Abstract
Mastocytosis is characterized by an abnormal accumulation of mast cells (MC) in various organs. In most patients, the disease is driven by the KIT D816V mutation, leading to activation of the KIT receptor and subsequent downstream signaling, including the JAK/STAT pathway. In recent years, KIT-targeting tyrosine kinase inhibitors (TKI) have emerged for the treatment of systemic mastocytosis; however, the overall response rate is often not sufficient. In this study, we investigated whether targeting the JAK/STAT pathway might be a novel treatment approach in mastocytosis. Using human MC lines carrying the KIT D816V mutation and human primary cord blood-derived MC, we examined the effects of different JAK inhibitors. Our findings revealed that the JAK inhibitors fedratinib and gandotinib decreased viability, reduced proliferation, and induced apoptosis in KIT D816V-positive MC lines (HMC-1.2 and ROSAKIT D816V). In contrast, ruxolitinib, baricitinib, upadacitinib and abrocitinib failed to affect MC functions. Combinatorial treatment with fedratinib, gandotinib and the two TKI avapritinib and midostaurin was more effective than treatment with TKI alone. Fedratinib also induced apoptosis and enhanced the efficacy of TKI in primary cord blood-derived MC. These results indicate that fedratinib and gandotinib, but not the other JAK inhibitors used in this study, can suppress viability and induce apoptosis in KIT D816V-mutant and KIT WT MC and increase effects of TKI. These findings suggest to explore fedratinib and gandotinib as novel treatment option in mastocytosis.
Keywords: Avapritinib, fedratinib, JAK inhibitors, KIT mutation, KIT D816V, mast cells, mastocytosis, midostaurin, tryptase, tyrosine kinase inhibitors
Introduction
Mastocytosis is a clonal disease characterized by abnormal expansion and accumulation of neoplastic mast cells (MC) affecting various organs, such as the skin, bone marrow (BM) and gastrointestinal tract [1]. The classification of mastocytosis encompasses cutaneous mastocytosis, systemic mastocytosis (SM) and MC sarcoma. SM can be subdivided according to symptoms and prognosis into non-advanced SM, which includes indolent SM (ISM), BM mastocytosis (BMM) and smoldering SM (SSM), and advanced SM, which comprises aggressive SM (ASM), SM with an associated hematologic neoplasm (SM-AHN) and mast cell leukemia (MCL) [2,3]. Patients with non-advanced forms suffer predominantly from MC mediator-related symptoms, whereas patients with advanced SM develop organ dysfunction and show a progressive course with poor prognosis [4,5].
Regardless of the subtype, more than 90% of SM cases are driven by the KIT D816V mutation [6]. In addition, several non-KIT somatic mutations, such as SRSF2, ASXL1, RUNX1 and others, can contribute to the pathophysiology of SM and are usually associated with more advanced forms of the disease and poor prognosis [7]. Treatment of SM includes tyrosine kinase inhibitors (TKI) like the KIT D816V-targeting TKI avapritinib [8,9] and the multikinase inhibitor midostaurin [10,11]. Furthermore, several clinical trials testing alternative KIT D816V-targeting TKI for the treatment of SM are currently ongoing [12,13]. Allogeneic hematopoietic stem cell transplantation (ASCT) is the only potentially curative option; however, the advantages of ASCT in the era of modern KIT inhibitors remain to be defined [14,15]. Despite these treatment options, many patients with SM are still not treated sufficiently or develop side effects, therefore, identifying novel therapeutic strategies for mastocytosis is of particular importance [5,16].
The KIT D816V mutation leads to enhanced proliferation and survival of neoplastic MC by stem cell factor (SCF)-independent activation of the KIT receptor. This, in turn, activates several downstream signaling pathways such as Janus kinase (JAK)/signal transducers and activators of transcription (STAT), phosphatydylinositol triphosphate (PI3)-kinase, protein kinase C (PKC), Ras/mitogen-activated protein kinase (MAPK), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and mammalian target of rapamycin (mTOR) [17-24].
The JAK-STAT pathway is a highly conserved signaling network. It comprises four JAK (JAK1, JAK2, JAK3 and Tyk2), and seven STAT proteins (STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b and STAT6) [25]. Dysregulation of the JAK/STAT pathway is associated with various diseases, especially cancers, allergic and autoimmune diseases. Therefore, several JAK inhibitors were developed, and are currently used in the therapy of atopic dermatitis, autoimmune diseases, myeloproliferative neoplasms and infectious diseases [26]. In diseases characterized by a hyperactivated JAK/STAT pathway, elevated proinflammatory cytokines in the serum were shown to respond well to JAK inhibitors. Also, JAK inhibitors were found to suppress MC activation and growth, suggesting their potential use in the setting of SM [27,28]. Considering the high efficacy of JAK inhibitors in a wide range of diseases and their relatively favorable safety profile, we sought to investigate their potential in the treatment of mastocytosis.
In the current study, we analyzed the impact of different JAK inhibitors in KIT D816V-mutant human MC lines and primary human MC. We also explored the effect of combinatorial treatments with JAK inhibitors and TKI in these preclinical cell models of mastocytosis.
Material and methods
MC lines
The human MC lines HMC-1.1 (carrying the KIT V560G mutation) and HMC-1.2 (carrying both KIT V560G and KIT D816V mutations) were kindly provided by Dr. J. H. Butterfield (Mayo Clinic, Rochester, MN) [29]. They were maintained in Iscove’s modified Dulbecco’s medium (IMDM, Sigma-Aldrich, Irvine, United Kingdom) supplemented with 10% heat-inactivated fetal calf serum (FCS), 1% (vol/vol) penicillin-streptomycin (BioConcept, Allschwil, Switzerland) and 1.2 mM 1-thioglycerol (Sigma-Aldrich, Irvine, United Kingdom). The human MC lines ROSAKIT D816V and ROSAKIT WT cells were generated and kept as previously described [30]. In brief, ROSAKIT D816V were cultured in IMDM supplemented with 10% FCS and 1% (vol/vol) penicillin-streptomycin. ROSAKIT WT and MCPV-1.1 [31], kindly provided by Prof. Dr. Peter Valent (Vienna, Austria), cells were cultured in IMDM supplemented with 10% FCS, 1% (vol/vol) penicillin-streptomycin and 10% SCF-containing supernatants of Chinese hamster ovary cells transfected with the murine scf (kl) gene (CHO-Kl). CHO-Kl cells were kindly provided by Dr. Patrice Dubreuil (Marseille, France) [32]. Cells were expanded in DMEM enriched with 10% (vol/vol) FCS for the collection of SCF-containing supernatant, which was filtered via 0.22 µM syringe filters and stored at -20°C. Cells were tested for mycoplasma contamination by conventional PCR using the following specific primers: forward (GGG AGC AAA CAG GAT TAG ATA CCC T) and reverse (TGC ACC ATC TGT CAC TCT GTT AAC CTC).
Generation of human cord blood-derived MC
The generation of human primary cord blood-derived MC (CBMC) was performed as described previously [33]. Briefly, human CD34+ hematopoietic progenitor cells were purified from cord blood mononuclear cells by density gradient separation (Density Diluent Medium mixed with High Density Spin Medium) (pluriSelect, Leipzig, Germany) followed by magnetic separation with CD34 MicroBead Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions, and after informed consent and institutional review board approval. Isolated CD34+ cells were cultured in serum-free medium (SFEM, STEMCELLTM Technologies, Vancouver, BC) supplemented with penicillin (100 units/mL), streptomycin (100 μg/mL), SCF-containing medium (3% vol/vol), interleukin-6 (IL-6, 50 ng/mL; PeproTech, Rocky Hill, USA) and interleukin-3 (IL-3, 10 ng/mL; PeproTech) for four weeks. Thereafter, cells were cultured for additional four to eight weeks in IMDM supplemented with insulin-transferrin-selenium, 2-mercaptoethanol, GlutaMAX-I (all from Gibco, New York, NY), penicillin-streptomycin, SCF (100 ng/mL) and IL-6 (50 ng/mL). Cell purity was confirmed by flow cytometry using anti-CD117 and -FcεRI antibodies (Table S1) on a CytoFLEX flow cytometry analyzer (Beckman Coulter, California, US).
