Abstract
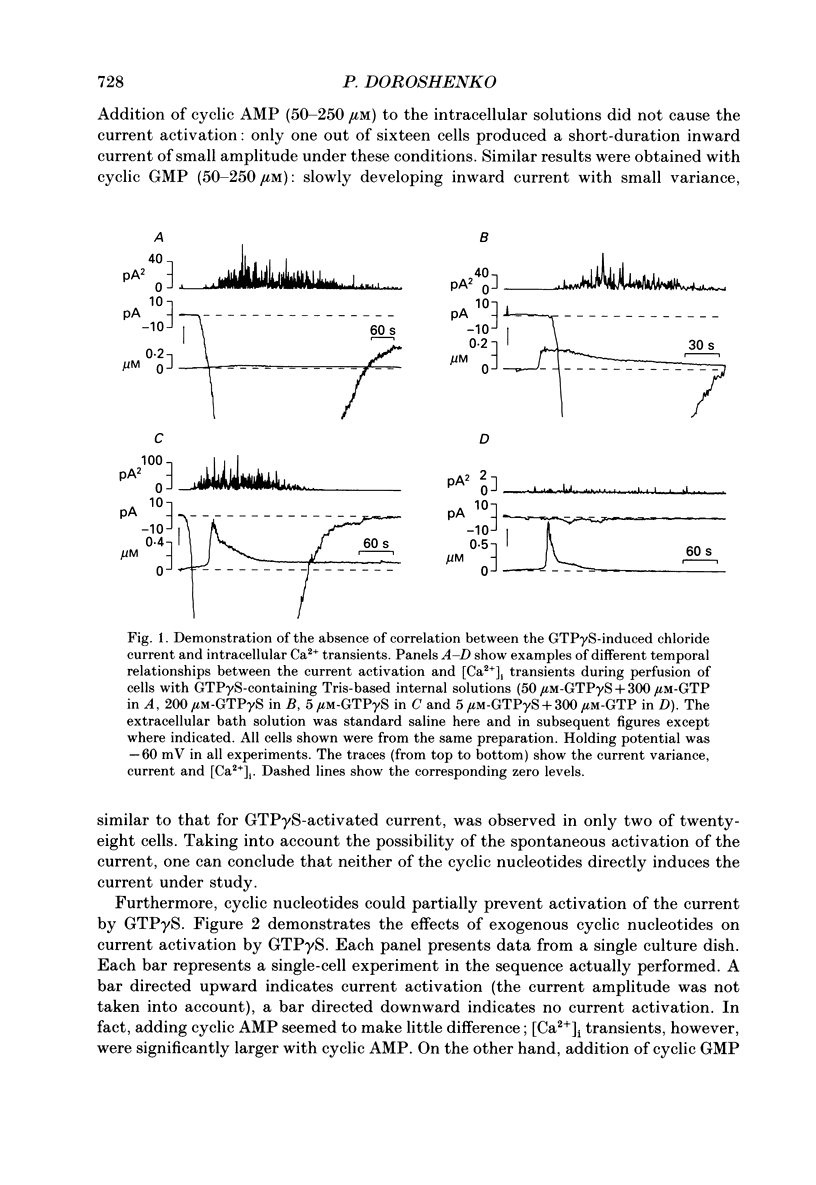
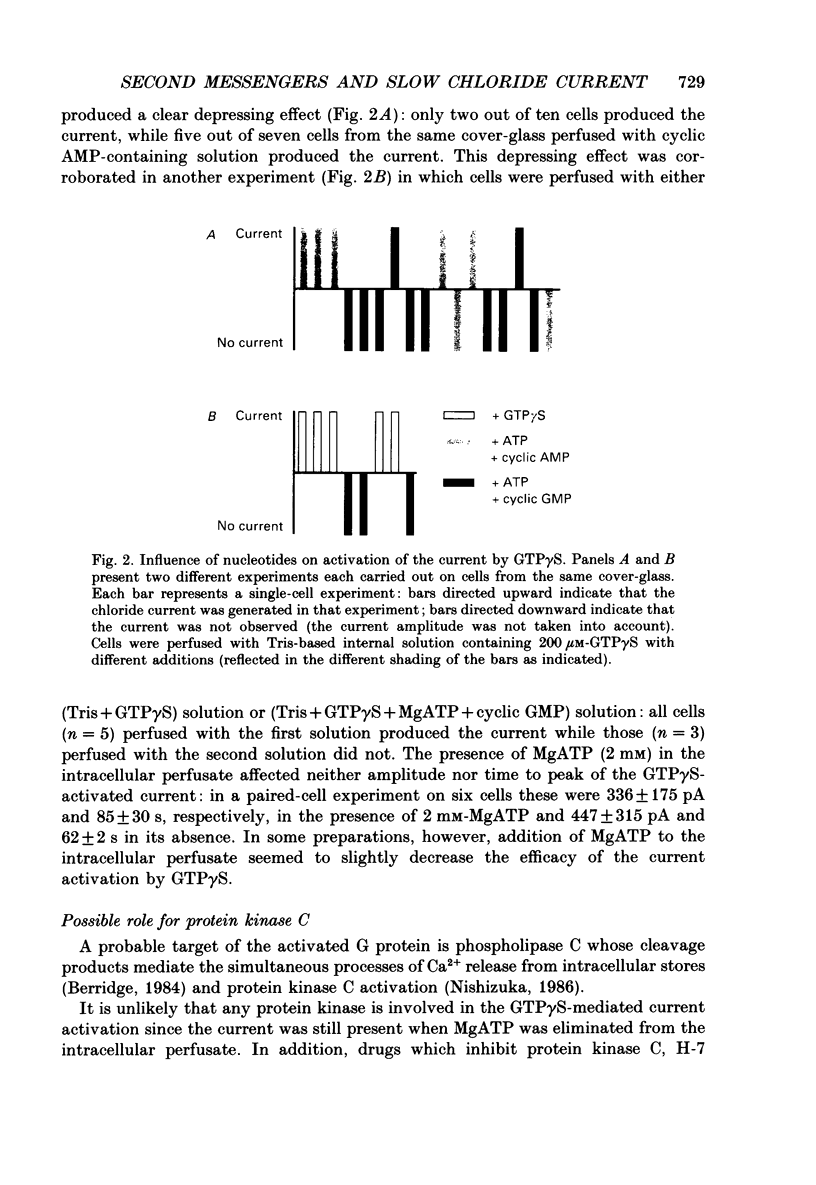
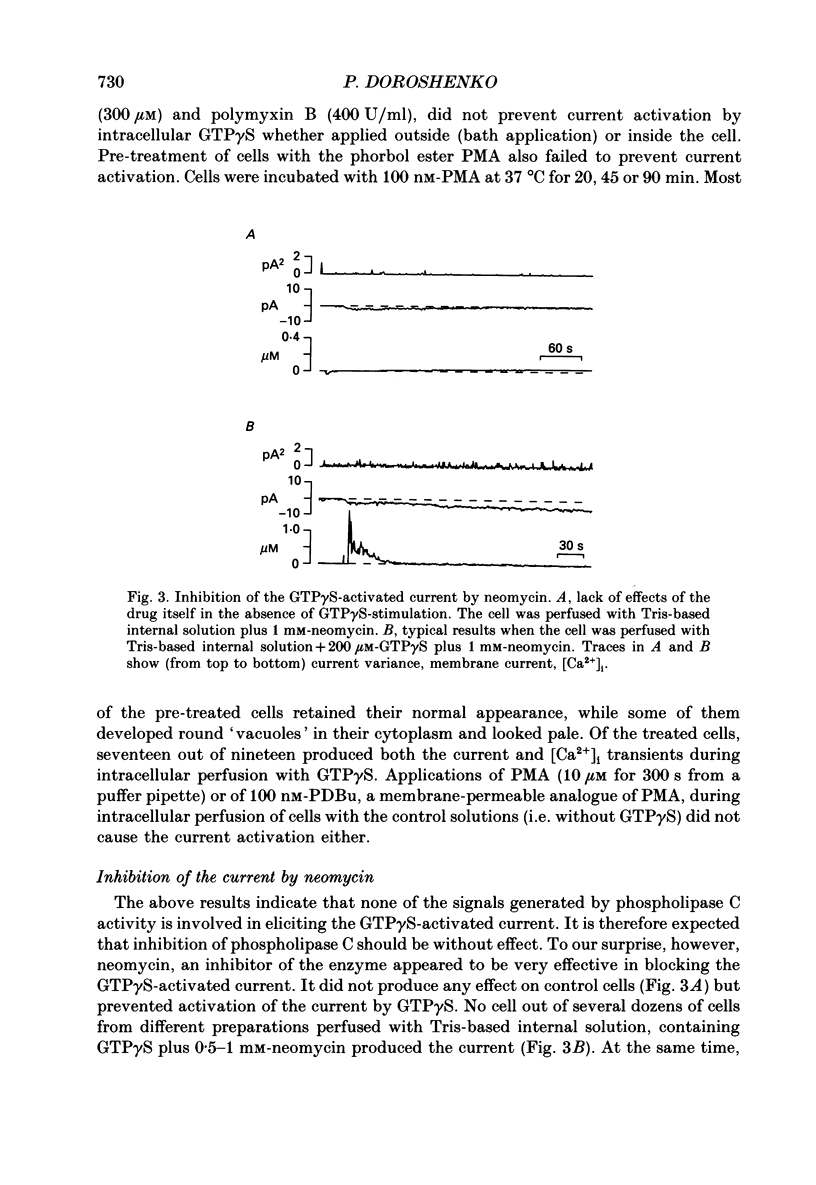
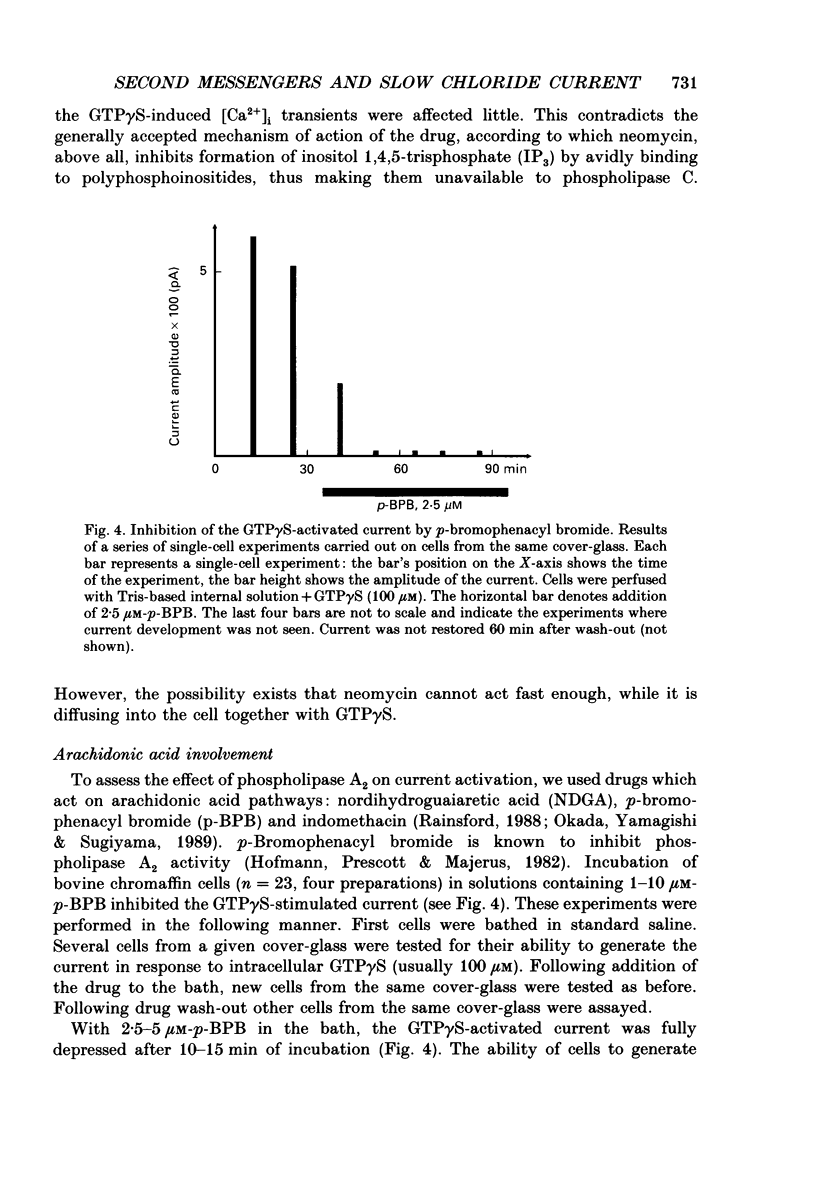
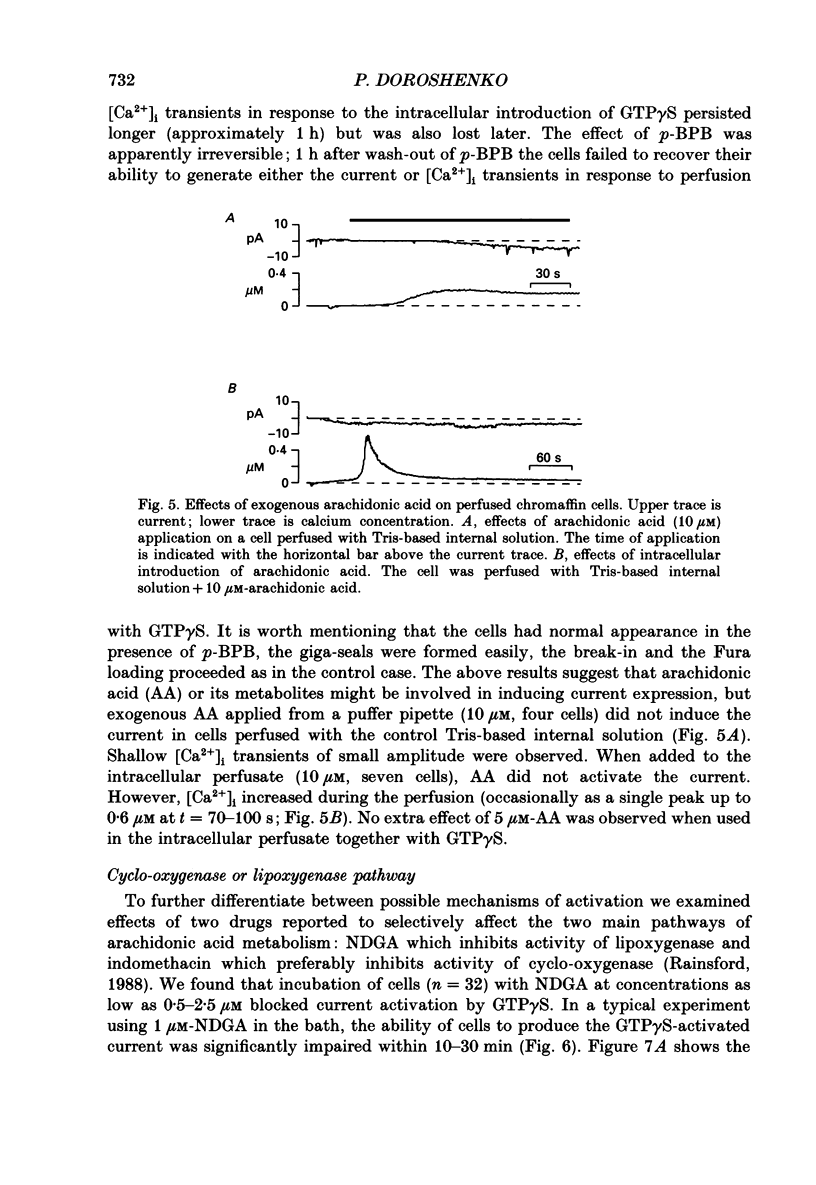
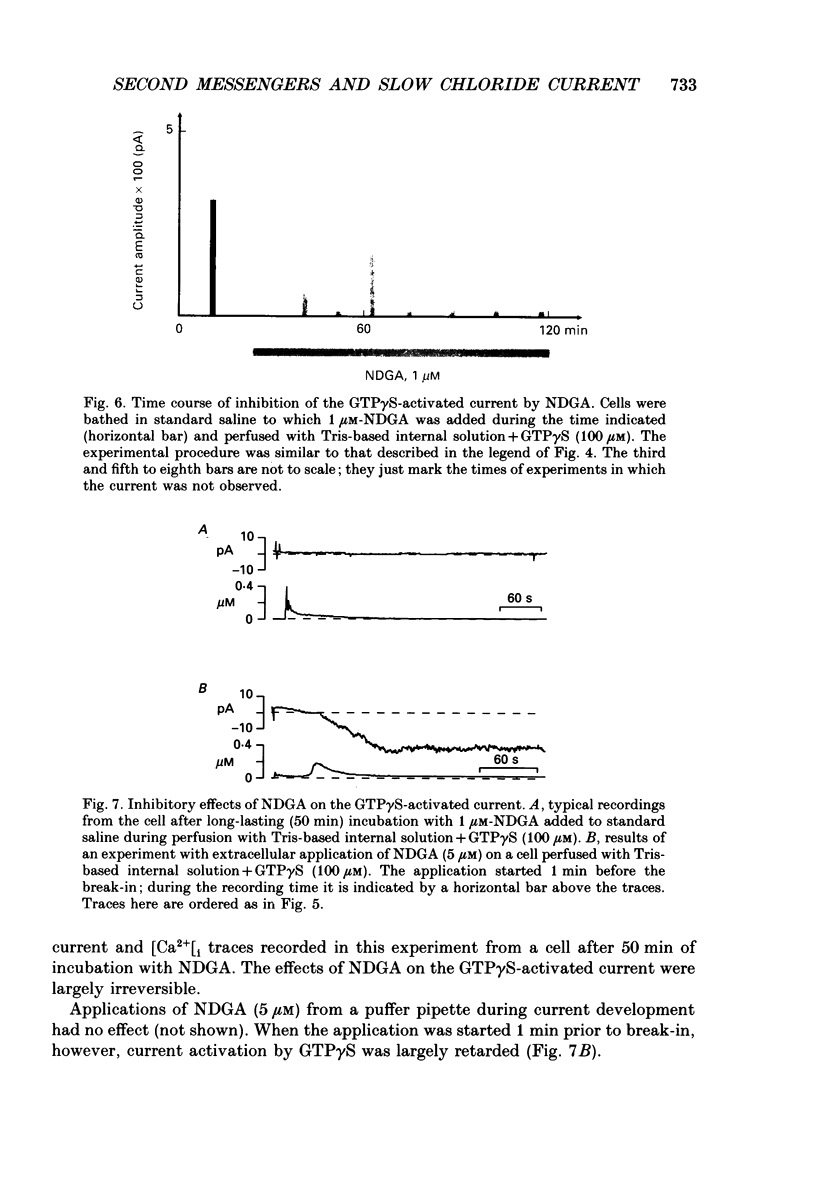
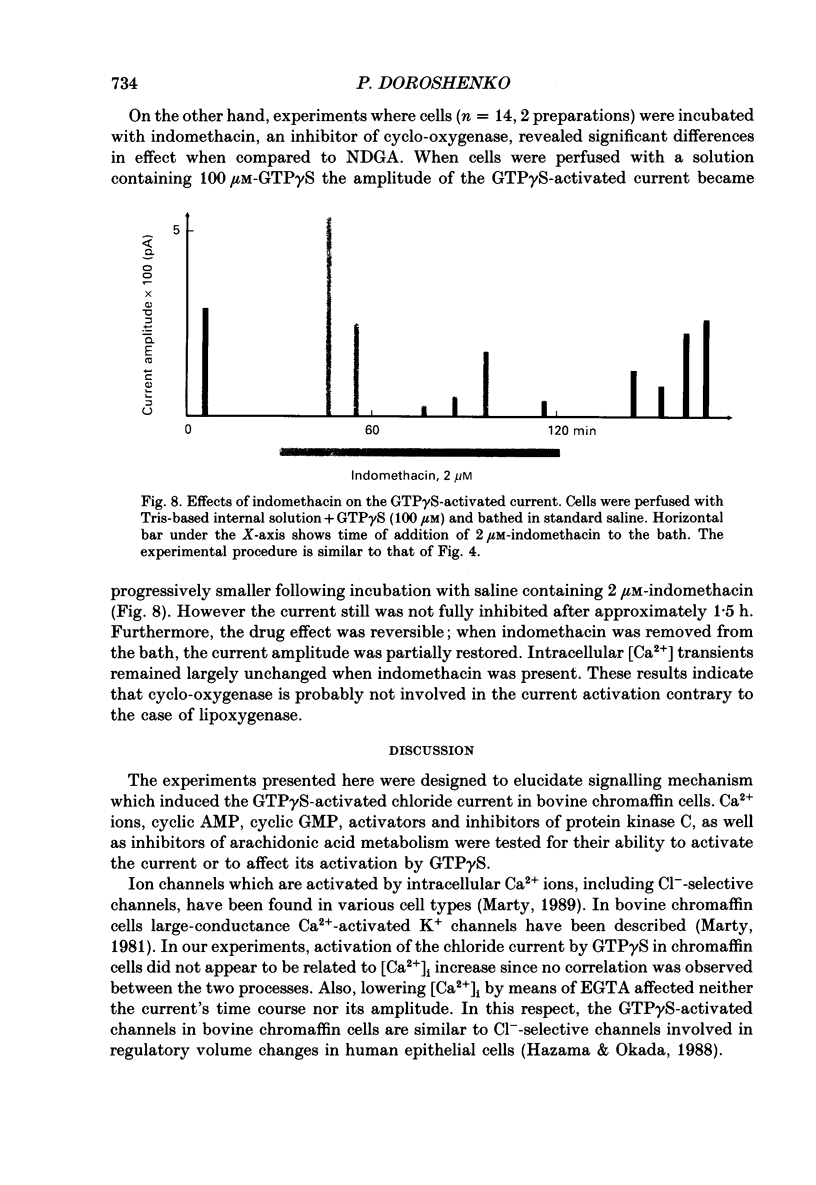
1. Intracellular mechanisms and second messengers involved in chloride current activation by intracellular GTP gamma S (guanosine 5'-O-(3-thiotriphosphate] in bovine chromaffin cells were studied using the whole-cell patch-clamp technique combined with measurements of intracellular calcium [Ca2+]i. 2. No correlation between the time of current activation and the appearance of [Ca2+]i transients was observed; intracellular introduction of sufficient EGTA (10 mM) to suppress the [Ca2+]i transients did not affect the current activation by GTP gamma S. 3. The cyclic nucleotides, cyclic AMP or cyclic GMP, did not activate the current when introduced intracellularly (50-250 microM). The ability of GTP gamma S to activate the current decreased when cyclic GMP (250 microM), together with MgATP (2 mM), was added to the perfusate. 