Abstract
Objective: To determine circulating levels of hypoxia-inducible factor-1α (HIF-1α), vascular endothelial growth factor (VEGF), and urokinase-type plasminogen activator (uPA) in the peripheral blood of patients with oral squamous cell carcinoma (OSCC) and to explore their relationship with clinicopathologic features and prognosis, in order to facilitate treatment. Methods: 160 OSCC patients and 51 control subjects were prospectively recruited, and serum HIF-1α, VEGF, and uPA levels were measured by enzyme-linked immunosorbent assay (ELISA). Preoperative threshold values of HIF-1α, VEGF, and uPA were determined by ROC curves. Kaplan-Meier curves were analyzed for overall survival and progression-free survival of patients. Univariate and multivariate Cox risk regression analyzed prognostic factors. Results: Serum HIF-1α, VEGF, and uPA were higher in OSCC patients compared to control subjects (P < 0.001). Critical values of HIF-1α, VEGF, and uPA were 99.8 pg/mL, 130.4 pg/mL, and 142.9 pg/mL, respectively. Serum levels of HIF-1α, VEGF, and uPA were associated with the overall pathologic status (TNM staging), neural invasion, extranodal extension, lymphovascular invasion, depth of invasion, and degree of cellular differentiation (P < 0.05). Patients with higher serum HIF-1α, VEGF, and uPA levels had poorer overall survival and shorter progression-free survival. Higher-than-threshold serum HIF-1α, VEGF, and uPA were independent prognostic factors for overall survival (P < 0.001, P < 0.001, P = 0.006) of and progression-free survival (P < 0.012, P < 0.001, P = 0.010). Conclusion: Higher circulating levels of HIF-1α, VEGF, and uPA were associated with clinicopathologic correlations of lymph nodes, metastasis, and were independent risk factors for survival and progression-free survival.
Keywords: Oral squamous cell carcinoma, clinicopathology, prognosis, serum biomarkers
Introduction
Head and neck squamous cell carcinoma (SCC) originates from the mucous epithelium of the mouth, pharynx, and larynx [1]. Oral SCC (OSCC) accounts for 40% of head and neck cancer [2-4], usually manifested by metastasis to cervical lymph nodes [5]. The main treatment strategy for OSCC is primary ablation. Adjuvant therapy such as radiotherapy or chemoradiotherapy is often considered at high risk of recurrence [6]. Overall, the five-year relative survival rate for patients with OSCC is close to 50%, and many patients also experience local or distal relapses [7]. At present, many studies have explored and confirmed clinically relevant prognostic factors related to OSCC, such as clinical stage, histologic characteristics, and degree of cell differentiation [8-10]. Abnormalities in blood markers often occur before abnormalities are observed on imaging, and marker detection is relatively simple, inexpensive, and less invasive. Commonly used tumor markers can not only be used as cancer diagnostic markers but also have prognostic value [11,12]. They may be useful for the diagnosis and treatment of OSCC.
Hypoxic cells exhibit adaptive changes that promote tumor progression [5] and hypoxic areas and tumor metastasis promotes tumor initiation and leads to poor prognosis of patients [13]. Hypoxia-inducible factor-1α (HIF-1α) is a transcriptional activator of several genes related to the adaptive response to hypoxia [14]. HIF-1α dysfunction is associated with malignant tumors, and hinders cancer treatment [15]. It has been found that activation of HIF-1α transcription complex upregulates vascular endothelial growth factor (VEGF) expression [16]. It is evident that VEGF stimulates angiogenesis, increases vascular permeability, changes tumor microenvironment, regulates cancer cell death, and enhances tumor development [17]. VEGF stimulates endothelial cells and activates the urokinase-type plasminogen activator (uPA) system. This system consists of uPA, its cell receptor uPAR and its inhibitor PAI-1, which participate in the above processes induced by VEGF [18]. uPAR forms a complex with a5β1 that distributes in focal adhesion to endothelial cells, resulting in matrix degradation and cell invasion [18,19].