JAK inhibitors and TKI
The JAK inhibitors ruxolitinib (INCB018424), baricitinib (LY3009104), upadacitinib (ABT-494), abrocitinib (PF-04965842), fedratinib (TG-101348) and gandotinib (LY2784544) and the TKI avapritinib (BLU-285) and midostaurin (PKC-412) were all purchased from MedChemExpress (Monmouth Junction, NJ, US). Ruxolitinib and baricitinib are known to target JAK1 and JAK2 (JAK1/2 inhibitors), while upadacitinib and abrocitinib predominantly target JAK1 (JAK1 inhibitors) and fedratinib and gandotinib predominantly JAK2 (JAK2 inhibitors).
XTT viability assay
HMC-1.2 and ROSAKIT D816V cells were seeded at a density of 3.5 × 105 cells/mL and treated with the indicated substances for 48 hours. After treatment, cell viability was determined with the CyQUANT XTT cell viability assay (ThermoFisher Scientific, Waltham, Massachusetts, US) following the manufacturer’s protocol. Cell viability was detected by measuring absorbance at 450 nm and 660 nm (SynergyH1 Hybrid Reader, BioTek, Vermont, US) and normalized to DMSO-treated cells. IC50 for inhibitors were calculated by non-linear regression analysis (Prism 9.0).
Proliferation assay
HMC-1.2 and ROSAKIT D816V cells were seeded at a density of 105/mL and treated with the indicated substances for 48 hours. After treatment, cells were incubated with EdU (5-ethynyl-2’-deoxyuridine, EdU FC 488 Kit from Baseclick, Munich, Germany) for 90 minutes, followed by fixation and permeabilization. The click reaction with 6-FAM azide (488 nm) was performed according to the manufacturer’s instructions. Proliferating cells (defined as EdU-positive cells) were evaluated by flow cytometry using a CytoFLEX flow cytometry analyzer.
Apoptosis assay
HMC-1.2 and ROSAKIT D816V cells were seeded at a density of 3.5 × 105 cells/mL and treated with the indicated substances for 48 hours. After treatment, cells were stained with the FITC Annexin V Apoptosis Detection Kit (BioLegend, San Diego, CA, US) according to the manufacturer’s instructions, and analyzed by flow cytometry as described before. CBMC were seeded at a density of 5 × 105 cells/mL at week 8 of cultivation and treated with the indicated substances for 48 hours. After treatment, cells were incubated with antibodies against CD117 and FcεRI, then with annexin V and 7-AAD antibodies according to the manufacturer’s instructions, and finally analyzed by flow cytometry. MC were identified as CD117+ FcεRI+. Apoptotic MC were identified as annexin V- and 7-AAD-positive cells within the MC population (CD117+ FcεRI+ cells).
Western blotting
Technical details are described in the ‘Methods’ Section of the Supplementary Materials.
Statistical analysis
Results are shown as mean ± SEM. Statistical significance was assessed by one-way ANOVA with Tukey post-hoc multiple comparison test or two-way ANOVA with Šidák post-hoc multiple comparison test (Prism 9.0). P values <0.05 were considered significant. No samples were excluded from the analysis.
Results
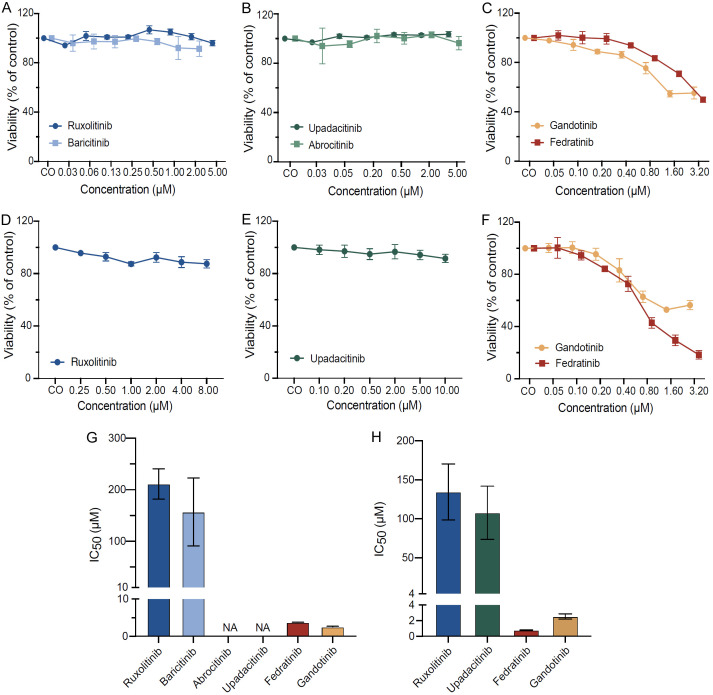
Fedratinib and gandotinib decrease viability in human neoplastic MC lines
To investigate the overall impact of JAK inhibitors on functions of human neoplastic MC, we conducted a pharmacological screening using the human neoplastic MC line HMC-1.2, which carries the KIT D816V and KIT V560G mutations (Figure 1A-C) [29]. First, we assessed the viability of HMC-1.2 cells after the treatment with various JAK inhibitors, including those predominantly targeting JAK1 (upadacitinib, abrocitinib), JAK2 (fedratinib, gandotinib) or both JAK1/2 (ruxolitinib, baricitinib). Upadacitinib, abrocitinib, ruxolitinib and baricitinib failed to affect MC viability (Figure 1A and 1B, respectively). In contrast, fedratinib and gandotinib decreased viability of HMC-1.2 (Figure 1C), with IC50 values of 3.74 µM for fedratinib and 2.65 µM for gandotinib (Figure 1G).
Figure 1.
Fedratinib and gandotinib decrease viability in human neoplastic MC lines. HMC-1.2 (A-C) and ROSAKIT D816V cells (D-F) were incubated in DMSO control medium (CO) or various concentrations of ruxolitinib, baricitinib (A, D), abrocitinib, upadacitinib (B, E), fedratinib and gandotinib (C, F) for 48 h. MC viability was evaluated by CyQUANT™ XTT Cell Viability Assay and normalized to DMSO-treated cells. IC50 of JAK inhibitors in HMC-1.2 (G) and ROSAKIT D816V cells (H) were calculated by nonlinear regression analysis. Results represent the mean and SEM of three to five independent experiments.
To validate these findings, the same set of experiments was performed using a second human MC line carrying the KIT D816V mutation, ROSAKIT D816V (Figure 1D-F). Similarly, in ROSAKIT D816V cells, ruxolitinib (Figure 1D) and upadacitinib (Figure 1E) did not affect MC viability at concentrations up to 8-10 µM, whereas fedratinib and gandotinib decreased viability with IC50 values of 0.8 µM for fedratinib and 2.32 µM for gandotinib (Figure 1F and 1H).
In the human MC lines HMC-1.1 and MCPV-1.1, a decrease in MC viability was observed only with fedratinib and gandotinib, consistent with the results obtained for the KIT D816V-carrying MC lines (data not shown). In ROSAKIT WT cells, fedratinib and gandotinib exhibited 15-25 times lower IC50 compared to the other JAK inhibitors (data not shown).
