked inhibition of these neurones on electrical stimulation of the hindpaw without attenuating the excitatory input from the same stimulus. 6. These results indicate that RVL-spinal 'vasomotor' neurones receive an input from cutaneous nociceptive afferents. This suggests that these neurones mediate, at least partly, the cardiovascular responses related to nociceptor stimulation.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besson J. M., Chaouch A. Peripheral and spinal mechanisms of nociception. Physiol Rev. 1987 Jan;67(1):67–186. doi: 10.1152/physrev.1987.67.1.67. [DOI] [PubMed] [Google Scholar]
- Bing Z., Villanueva L., Le Bars D. Ascending pathways in the spinal cord involved in the activation of subnucleus reticularis dorsalis neurons in the medulla of the rat. J Neurophysiol. 1990 Mar;63(3):424–438. doi: 10.1152/jn.1990.63.3.424. [DOI] [PubMed] [Google Scholar]
- Brown D. L., Guyenet P. G. Electrophysiological study of cardiovascular neurons in the rostral ventrolateral medulla in rats. Circ Res. 1985 Mar;56(3):359–369. doi: 10.1161/01.res.56.3.359. [DOI] [PubMed] [Google Scholar]
- Caverson M. M., Ciriello J., Calaresu F. R. Direct pathway from cardiovascular neurons in the ventrolateral medulla to the region of the intermediolateral nucleus of the upper thoracic cord: an anatomical and electrophysiological investigation in the cat. J Auton Nerv Syst. 1983 Nov;9(2-3):451–475. doi: 10.1016/0165-1838(83)90007-3. [DOI] [PubMed] [Google Scholar]
- Dampney R. A., Moon E. A. Role of ventrolateral medulla in vasomotor response to cerebral ischemia. Am J Physiol. 1980 Sep;239(3):H349–H358. doi: 10.1152/ajpheart.1980.239.3.H349. [DOI] [PubMed] [Google Scholar]
- Feldberg W. The ventral surface of the brain stem: a scarcely explored region of pharmacological sensitivity. Neuroscience. 1976 Dec;1(6):427–441. doi: 10.1016/0306-4522(76)90093-2. [DOI] [PubMed] [Google Scholar]
- Granata A. R., Ruggiero D. A., Park D. H., Joh T. H., Reis D. J. Brain stem area with C1 epinephrine neurons mediates baroreflex vasodepressor responses. Am J Physiol. 1985 Apr;248(4 Pt 2):H547–H567. doi: 10.1152/ajpheart.1985.248.4.H547. [DOI] [PubMed] [Google Scholar]
- Haselton J. R., Guyenet P. G. Central respiratory modulation of medullary sympathoexcitatory neurons in rat. Am J Physiol. 1989 Mar;256(3 Pt 2):R739–R750. doi: 10.1152/ajpregu.1989.256.3.R739. [DOI] [PubMed] [Google Scholar]
- Hilton S. M. Hypothalamic regulation of the cardiovascular system. Br Med Bull. 1966 Sep;22(3):243–248. doi: 10.1093/oxfordjournals.bmb.a070481. [DOI] [PubMed] [Google Scholar]
- Horeyseck G., Jänig W. Reflex activity in postganglionic fibres within skin and muscle nerves elicited by somatic stimuli in chronic spinal cats. Exp Brain Res. 1974;21(2):155–168. doi: 10.1007/BF00234387. [DOI] [PubMed] [Google Scholar]
- Hylden J. L., Anton F., Nahin R. L. Spinal lamina I projection neurons in the rat: collateral innervation of parabrachial area and thalamus. Neuroscience. 1989;28(1):27–37. doi: 10.1016/0306-4522(89)90229-7. [DOI] [PubMed] [Google Scholar]
- Jänig W. Organization of the lumbar sympathetic outflow to skeletal muscle and skin of the cat hindlimb and tail. Rev Physiol Biochem Pharmacol. 1985;102:119–213. doi: 10.1007/BFb0034086. [DOI] [PubMed] [Google Scholar]