4. Neomycin (0.5-1 mM), a presumed inhibitor of phospholipase C effectively prevented the current activation by GTP gamma S but it did not prevent [Ca2+]i transients. 5. Modulation of protein kinase C activity using specific inhibitors (H-7, 300 microM; polymyxin B, 400 U/ml) or activators (phorbol ester PMA, 100 nM, 20-90 min at 37 degrees C) did not affect the current activation by GTP gamma S nor did it cause current activation in the absence of GTP gamma S. 6. Activation of the current by GTP gamma S could be prevented by incubating the cells for 10-15 min with 2.5 microM p-bromophenacyl bromide (p-BPB), an inhibitor of phospholipase A2 activity. Exogenous arachidonic acid (5-10 microM), applied extracellularly or intracellularly, neither activated the current itself nor did it interfere with its activation by GTP gamma S. 7. Activation of the current by GTP gamma S could also be prevented by incubating the cells with 1 microM-nordihydroguaiaretic acid (NDGA), an inhibitor of lipoxygenase, but not with indomethacin (2 microM), an inhibitor of cyclo-oxygenase pathway of arachidonic acid metabolism. 8. It is suggested that chloride current activation by GTP gamma S in bovine chromaffin cells involves G protein-mediated stimulation of phospholipase A2 activity and subsequent formation of lipoxygenase product(s) of arachidonic acid metabolism.