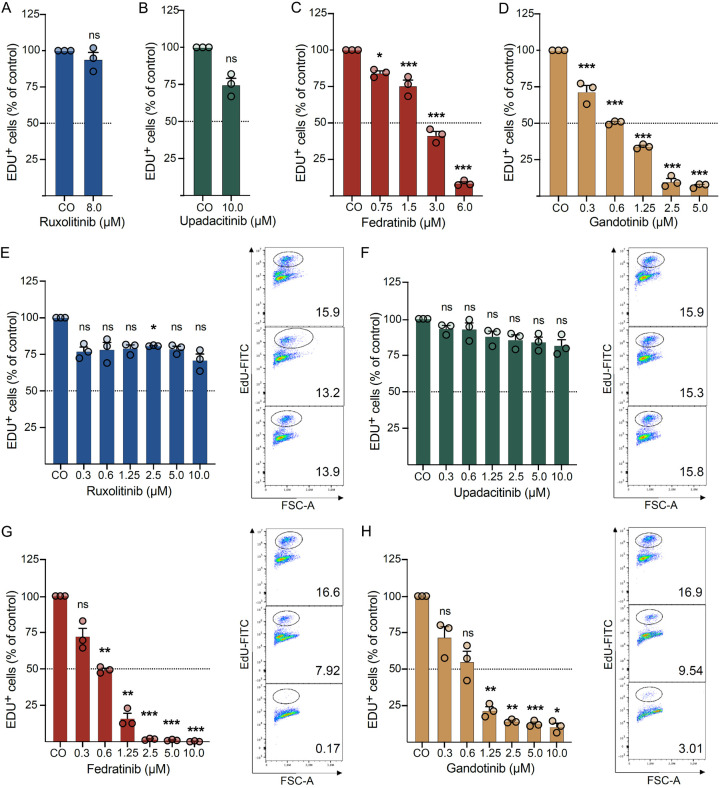
Fedratinib and gandotinib decrease proliferation in human neoplastic MC lines
In line with what was observed for viability, ruxolitinib (Figure 2A and 2E) and upadacitinib (Figure 2B and 2F) failed to affect proliferation in the two MC lines HMC-1.2 and ROSAKIT D816V up to 8-10 µM concentrations. On the contrary, fedratinib and gandotinib demonstrated dose-dependent inhibition of proliferation in both HMC-1.2 (Figure 2C, 2D) and ROSAKIT D816V (Figure 2G, 2H) cells. In HMC-1.2 cells, a significant reduction in proliferation was observed already at 0.75 µM for fedratinib and 0.3 µM for gandotinib, while in ROSAKIT D816V cells, significant anti-proliferative effects were observed at 0.6 µM for fedratinib and 1.25 µM for gandotinib.
Figure 2.
Fedratinib and gandotinib decrease proliferation in human neoplastic MC lines. HMC-1.2 (A-D) and ROSAKIT D816V (E-H) cells were incubated in a DMSO control medium (CO) or various concentrations of ruxolitinib (A, E), upadacitinib (B, F), fedratinib (C, G) and gandotinib (D, H) for 48 h. Proliferating cells were considered as EdU+ (Edu-FITC) and normalized to DMSO-treated cells. Results represent the mean and SEM of three independent experiments. *P<0.05, **P<0.01, ***P<0.001. (E-H) Right panel. Representative flow cytometry plots for corresponding JAK inhibitors are also shown (from top to bottom): CO, 0.6 µM, 2.5 µM.
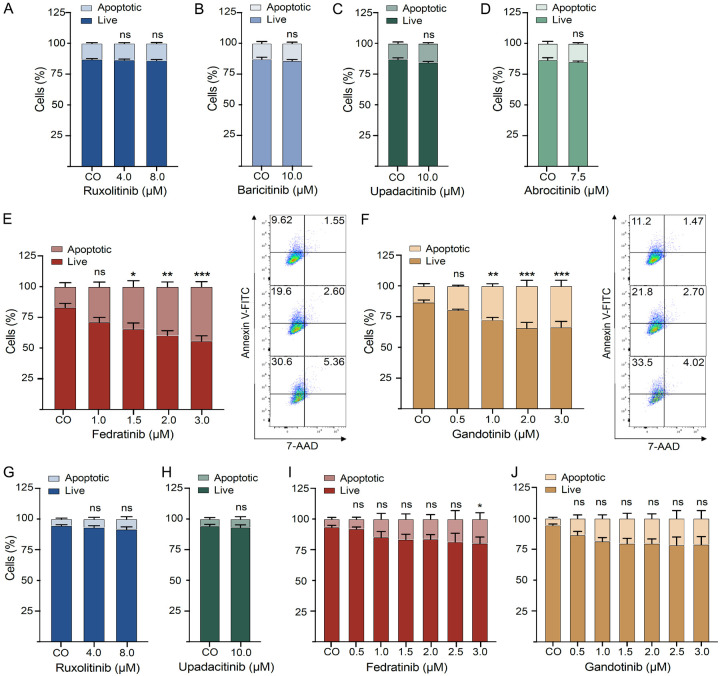
Fedratinib and gandotinib induce apoptosis in human neoplastic MC lines
To evaluate how JAK inhibitors affect the survival of human neoplastic MC, the number of apoptotic cells was evaluated in HMC-1.2 cells after treatment with different JAK inhibitors (Figure 3A-F). Ruxolitinib, baricitinib, upadacitinib and abrocitinib (Figure 3A-D) failed to affect apoptosis of HMC-1.2 cells up to concentrations of 7.5-10 µM. By contrast, fedratinib and gandotinib significantly induced apoptosis in a dose-dependent manner at pharmacologically meaningful concentrations (1.5 µM for fedratinib and 1.0 µM for gandotinib), as evidenced by flow cytometry (Figure 3E, 3F).
Figure 3.
Fedratinib and gandotinib induce apoptosis in human neoplastic MC lines. HMC-1.2 (A-F) and ROSAKIT D816V (G-J) cells were incubated in a DMSO control medium (CO) or various concentrations of ruxolitinib (A, G), baricitinib (B), upadacitinib (C, H), abrocitinib (D), fedratinib (E, I) and gandotinib (F, J) for 48 h. Apoptotic cells were defined as annexin+ and annexin+7-AAD+ cells by flow cytometry as described in ‘Materials and Methods’. Results represent the mean and SEM of three to five independent experiments. *P<0.05, **P<0.01, ***P<0.001. Representative flow cytometry plots for corresponding JAK inhibitors are shown (from top to bottom): (E) CO, 1.5 µM, 3.0 µM; (F) CO, 1.0 µM, 3.0 µM.
In ROSAKIT D816V cells, apoptosis-inducing activity was less prominent, but also reached statistical significance in cells treated with fedratinib at a concentration of 3 µM (Figure 3I). Notably, ruxolitinib (Figure 3G) and upadacitinib (Figure 3H) failed to exert any impact on MC survival. Although treatment with gandotinib showed a slight increase in the apoptotic cell fraction, this effect was not statistically significant (Figure 3J).
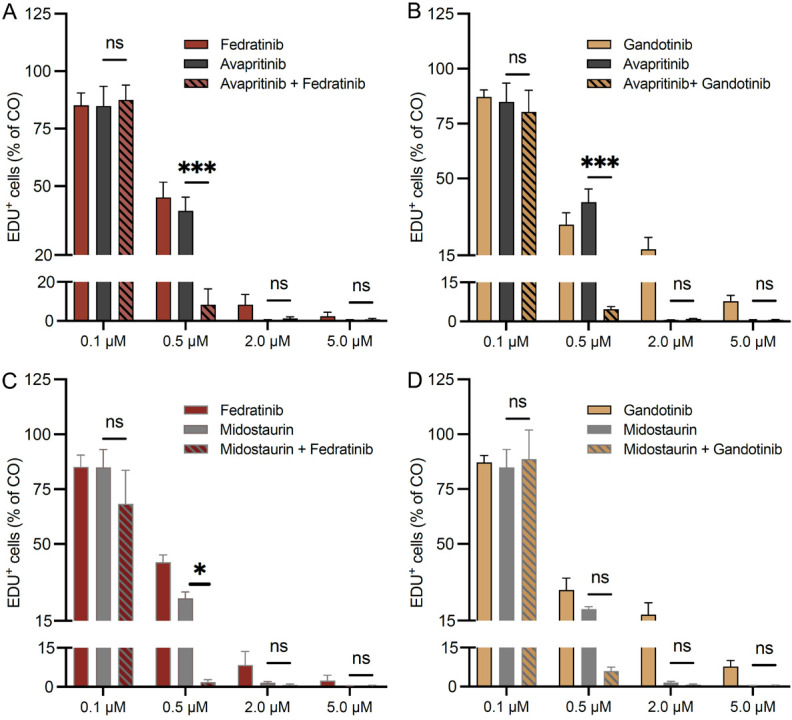
Fedratinib and gandotinib enhance the viability-reducing effect of TKI in human neoplastic MC lines
In mastocytosis, especially in advanced forms, the use of drug combinations may be an effective approach to increase the response rate to treatment, overcome resistance to TKI and minimize side effects. Currently, two TKI, namely the KIT D816V-selective inhibitor avapritinib and the multikinase inhibitor midostaurin, are used in clinical routine for the treatment of SM. Therefore, we explored the combination of TKI with fedratinib and gandotinib (Figure 4), since the latter were found to impact viability, proliferation, and apoptosis of neoplastic MC in single-treatment experiments (Figures 1, 2 and 3).