- Koizumi K., Brooks C. M. The integration of autonomic system reactions: a discussion of autonomic reflexes, their control and their association with somatic reactions. Ergeb Physiol. 1972;67:1–68. [PubMed] [Google Scholar]
- Koizumi K., Collin R., Kaufman A., Brooks C. M. Contribution of unmyelinated afferent excitation to sympathetic reflexes. Brain Res. 1970 May 20;20(1):99–106. doi: 10.1016/0006-8993(70)90158-7. [DOI] [PubMed] [Google Scholar]
- Koizumi K., Sato A., Kaufman A., Brooks C. M. Studies of sympathetic neuron discharges modified by central and peripheral excitation. Brain Res. 1968 Oct;11(1):212–224. doi: 10.1016/0006-8993(68)90082-6. [DOI] [PubMed] [Google Scholar]
- McMahon S. B., Wall P. D. A system of rat spinal cord lamina 1 cells projecting through the contralateral dorsolateral funiculus. J Comp Neurol. 1983 Feb 20;214(2):217–223. doi: 10.1002/cne.902140209. [DOI] [PubMed] [Google Scholar]
- Riley M. V., Winkler B. S. Strong Pasteur effect in rabbit corneal endothelium preserves fluid transport under anaerobic conditions. J Physiol. 1990 Jul;426:81–93. doi: 10.1113/jphysiol.1990.sp018127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M. K., Guyenet P. G. Arterial baroreceptor and vagal inputs to sympathoexcitatory neurons in rat medulla. Am J Physiol. 1987 Apr;252(4 Pt 2):R699–R709. doi: 10.1152/ajpregu.1987.252.4.R699. [DOI] [PubMed] [Google Scholar]
- Sun M. K., Guyenet P. G. GABA-mediated baroreceptor inhibition of reticulospinal neurons. Am J Physiol. 1985 Dec;249(6 Pt 2):R672–R680. doi: 10.1152/ajpregu.1985.249.6.R672. [DOI] [PubMed] [Google Scholar]
- Sun M. K., Guyenet P. G. Hypothalamic glutamatergic input to medullary sympathoexcitatory neurons in rats. Am J Physiol. 1986 Oct;251(4 Pt 2):R798–R810. doi: 10.1152/ajpregu.1986.251.4.R798. [DOI] [PubMed] [Google Scholar]
- Sun M. K., Guyenet P. G. Medullospinal sympathoexcitatory neurons in normotensive and spontaneously hypertensive rats. Am J Physiol. 1986 May;250(5 Pt 2):R910–R917. doi: 10.1152/ajpregu.1986.250.5.R910. [DOI] [PubMed] [Google Scholar]
- Sun M. K., Hackett J. T., Guyenet P. G. Sympathoexcitatory neurons of rostral ventrolateral medulla exhibit pacemaker properties in the presence of a glutamate-receptor antagonist. Brain Res. 1988 Jan 12;438(1-2):23–40. doi: 10.1016/0006-8993(88)91320-0. [DOI] [PubMed] [Google Scholar]
- Sun M. K., Young B. S., Hackett J. T., Guyenet P. G. Reticulospinal pacemaker neurons of the rat rostral ventrolateral medulla with putative sympathoexcitatory function: an intracellular study in vitro. Brain Res. 1988 Mar 1;442(2):229–239. doi: 10.1016/0006-8993(88)91508-9. [DOI] [PubMed] [Google Scholar]
- Sun M. K., Young B. S., Hackett J. T., Guyenet P. G. Rostral ventrolateral medullary neurons with intrinsic pacemaker properties are not catecholaminergic. Brain Res. 1988 Jun 7;451(1-2):345–349. doi: 10.1016/0006-8993(88)90781-0. [DOI] [PubMed] [Google Scholar]
- TORVIK A. Afferent connections to the sensory trigeminal nuclei, the nucleus of the solitary tract and adjacent structures; an experimental study in the rat. J Comp Neurol. 1956 Nov;106(1):51–141. doi: 10.1002/cne.901060104. [DOI] [PubMed] [Google Scholar]
- Zemlan F. P., Leonard C. M., Kow L. M., Pfaff D. W. Ascending tracts of the lateral columns of the rat spinal cord: a study using the silver impregnation and horseradish peroxidase techniques. Exp Neurol. 1978 Nov;62(2):298–334. doi: 10.1016/0014-4886(78)90059-6. [DOI] [PubMed] [Google Scholar]