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldenhoff J. B., Hofmeier G., Lux H. D., Swandulla D. Stimulation of a sodium influx by cAMP in Helix neurons. Brain Res. 1983 Oct 16;276(2):289–296. doi: 10.1016/0006-8993(83)90736-9. [DOI] [PubMed] [Google Scholar]
- Belardetti F., Siegelbaum S. A. Up- and down-modulation of single K+ channel function by distinct second messengers. Trends Neurosci. 1988 May;11(5):232–238. doi: 10.1016/0166-2236(88)90132-4. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune K., Rainsford K. D., Wagner K., Peskar B. A. Inhibition of anti-inflammatory drugs of prostaglandin production in cultured macrophages. Naunyn Schmiedebergs Arch Pharmacol. 1981 Jan;315(3):269–276. doi: 10.1007/BF00499844. [DOI] [PubMed] [Google Scholar]
- Burch R. M., Axelrod J. Dissociation of bradykinin-induced prostaglandin formation from phosphatidylinositol turnover in Swiss 3T3 fibroblasts: evidence for G protein regulation of phospholipase A2. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6374–6378. doi: 10.1073/pnas.84.18.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch R. M., Luini A., Axelrod J. Phospholipase A2 and phospholipase C are activated by distinct GTP-binding proteins in response to alpha 1-adrenergic stimulation in FRTL5 thyroid cells. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7201–7205. doi: 10.1073/pnas.83.19.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan M. D., Lewis R. S. Role of potassium and chloride channels in volume regulation by T lymphocytes. Soc Gen Physiol Ser. 1988;43:281–301. [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Role of guanine nucleotide binding protein in the activation of polyphosphoinositide phosphodiesterase. Nature. 1985 Apr 11;314(6011):534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Stutchfield J. G-proteins, the inositol lipid signalling pathway, and secretion. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):247–265. doi: 10.1098/rstb.1988.0075. [DOI] [PubMed] [Google Scholar]
- Diamant S., Lev-Ari I., Uzielli I., Atlas D. Muscarinic agonists evoke neurotransmitter release: possible roles for phosphatidyl inositol bisphosphate breakdown products in neuromodulation. J Neurochem. 1988 Sep;51(3):795–802. doi: 10.1111/j.1471-4159.1988.tb01814.x. [DOI] [PubMed] [Google Scholar]