Figure 4.
Fedratinib and gandotinib enhance the viability-reducing effect of TKI in human neoplastic MC lines. HMC-1.2 (A-D) and ROSAKIT D816V (E-H) cells were incubated in a DMSO control medium, various concentrations of the TKI avapritinib (A, C, E, G) and midostaurin (B, D, F, H), either alone or in combination with fedratinib (A, B, E, F) and gandotinib (C, D, G, H) for 48 h. MC viability was evaluated by CyQUANT™ XTT Cell Viability Assay and normalized to the DMSO control medium. Results represent the mean and SEM of three to five independent experiments. As ROSAKIT D816V cells did not respond to avapritinib (E, G), the two-way ANOVA statistical test was performed between the JAK inhibitor group and their combinations with avapritinib. *P<0.05, **P<0.01, ***P<0.001.
In HMC-1.2 cells, avapritinib in combination with either fedratinib (Figure 4A) or gandotinib (Figure 4C) was significantly more efficient in reducing MC viability at a concentration of 0.5 µM compared to single treatment with avapritinib. Similarly, the combinatorial treatment of midostaurin with fedratinib at concentrations of 0.2 µM and 0.5 µM was significantly more effective in reducing MC viability compared to single treatment with midostaurin (Figure 4B). The combination of midostaurin with gandotinib did not demonstrate a significant effect on viability compared to single treatment with midostaurin (Figure 4D).
ROSAKIT D816V cells did not respond to single treatment with avapritinib, at least at concentrations up to 2 µM (Figure 4E and 4G). However, when avapritinib was combined with gandotinib, cell viability was significantly reduced compared to single treatment with gandotinib (Figure 4G). Combinations of midostaurin with either fedratinib (Figure 4F) or gandotinib (Figure 4H) did not show an additional decrease in viability of ROSAKIT D816V cells compared to single treatment.
Fedratinib and gandotinib enhance the proliferation-inhibiting effect of TKI in human neoplastic MC lines
Given the previous finding that fedratinib and gandotinib increased the effect of TKI on viability of MC lines, we next tested whether combined JAK and KIT targeting had a similar effect on MC proliferation (Figure 5). Here, we found that fedratinib (Figure 5A) and gandotinib (Figure 5B) both showed cooperative effects with avapritinib on proliferation in HMC-1.2 cells, with a statistically significant decrease of proliferation at a concentration of 0.5 µM, compared to single treatment with avapritinib. Similarly, combining fedratinib with midostaurin significantly reduced proliferation at 0.5 µM compared to single treatment with midostaurin (Figure 5C), while the combination of midostaurin with gandotinib did not demonstrate a significant effect on proliferation (Figure 5D).
Figure 5.
Fedratinib and gandotinib enhance the growth-inhibitory effect of TKI in human neoplastic MC lines. HMC-1.2 cells were incubated in a DMSO control medium or various concentrations of avapritinib (A, B) and midostaurin (C, D), alone and in combination with fedratinib and gandotinib, for 48 h. Proliferating cells were considered as EdU+ and normalized to DMSO control as described in the ‘Materials and Methods’ section. Results represent the mean and SEM of three independent experiments. *P<0.05, **P<0.01, ***P<0.001.
Fedratinib inhibits phosphorylation of signaling molecules downstream of KIT in human neoplastic MC lines
To explore the mechanism of decreased MC functions in response to JAK inhibitors, we next performed Western blotting to analyze the expression of several signaling molecules downstream of KIT, including STAT5, STAT3 and AKT, after incubation with fedratinib, avapritinib and combined fedratinib and avapritinib (Figure S1). In these experiments, we observed that the KIT D816V-targeting TKI avapritinib, as expected, suppressed phosphorylation of pKIT in both HMC-1.2 (Figure S1A) and ROSAKIT D816V (Figure S1B) cells. In addition, avapritinib decreased phosphorylation of pSTAT5 and pSTAT3 in HMC-1.2, but not in ROSAKIT D816V cells. Fedratinib was also found to reduce phosphorylation of pSTAT5 and pSTAT3 in both cell lines. Furthermore, combinatorial treatment with fedratinib and avapritinib decreased phosphorylation of pKIT, pSTAT5 and pSTAT3 in both cell lines and pAKT in HMC-1.2 cells.
Fedratinib enhances apoptosis induced by TKI in human cord blood-derived MC
To further expand our findings, we analyzed the effect of various JAK inhibitors, TKI and their combination on apoptosis of primary human MC (Figure 6). In 8-week cultured CBMC, ruxolitinib (Figure 6A, 6B) and upadacitinib (Figure 6C, 6D) did not affect the number of apoptotic cells. The TKI avapritinib (Figure 6A, 6C, 6E) and midostaurin (Figure 6B, 6D, 6F) both induced apoptosis at higher concentrations (1.0 µM) in CBMC. There was no increase in apoptosis when treatments with either ruxolitinib or upadacitinib were combined with TKI. In contrast, treatment with fedratinib showed a trend, albeit not statistically significant, for inducing apoptosis in CBMC (Figure 6E, 6F). Moreover, we observed a significant increase in apoptosis upon combinatorial treatment with fedratinib and midostaurin at 1.0 µM in CBMC (Figure 6F).
Figure 6.
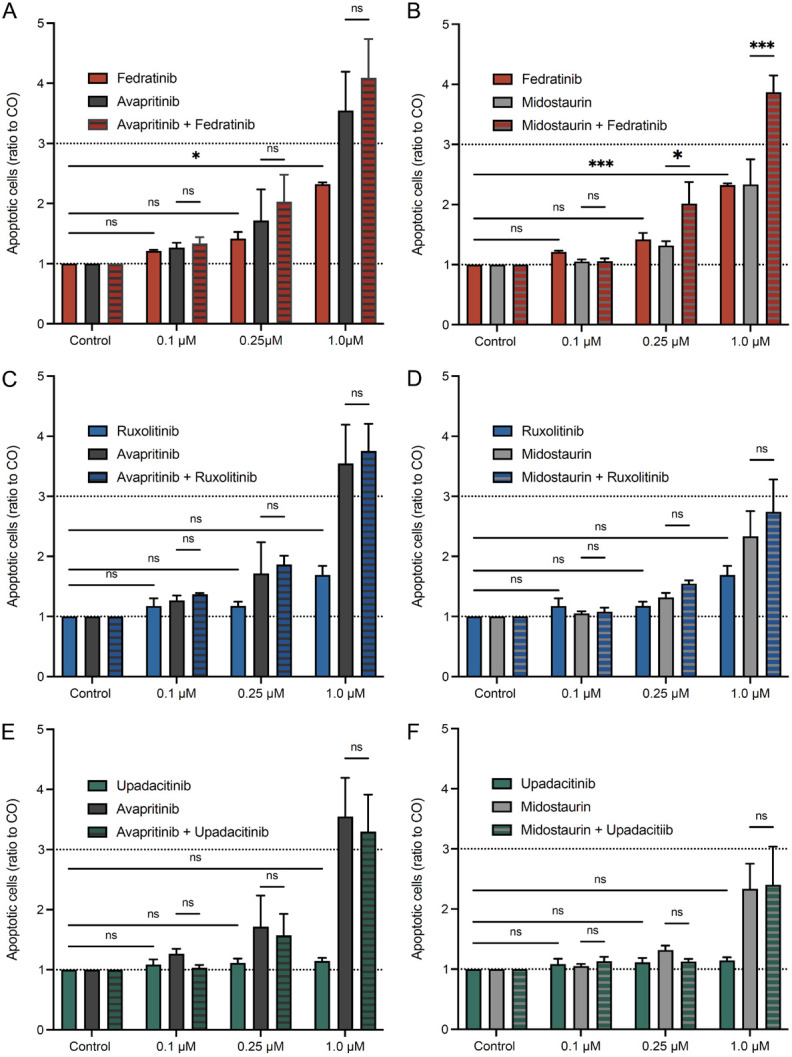
Fedratinib induces apoptosis and increases the effect of the TKI midostaurin in human CBMC. Primary human CBMC, cultured for eight weeks, were incubated in DMSO control medium or medium containing various concentrations of ruxolitinib (A, B), upadacitinib (C, D), fedratinib (E, F), avapritinib (A, C, E) and midostaurin (B, D, F) as well as combinations of JAK inhibitors and TKI for 48 h. CBMC were identified as CD117+FceRI+ cells, and apoptotic CBMC were defined as annexin+ and annexin+7-AAD+ cells by flow cytometry, as described in the section ‘Materials and Methods’ of this manuscript. Results represent the mean and SEM of three independent experiments. *P<0.05, **P<0.01, ***P<0.001.
Discussion
In the present study, we show that fedratinib and gandotinib, but not the other JAK inhibitors tested in our study (ruxolitinib, baricitinib, upadacitinib and abrocitinib), reduce viability and proliferation and induce apoptosis in human KIT D816V-mutant MC lines. We also found that fedratinib and gandotinib induce apoptosis in primary human CBMC. Furthermore, we demonstrate that fedratinib and gandotinib enhance the anti-proliferative and cytotoxic effects of TKI, both in human neoplastic MC lines as well as in CBMC. These findings provide a rationale for exploring the potential of JAK inhibitors, especially fedratinib and gandotinib, in MC-associated diseases. In particular, our data on KIT D816V-mutant MC suggest the potential of using these JAK inhibitors, either alone or in combination with TKI, in the treatment of mastocytosis.
Numerous studies have shown antineoplastic effects of JAK inhibitors in various cancer cell lines (summarized e.g. by Qureshy et al.) [34]. JAK1 inhibitors (AZD4205, itacitinib, filgotinib), JAK1/2 inhibitors (ruxolitinib, momelotinib) as well as JAK2 inhibitors (fedratinib, pacritinib, WP1066) have been found to have growth-inhibitory effects in preclinical solid tumor models. Furthermore, fedratinib and ruxolitinib have demonstrated antineoplastic effects in myeloproliferative neoplasms (MPN) in both preclinical models and clinical trials, and are currently FDA-approved for the treatment of MPN patients [34]. Emerging data suggest that JAK inhibitors increase the efficacy of chemotherapeutic and other targeted agents, mostly by preventing STAT3 activation, which is involved in chemotherapy insensitivity [35]. In addition, Mathew and colleagues described that itacitinib improved clinical and immune responses to anti-PD1 treatment via promotion of CD8 T cell plasticity and therapeutic responses of exhausted and effector memory - like T cell clonotypes [36].
To date, suppressive effects of JAK inhibitors have furthermore been demonstrated in various types of cells secreting pro-inflammatory cytokines and involved in the pathogenesis of allergic and autoimmune diseases. Nyireida et al. showed that tofacitinib, ruxolitinib and AG490 (tyrphostin) decreased the production of proinflammatory cytokines by cytokine-activated T cells (Tck) and Tck cell-activated macrophages. De Vries et al. found that tofacitinib and a JAK1 inhibitor affected macrophage activation and skewed polarization towards M2-like macrophages [37]. In addition, Kurowski et al. showed that the JAK1/2 inhibitor baricitinib suppressed basophil activation and degranulation in vitro [38].
However, there are only a few studies that investigated the impact of JAK inhibitors on MC functions. Hermans et al. showed that ruxolitinib does not have a cytotoxic effect on HMC-1.2 cells and the human MC line LAD2 at concentrations up to 50 µM. On the other hand, they observed that ruxolitinib inhibited codeine- and substance P-induced degranulation and the production of IL-6, TNF-α and MCP-1 in the two MC lines [28]. Tobio et al. demonstrated that the KIT D816V mutation is associated with increased secretion of IL-6 in both cells from mastocytosis patients and HMC-1 cells, and that ruxolitinib and fedratinib were able to inhibit the IL-6 production from HMC-1.2 cells [28,39]. Furthermore, Lasho et al. reported that fedratinib was able to reduce phosphorylation of proteins downstream of JAK/STAT in HMC-1.1 and HMC-1.2 cells and that fedratinib acted synergistically with the TKI dasatinib to inhibit proliferation of HMC-1.2 cells, however, the effect of fedratinib on other cellular functions and on primary MC was not explored [27]. In addition, Keller et al. found that several JAK inhibitors, including fedratinib, decreased proliferation and induced apoptosis in canine KIT-mutant mastocytoma cell lines [40].
Taken together, these studies indicate that JAK inhibitors may not only exert direct cytostatic and cytotoxic effects on human neoplastic MC, but could also have prominent anti-inflammatory effects in mastocytosis. Patients with SM are known to show constitutive activation of functionally impaired blood monocytes and increased plasma levels of IL-1β, IL-6, IL-8, TNFα and IL-10 secreted by blood monocytes, indicating a broad activation of the innate immune response [41]. Recently, an altered distribution of leukocyte subsets and a proinflammatory proteome were described in two cohorts of patients with ISM [42]. Furthermore, increased plasma levels of IL-6 in patients with ISM were found to be associated with a high risk of later developing advanced forms of SM [43]. It was also demonstrated that TNF promotes the expansion of neoplastic MC via suppression of normal myeloid cells, and high levels of TNF correlated with inferior survival in SM [44]. Thus, patients with mastocytosis, particularly those with advanced SM, are also characterized by inflammation, which could be targeted by JAK inhibitors. In line, a recent study showed that the JAK2 inhibitor AG490 reduced TNF-induced IL-18 bioactivity by blocking caspase-1 in fibroblasts [45]; and IL-18 was found to also play an important role in the generation and maturation of MC and basophils from murine bone marrow progenitors [46].
Data on the usage of JAK inhibitors for the treatment of mastocytosis are limited to the description of a few clinical cases. In one case of ASM associated with myelofibrosis and a rare KIT K509I mutation, ruxolitinib was added to imatinib for better control of constitutional symptoms [47]. Another clinical report described a patient with SM-AHN (SM with refractory cytopenia with multilineage dysplasia) without KIT D816V mutation that achieved a good response upon ruxolitinib treatment and successfully underwent allogeneic stem cell transplantation [48]. However, in both cases, ruxolitinib was used off-label for the treatment of KIT D816V-negative forms of SM and never in combination with TKI. To our knowledge, previous pre-clinical studies mainly focused on ruxolitinib, while studies with other JAK inhibitors are very limited. As a result, the potential effects of other JAK inhibitors may have been overlooked, and they were therefore not considered for either single-agent or combinatorial treatment approaches in patients with mastocytosis. Moreover, topical JAK inhibitors have been demonstrated to be effective and relatively safe for treating various skin diseases such as atopic dermatitis [49-51] and vitiligo [52]. However, their use has not been explored yet in cutaneous mastocytosis, where they might have potential, particularly in the treatment of mastocytoma.
In summary, our data show that fedratinib and gandotinib inhibit viability and proliferation and induce apoptosis in neoplastic MC, when used alone and in combination with TKI currently used for the therapy of SM. Hence, our findings suggest to explore whether this treatment is beneficial in patients with SM. In such study, it will also be of particular interest to investigate the anti-inflammatory potential of JAK inhibition, in addition to their direct cytotoxic effects on neoplastic MC, as a proinflammatory phenotype is associated with disease progression and poor survival in patients with SM.
Acknowledgements
AM is funded in part by the EU-H2020-MSCA-COFUND EURIdoc programme (No. 101034170). KH received research support from the Swiss National Science Foundation (SNSF; grant 310030_207705), the Swiss Cancer Research Foundation (grant KFS-5979-08-2023) and the EU-H2020-MSCA-COFUND EURIdoc programme (No. 101034170).
Disclosure of conflict of interest
MA has consulted for and received honoraria from AB Science, Blueprint Medicines and Thermo Fisher. KH has consulted for and received honoraria from ALK, Allergopharma, BioCryst, Blueprint, Cogent, Galderma, KalVista, Leo, Menarini, Novartis, Pfizer, Sanofi, Takeda and Thermo Fisher.