- Doroshenko P., Penner R., Neher E. Novel chloride conductance in the membrane of bovine chromaffin cells activated by intracellular GTP gamma S. J Physiol. 1991 May;436:711–724. doi: 10.1113/jphysiol.1991.sp018575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain J. N., Wallace M. A., Wojcikiewicz R. J. Evidence for involvement of guanine nucleotide-binding regulatory proteins in the activation of phospholipases by hormones. FASEB J. 1988 Jul;2(10):2569–2574. doi: 10.1096/fasebj.2.10.2838362. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hazama A., Okada Y. Ca2+ sensitivity of volume-regulatory K+ and Cl- channels in cultured human epithelial cells. J Physiol. 1988 Aug;402:687–702. doi: 10.1113/jphysiol.1988.sp017229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann S. L., Prescott S. M., Majerus P. W. The effects of mepacrine and p-bromophenacyl bromide on arachidonic acid release in human platelets. Arch Biochem Biophys. 1982 Apr 15;215(1):237–244. doi: 10.1016/0003-9861(82)90300-9. [DOI] [PubMed] [Google Scholar]
- Jelsema C. L. Light activation of phospholipase A2 in rod outer segments of bovine retina and its modulation by GTP-binding proteins. J Biol Chem. 1987 Jan 5;262(1):163–168. [PubMed] [Google Scholar]
- Kim D., Clapham D. E. Potassium channels in cardiac cells activated by arachidonic acid and phospholipids. Science. 1989 Jun 9;244(4909):1174–1176. doi: 10.1126/science.2727703. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Ito H., Sugimoto T., Shimizu T., Miki I., Ui M. Arachidonic acid metabolites as intracellular modulators of the G protein-gated cardiac K+ channel. Nature. 1989 Feb 9;337(6207):555–557. doi: 10.1038/337555a0. [DOI] [PubMed] [Google Scholar]
- Lambert I. H. Effect of arachidonic acid, fatty acids, prostaglandins, and leukotrienes on volume regulation in Ehrlich ascites tumor cells. J Membr Biol. 1987;98(3):207–221. doi: 10.1007/BF01871184. [DOI] [PubMed] [Google Scholar]
- Marty A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 1981 Jun 11;291(5815):497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- Marty A. The physiological role of calcium-dependent channels. Trends Neurosci. 1989 Nov;12(11):420–424. doi: 10.1016/0166-2236(89)90090-8. [DOI] [PubMed] [Google Scholar]