Abbreviations
- advSM
advanced systemic mastocytosis
- ASCT
allogeneic hematopoietic stem cell transplantation
- ASM
aggressive systemic mastocytosis
- BM
bone marrow
- BMM
bone marrow mastocytosis
- BSA
bovine serum albumin
- CBMC
cord blood-derived mast cells
- FCS
fetal calf serum
- FcεRI
high-affinity receptor for IgE
- ISM
indolent systemic mastocytosis
- MC
mast cells
- MCL
mast cell leukemia
- MNC
mononuclear cells
- MPN
myeloproliferative neoplasms
- SCF
stem cell factor
- SM
systemic mastocytosis
- SM-AHN
systemic mastocytosis with an associated hematologic neoplasm
- SSM
smoldering systemic mastocytosis
- TKI
tyrosine kinase inhibitors
Supporting Information
References
- 1.Valent P, Akin C, Hartmann K, Alvarez-Twose I, Brockow K, Hermine O, Niedoszytko M, Schwaab J, Lyons JJ, Carter MC, Elberink HO, Butterfield JH, George TI, Greiner G, Ustun C, Bonadonna P, Sotlar K, Nilsson G, Jawhar M, Siebenhaar F, Broesby-Olsen S, Yavuz S, Zanotti R, Lange M, Nedoszytko B, Hoermann G, Castells M, Radia DH, Muñoz-Gonzalez JI, Sperr WR, Triggiani M, Kluin-Nelemans HC, Galli SJ, Schwartz LB, Reiter A, Orfao A, Gotlib J, Arock M, Horny HP, Metcalfe DD. Updated diagnostic criteria and classification of mast cell disorders: a consensus proposal. Hemasphere. 2021;5:e646. doi: 10.1097/HS9.0000000000000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, Bejar R, Berti E, Busque L, Chan JKC, Chen W, Chen X, Chng WJ, Choi JK, Colmenero I, Coupland SE, Cross NCP, De Jong D, Elghetany MT, Takahashi E, Emile JF, Ferry J, Fogelstrand L, Fontenay M, Germing U, Gujral S, Haferlach T, Harrison C, Hodge JC, Hu S, Jansen JH, Kanagal-Shamanna R, Kantarjian HM, Kratz CP, Li XQ, Lim MS, Loeb K, Loghavi S, Marcogliese A, Meshinchi S, Michaels P, Naresh KN, Natkunam Y, Nejati R, Ott G, Padron E, Patel KP, Patkar N, Picarsic J, Platzbecker U, Roberts I, Schuh A, Sewell W, Siebert R, Tembhare P, Tyner J, Verstovsek S, Wang W, Wood B, Xiao W, Yeung C, Hochhaus A. The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36:1703–1719. doi: 10.1038/s41375-022-01613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, Wang SA, Bagg A, Barbui T, Branford S, Bueso-Ramos CE, Cortes JE, Dal Cin P, DiNardo CD, Dombret H, Duncavage EJ, Ebert BL, Estey EH, Facchetti F, Foucar K, Gangat N, Gianelli U, Godley LA, Gökbuget N, Gotlib J, Hellström-Lindberg E, Hobbs GS, Hoffman R, Jabbour EJ, Kiladjian JJ, Larson RA, Le Beau MM, Loh ML, Löwenberg B, Macintyre E, Malcovati L, Mullighan CG, Niemeyer C, Odenike OM, Ogawa S, Orfao A, Papaemmanuil E, Passamonti F, Porkka K, Pui CH, Radich JP, Reiter A, Rozman M, Rudelius M, Savona MR, Schiffer CA, Schmitt-Graeff A, Shimamura A, Sierra J, Stock WA, Stone RM, Tallman MS, Thiele J, Tien HF, Tzankov A, Vannucchi AM, Vyas P, Wei AH, Weinberg OK, Wierzbowska A, Cazzola M, Döhner H, Tefferi A. International consensus classification of myeloid neoplasms and acute leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140:1200–1228. doi: 10.1182/blood.2022015850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valent P, Akin C, Sperr WR, Horny HP, Arock M, Metcalfe DD, Galli SJ. New insights into the pathogenesis of mastocytosis: emerging concepts in diagnosis and therapy. Annu Rev Pathol. 2023;18:361–386. doi: 10.1146/annurev-pathmechdis-031521-042618. [DOI] [PubMed] [Google Scholar]
- 5.Pardanani A. Systemic mastocytosis in adults: 2023 update on diagnosis, risk stratification and management. Am J Hematol. 2023;98:1097–1116. doi: 10.1002/ajh.26962. [DOI] [PubMed] [Google Scholar]
- 6.Furitsu T, Tsujimura T, Tono T, Ikeda H, Kitayama H, Koshimizu U, Sugahara H, Butterfield JH, Ashman LK, Kanayama Y, et al. Identification of mutations in the coding sequence of the proto-oncogene c-kit in a human mast cell leukemia cell line causing ligand-independent activation of c-kit product. J Clin Invest. 1993;92:1736–1744. doi: 10.1172/JCI116761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoermann G, Sotlar K, Jawhar M, Kristensen T, Bachelot G, Nedoszytko B, Carter MC, Horny HP, Bonadonna P, Sperr WR, Hartmann K, Brockow K, Lyons JJ, Kluin-Nelemans HC, Hermine O, Akin C, Broesby-Olsen S, Triggiani M, Butterfield JH, Schwaab J, Reiter A, Gotlib J, Metcalfe DD, George TI, Orfao A, Valent P, Arock M. Standards of genetic testing in the diagnosis and prognostication of systemic mastocytosis in 2022: recommendations of the EU-US cooperative group. J Allergy Clin Immunol Pract. 2022;10:1953–1963. doi: 10.1016/j.jaip.2022.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Gotlib J, Reiter A, DeAngelo DJ. Avapritinib for advanced systemic mastocytosis. Blood. 2022;140:1667–1673. doi: 10.1182/blood.2021014612. [DOI] [PubMed] [Google Scholar]
- 9.Gotlib J, Castells M, Elberink HO, Siebenhaar F, Hartmann K, Broesby-Olsen S, George TI, Panse J, Alvarez-Twose I, Radia DH, Tashi T, Bulai Livideanu C, Sabato V, Heaney M, Van Daele P, Cerquozzi S, Dybedal I, Reiter A, Pongdee T, Barete S, Ustun C, Schwartz L, Ward BR, Schafhausen P, Vadas P, Bose P, DeAngelo DJ, Rein L, Vachhani P, Triggiani M, Bonadonna P, Rafferty M, Butt NM, Oh ST, Wortmann F, Ungerstedt J, Guilarte M, Taparia M, Kuykendall AT, Arana Yi C, Ogbogu P, Gaudy-Marqueste C, Mattsson M, Shomali W, Giannetti MP, Bidollari I, Lin HM, Sulllivan E, Mar B, Scherber R, Roche M, Akin C, Maurer M. Avapritinib versus placebo in indolent systemic mastocytosis. NEJM Evid. 2023;2:EVIDoa2200339. doi: 10.1056/EVIDoa2200339. [DOI] [PubMed] [Google Scholar]
- 10.Gotlib J, Kluin-Nelemans HC, George TI, Akin C, Sotlar K, Hermine O, Awan FT, Hexner E, Mauro MJ, Sternberg DW, Villeneuve M, Huntsman Labed A, Stanek EJ, Hartmann K, Horny HP, Valent P, Reiter A. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N Engl J Med. 2016;374:2530–2541. doi: 10.1056/NEJMoa1513098. [DOI] [PubMed] [Google Scholar]
- 11.Hartmann K, Gotlib J, Akin C, Hermine O, Awan FT, Hexner E, Mauro MJ, Menssen HD, Redhu S, Knoll S, Sotlar K, George TI, Horny HP, Valent P, Reiter A, Kluin-Nelemans HC. Midostaurin improves quality of life and mediator-related symptoms in advanced systemic mastocytosis. J Allergy Clin Immunol. 2020;146:356–366. e354. doi: 10.1016/j.jaci.2020.03.044. [DOI] [PubMed] [Google Scholar]
- 12.DeAngelo DJ, Pullarkat V, Piris-Villaespesa M, George TI, Patel JL, Ustun C, Bose P, Heaney ML, Pilla A, Massaro M, Exter B, Jolin HA, Mikhak Z, Tashi T. P1049: a phase 2 study of bezuclastinib (CGT9486), a novel, highly selective, potent KIT D816V inhibitor, in adults with advanced systemic mastocytosis (APEX): methods, baseline data, and early insights. Hemasphere. 2022;6(Suppl):939–940. [Google Scholar]
- 13.Tashi T, Hermine O, Castells M, Guilarte M, Sabato V, Maurer M, Panse J, Alvarez-Twose I, Cabral R, Bird R, Barete S, Bouillet L, Hermans M, Van Daele P, González-De-Olano D, Griffiths EA, Jurcic J, Dybedal I, Damaj GL, Schafhausen P, Elena C, Teh TC, Vachhani P, Labe C, Venugopal S, Kevin He, Muñoz-González J, Lampson B, Scherber R, Bose P, Grattan C, Pongdee T, George TI, Livideanu CB. Elenestinib, an investigational, next generation KIT D816V inhibitor, reduces mast cell burden, improves symptoms, and has a favorable safety profile in patients with indolent systemic mastocytosis: analysis of the harbor trial. Blood. 2023;142(Suppl 1):76. [Google Scholar]
- 14.Ustun C, Reiter A, Scott BL, Nakamura R, Damaj G, Kreil S, Shanley R, Hogan WJ, Perales MA, Shore T, Baurmann H, Stuart R, Gruhn B, Doubek M, Hsu JW, Tholouli E, Gromke T, Godley LA, Pagano L, Gilman A, Wagner EM, Shwayder T, Bornhäuser M, Papadopoulos EB, Böhm A, Vercellotti G, Van Lint MT, Schmid C, Rabitsch W, Pullarkat V, Legrand F, Yakoub-Agha I, Saber W, Barrett J, Hermine O, Hagglund H, Sperr WR, Popat U, Alyea EP, Devine S, Deeg HJ, Weisdorf D, Akin C, Valent P. Hematopoietic stem-cell transplantation for advanced systemic mastocytosis. J. Clin. Oncol. 2014;32:3264–3274. doi: 10.1200/JCO.2014.55.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ustun C, Gotlib J, Popat U, Artz A, Litzow M, Reiter A, Nakamura R, Kluin-Nelemans HC, Verstovsek S, Gajewski J, Perales MA, George T, Shore T, Sperr W, Saber W, Kota V, Yavuz AS, Pullarkat V, Rogosheske J, Hogan W, Van Besien K, Hagglund H, Damaj G, Arock M, Horny HP, Metcalfe DD, Deeg HJ, Devine S, Weisdorf D, Akin C, Valent P. Consensus opinion on allogeneic hematopoietic cell transplantation in advanced systemic mastocytosis. Biol Blood Marrow Transplant. 2016;22:1348–1356. doi: 10.1016/j.bbmt.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Gotlib J. Available and emerging therapies for bona fide advanced systemic mastocytosis and primary eosinophilic neoplasms. Hematology Am Soc Hematol Educ Program. 2022;2022:34–46. doi: 10.1182/hematology.2022000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chian R, Young S, Danilkovitch-Miagkova A, Rönnstrand L, Leonard E, Ferrao P, Ashman L, Linnekin D. Phosphatidylinositol 3 kinase contributes to the transformation of hematopoietic cells by the D816V c-Kit mutant. Blood. 2001;98:1365–1373. doi: 10.1182/blood.v98.5.1365. [DOI] [PubMed] [Google Scholar]
- 18.Harir N, Boudot C, Friedbichler K, Sonneck K, Kondo R, Martin-Lannerée S, Kenner L, Kerenyi M, Yahiaoui S, Gouilleux-Gruart V, Gondry J, Bénit L, Dusanter-Fourt I, Lassoued K, Valent P, Moriggl R, Gouilleux F. Oncogenic Kit controls neoplastic mast cell growth through a Stat5/PI3-kinase signaling cascade. Blood. 2008;112:2463–2473. doi: 10.1182/blood-2007-09-115477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka A, Konno M, Muto S, Kambe N, Morii E, Nakahata T, Itai A, Matsuda H. A novel NF-kappaB inhibitor, IMD-0354, suppresses neoplastic proliferation of human mast cells with constitutively activated c-kit receptors. Blood. 2005;105:2324–2331. doi: 10.1182/blood-2004-08-3247. [DOI] [PubMed] [Google Scholar]
- 20.Smrz D, Kim MS, Zhang S, Mock BA, Smrzová S, DuBois W, Simakova O, Maric I, Wilson TM, Metcalfe DD, Gilfillan AM. mTORC1 and mTORC2 differentially regulate homeostasis of neoplastic and non-neoplastic human mast cells. Blood. 2011;118:6803–6813. doi: 10.1182/blood-2011-06-359984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baumgartner C, Cerny-Reiterer S, Sonneck K, Mayerhofer M, Gleixner KV, Fritz R, Kerenyi M, Boudot C, Gouilleux F, Kornfeld JW, Sillaber C, Moriggl R, Valent P. Expression of activated STAT5 in neoplastic mast cells in systemic mastocytosis: subcellular distribution and role of the transforming oncoprotein KIT D816V. Am J Pathol. 2009;175:2416–2429. doi: 10.2353/ajpath.2009.080953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mueller N, Wicklein D, Eisenwort G, Jawhar M, Berger D, Stefanzl G, Greiner G, Boehm A, Kornauth C, Muellauer L, Sehner S, Hoermann G, Sperr WR, Staber PB, Jaeger U, Zuber J, Arock M, Schumacher U, Reiter A, Valent P. CD44 is a RAS/STAT5-regulated invasion receptor that triggers disease expansion in advanced mastocytosis. Blood. 2018;132:1936–1950. doi: 10.1182/blood-2018-02-833582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trieselmann NZ, Soboloff J, Berger SA. Mast cells stimulated by membrane-bound, but not soluble, steel factor are dependent on phospholipase C activation. Cell Mol Life Sci. 2003;60:759–766. doi: 10.1007/s00018-003-2349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnstein BO, Li G, Wang Z, Kennedy S, Chalfant C, Nakajima H, Bunting KD, Ryan JJ. Stat5 expression is required for IgE-mediated mast cell function. J Immunol. 2006;177:3421–3426. doi: 10.4049/jimmunol.177.5.3421. [DOI] [PubMed] [Google Scholar]
- 25.O’Shea JJ, Schwartz DM, Villarino AV, Gadina M, McInnes IB, Laurence A. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311–328. doi: 10.1146/annurev-med-051113-024537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu X, Li J, Fu M, Zhao X, Wang W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther. 2021;6:402. doi: 10.1038/s41392-021-00791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lasho T, Tefferi A, Pardanani A. Inhibition of JAK-STAT signaling by TG101348: a novel mechanism for inhibition of KITD816V-dependent growth in mast cell leukemia cells. Leukemia. 2010;24:1378–80. doi: 10.1038/leu.2010.109. [DOI] [PubMed] [Google Scholar]
- 28.Hermans MAW, Schrijver B, van Holten-Neelen CCPA, Gerth van Wijk R, van Hagen PM, van Daele PLA, Dik WA. The JAK1/JAK2- inhibitor ruxolitinib inhibits mast cell degranulation and cytokine release. Clin Exp Allergy. 2018;48:1412–1420. doi: 10.1111/cea.13217. [DOI] [PubMed] [Google Scholar]
- 29.Butterfield JH, Weiler D, Dewald G, Gleich GJ. Establishment of an immature mast cell line from a patient with mast cell leukemia. Leuk Res. 1988;12:345–355. doi: 10.1016/0145-2126(88)90050-1. [DOI] [PubMed] [Google Scholar]
- 30.Saleh R, Wedeh G, Herrmann H, Bibi S, Cerny-Reiterer S, Sadovnik I, Blatt K, Hadzijusufovic E, Jeanningros S, Blanc C, Legarff-Tavernier M, Chapiro E, Nguyen-Khac F, Subra F, Bonnemye P, Dubreuil P, Desplat V, Merle-Béral H, Willmann M, Rülicke T, Valent P, Arock M. A new human mast cell line expressing a functional IgE receptor converts to tumorigenic growth by KIT D816V transfection. Blood. 2014;124:111–120. doi: 10.1182/blood-2013-10-534685. [DOI] [PubMed] [Google Scholar]
- 31.Hoermann G, Blatt K, Greiner G, Putz EM, Berger A, Herrmann H, Cerny-Reiterer S, Gleixner KV, Walz C, Hoetzenecker K, Müllauer L, Reiter A, Sotlar K, Sexl V, Valent P, Mayerhofer M. CD52 is a molecular target in advanced systemic mastocytosis. FASEB J. 2014;28:3540–3551. doi: 10.1096/fj.14-250894. [DOI] [PubMed] [Google Scholar]