- Matthews G., Neher E., Penner R. Chloride conductance activated by external agonists and internal messengers in rat peritoneal mast cells. J Physiol. 1989 Nov;418:131–144. doi: 10.1113/jphysiol.1989.sp017831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt J. E., Taylor C. W., Rubin R. P., Putney J. W., Jr Evidence suggesting that a novel guanine nucleotide regulatory protein couples receptors to phospholipase C in exocrine pancreas. Biochem J. 1986 Jun 1;236(2):337–343. doi: 10.1042/bj2360337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Okada D., Yamagishi S., Sugiyama H. Differential effects of phospholipase inhibitors in long-term potentiation in the rat hippocampal mossy fiber synapses and Schaffer/commissural synapses. Neurosci Lett. 1989 May 22;100(1-3):141–146. doi: 10.1016/0304-3940(89)90674-5. [DOI] [PubMed] [Google Scholar]
- Reid D. G., Gajjar K. A proton and carbon 13 nuclear magnetic resonance study of neomycin B and its interactions with phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 1987 Jun 15;262(17):7967–7972. [PubMed] [Google Scholar]
- Slivka S. R., Insel P. A. Phorbol ester and neomycin dissociate bradykinin receptor-mediated arachidonic acid release and polyphosphoinositide hydrolysis in Madin-Darby canine kidney cells. Evidence that bradykinin mediates noninterdependent activation of phospholipases A2 and C. J Biol Chem. 1988 Oct 15;263(29):14640–14647. [PubMed] [Google Scholar]
- Stoehr S. J., Smolen J. E., Holz R. W., Agranoff B. W. Inositol trisphosphate mobilizes intracellular calcium in permeabilized adrenal chromaffin cells. J Neurochem. 1986 Feb;46(2):637–640. doi: 10.1111/j.1471-4159.1986.tb13014.x. [DOI] [PubMed] [Google Scholar]
- Stryer L., Bourne H. R. G proteins: a family of signal transducers. Annu Rev Cell Biol. 1986;2:391–419. doi: 10.1146/annurev.cb.02.110186.002135. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Neher E., Sakmann B. Rat brain serotonin receptors in Xenopus oocytes are coupled by intracellular calcium to endogenous channels. Proc Natl Acad Sci U S A. 1987 Jul;84(14):5063–5067. doi: 10.1073/pnas.84.14.5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf B. A., Turk J., Sherman W. R., McDaniel M. L. Intracellular Ca2+ mobilization by arachidonic acid. Comparison with myo-inositol 1,4,5-trisphosphate in isolated pancreatic islets. J Biol Chem. 1986 Mar 15;261(8):3501–3511. [PubMed] [Google Scholar]