- 32.Tjio JH, Puck TT. Genetics of somatic mammalian cells. II. Chromosomal constitution of cells in tissue culture. J Exp Med. 1958;108:259–68. doi: 10.1084/jem.108.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Folkerts J, Gaudenzio N, Maurer M, Hendriks RW, Stadhouders R, Tam SY, Galli SJ. Rapid identification of human mast cell degranulation regulators using functional genomics coupled to high-resolution confocal microscopy. Nat Protoc. 2020;15:1285–1310. doi: 10.1038/s41596-019-0288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qureshy Z, Johnson DE, Grandis JR. Targeting the JAK/STAT pathway in solid tumors. J Cancer Metastasis Treat. 2020;6:27. [PMC free article] [PubMed] [Google Scholar]
- 35.Mathew D, Marmarelis ME, Foley C, Bauml JM, Ye D, Ghinnagow R, Ngiow SF, Klapholz M, Jun S, Zhang Z, Zorc R, Davis CW, Diehn M, Giles JR, Huang AC, Hwang WT, Zhang NR, Schoenfeld AJ, Carpenter EL, Langer CJ, Wherry EJ, Minn AJ. Combined JAK inhibition and PD-1 immunotherapy for non-small cell lung cancer patients. Science. 2024;384:eadf1329. doi: 10.1126/science.adf1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee HJ, Zhuang G, Cao Y, Du P, Kim HJ, Settleman J. Drug resistance via feedback activation of Stat3 in oncogene-addicted cancer cells. Cancer Cell. 2014;26:207–221. doi: 10.1016/j.ccr.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 37.De Vries LCS, Duarte JM, De Krijger M, Welting O, Van Hamersveld PHP, Van Leeuwen-Hilbers FWM, Moerland PD, Jongejan A, D’Haens GR, De Jonge WJ, Wildenberg ME. A JAK1 selective kinase inhibitor and tofacitinib affect macrophage activation and function. Inflamm Bowel Dis. 2019;25:647–660. doi: 10.1093/ibd/izy364. [DOI] [PubMed] [Google Scholar]
- 38.Peng W, Benfadal S, Yu C, Wenzel J, Oldenburg J, Novak N. JAK1/2 inhibitor but not IL-4 receptor alpha antibody suppresses allergen-mediated activation of human basophils in vitro. Allergy. 2022;77:2253–2256. doi: 10.1111/all.15322. [DOI] [PubMed] [Google Scholar]
- 39.Tobío A, Bandara G, Morris DA, Kim DK, O’Connell MP, Komarow HD, Carter MC, Smrz D, Metcalfe DD, Olivera A. Oncogenic D816V-KIT signaling in mast cells causes persistent IL-6 production. Haematologica. 2020;105:124–135. doi: 10.3324/haematol.2018.212126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keller A, Wingelhofer B, Peter B, Bauer K, Berger D, Gamperl S, Reifinger M, Cerny-Reiterer S, Moriggl R, Willmann M, Valent P, Hadzijusufovic E. The JAK2/STAT5 signaling pathway as a potential therapeutic target in canine mastocytoma. Vet Comp Oncol. 2018;16:55–68. doi: 10.1111/vco.12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pérez-Pons A, Jara-Acevedo M, Henriques A, Navarro-Navarro P, García-Montero AC, Álvarez-Twose I, Pedreira CE, Sánchez-Muñoz L, Damasceno D, Caldas C, Muñoz-González JI, Matito A, Flores-Montero J, González-López O, Criado I, Mayado A, Orfao A. Altered innate immune profile in blood of systemic mastocytosis patients. Clin Transl Allergy. 2022;12:e12167. doi: 10.1002/clt2.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hermans MAW, Heeringa JJ, Swagemakers SGA, Schrijver B, van Daele PLA, van der Spek PJ, van Hagen PM, van Zelm MC, Dik WA. Altered leukocyte subsets and immune proteome indicate proinflammatory mechanisms in mastocytosis. J Allergy Clin Immunol. 2022;150:146–156. e110. doi: 10.1016/j.jaci.2021.12.786. [DOI] [PubMed] [Google Scholar]
- 43.Mayado A, Teodosio C, Garcia-Montero AC, Matito A, Rodriguez-Caballero A, Morgado JM, Muñiz C, Jara-Acevedo M, Álvarez-Twose I, Sanchez-Muñoz L, Matarraz S, Caldas C, Muñoz-González JI, Escribano L, Orfao A. Increased IL6 plasma levels in indolent systemic mastocytosis patients are associated with high risk of disease progression. Leukemia. 2016;30:124–130. doi: 10.1038/leu.2015.176. [DOI] [PubMed] [Google Scholar]
- 44.Greiner G, Witzeneder N, Klein K, Tangermann S, Kodajova P, Jaeger E, Ratzinger F, Gerner MC, Jawhar M, Baumgartner S, Fruehwirth K, Schmetterer KG, Zuber J, Gleixner KV, Mayerhofer M, Schwarzinger I, Simonitsch-Klupp I, Esterbauer H, Baer C, Walter W, Meggendorfer M, Strassl R, Haferlach T, Hartmann K, Kenner L, Sperr WR, Reiter A, Sexl V, Arock M, Valent P, Hoermann G. TNF alpha promotes clonal dominance of KIT D816V+ cells in mastocytosis: role of survivin and impact on prognosis. Blood. 2024;143:1006–1017. doi: 10.1182/blood.2023020515. [DOI] [PubMed] [Google Scholar]
- 45.Marotte H, Tsou PS, Fedorova T, Pinney AJ, Lewis B, Koch AE. Blocking the janus-activated kinase pathway reduces tumor necrosis factor alpha-induced interleukin-18 bioactivity by caspase-1 inhibition. Arthritis Res Ther. 2014;16:R102. doi: 10.1186/ar4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sandersa NL, Venkateshaiah SU, Manohar M, Verma AK, Kandikattu HK, Mishra A. Interleukin-18 has an important role in differentiation and maturation of mucosal mast cells. J Mucosal Immunol Res. 2018;2:109. [PMC free article] [PubMed] [Google Scholar]
- 47.Santos FPS, Helman R, Pereira WO, Puga RD, Nakashima SS, Bello IC, Diniz MS, Hamerschlak N, Campregher PV. Activity of a JAK1/JAK2 inhibitor in a patient with KIT-mutated systemic mastocytosis (SM) associated with myelofibrosis. Blood. 2013;122:5246–5246. [Google Scholar]
- 48.Dowse R, Ibrahim M, McLornan DP, Moonim MT, Harrison CN, Radia DH. Beneficial effects of JAK inhibitor therapy in systemic mastocytosis. Br J Haematol. 2017;176:324–327. doi: 10.1111/bjh.13951. [DOI] [PubMed] [Google Scholar]
- 49.Katoh N, Ohya Y, Murota H, Ikeda M, Hu X, Ikeda K, Liu J, Sasaki T, Chu AD, Teixeira HD, Saeki H. A phase 3 randomized, multicenter, double-blind study to evaluate the safety of upadacitinib in combination with topical corticosteroids in adolescent and adult patients with moderate-to-severe atopic dermatitis in Japan (Rising Up): an interim 24-week analysis. JAAD Int. 2022;6:27–36. doi: 10.1016/j.jdin.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silverberg JI, Simpson EL, Thyssen JP, Gooderham M, Chan G, Feeney C, Biswas P, Valdez H, DiBonaventura M, Nduaka C, Rojo R. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863–873. doi: 10.1001/jamadermatol.2020.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Papp K, Szepietowski JC, Kircik L, Toth D, Eichenfield LF, Forman SB, Kuligowski ME, Kallender H, Sun K, Ren H, Simpson EL. Long-term safety and disease control with ruxolitinib cream in atopic dermatitis: results from two phase 3 studies. J Am Acad Dermatol. 2023;88:1008–1016. doi: 10.1016/j.jaad.2022.09.060. [DOI] [PubMed] [Google Scholar]
- 52.Rosmarin D, Passeron T, Pandya AG, Grimes P, Harris JE, Desai SR, Lebwohl M, Ruer-Mulard M, Seneschal J, Wolkerstorfer A, Kornacki D, Sun K, Butler K, Ezzedine K TRuE-V Study Group. Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. N Engl J Med. 2022;387:1445–1455. doi: 10.1056/NEJMoa2118828. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.