This study measured circulating HIF-1α, VEGF, and uPA in OSCC patients, and analyzed the relationship between these factors and clinicopathologic features and prognosis in OSCC based on the cutoff values of these factors. In addition, the predictive value of these factors was evaluated by univariate and multivariate approaches to help surgeons adopt strategic treatment.
Materials and methods
Subjects
This was a prospective study. The study population included all patients treated at Jinhua Municipal Central Hospital, who were pathologically diagnosed with OSCC between January 2021 and December 2022. Patients had primary tumors and treated primarily by surgery. No treatment was received before surgery. Patients with a history of any prior malignancy, a second primary cancer, or a history of neoadjuvant radiotherapy or chemotherapy were excluded. Ultimately, a total of 160 patients were enrolled and comprehensively evaluated for factors including age, gender, lifestyle habits, laboratory data, chest radiography, imaging data, liver ultrasound, bone scans, or positron emission tomography. This study was conducted under ethical principles of the Declaration of Helsinki, and was approved by the Jinhua Municipal Central Hospital, Institutional Review Committee. Written informed consent was obtained from all subjects.
Definitions: (1) Drinkers: alcohol consumption once a week or more for at least six months [20]; (2) Smokers: smoking daily for at least 1 year [21]; (3) Betel chewers: chewing ≥ 2 betel nuts daily for at least 1 year [22].
The main outcome evaluation parameters were overall survival (OS, time from diagnosis to death) and progression-free survival (PFS, time from diagnosis to tumor progression or death). All patients were followed up until September 2022 or death, a total of 18 months from the time the first patient was enrolled to the end of follow-up. Pathologic staging was graded by the staging criteria of UICC/AJCC in 2017 [23]. In addition, nerve invasion, extranodal extension, lymphatic vessel invasion, and invasion depth were also included as clinical features of OSCC. Another 51 age- and gender-matched control subjects were enrolled. Clinical study design is shown in Figure 1.
Figure 1.
Clinical study design process.
Blood and urine samples
In control subjects, blood samples were collected during blood drawing for other specified medical reasons. Blood samples were collected from all subjects after fasting overnight. Blood was centrifuged at 4°C at 1300 g for 20 min. The serum portion was then equalized and stored at -80°C.
Laboratory tests
HIF-1α, VEGF and uPA in serum were measured by enzyme-linked immunosorbent assay (ELISA) kits (MLBIO, Shanghai, China), expressed as picograms per milliliter (pg/mL). All samples were repeated in one assay to avoid interassay variation. ELISA measured less than 3% intra-assay variation.
Data analysis
Data analysis was conducted by SPSS software 22.0 (SPSS, Chicago, IL, USA). The data analysis was performed utilizing SPSS software version 22.0. Counted data were presented as frequency and ratio (N, %), with intergroup comparisons conducted using the Chi-square test. The normality of continuous values was assessed using the Shapiro-Wilk test. Measured data were expressed as mean ± standard deviation (X ± S), and intergroup comparisons for normally distributed continuous variable data were conducted using the Student’s t-test. For continuous variable data with skewed distribution, intergroup comparisons were made using the Mann-Whitney U test. Spearman correlation coefficient was adopted to analyze the correlation among factors (p-values were Bonferroni-corrected). Area under the Receiver Operating Characteristic (ROC) curve was calculated using the ROC analysis, and the best cutoff values for serum indicators (HIF-1α, VEGF, and uPA) were selected using the Youden J statistic and the selected Youden index. The survival rate was analyzed by Kaplan-Meier and tested by logarithmic rank test. Univariate and multivariate Cox regression models were employed to further analyze the correlation between variables and survival. Bilateral P < 0.05 indicated statistical significance.
Results
Clinicopathologic data
160 patients with OSCC were enrolled, with a mean age of 52.6 years, and 122 (76.2%) were male. Drinkers accounted for 57.5%, smokers 81.9%, and betel nut chewers 78.1%. Table 1 presents clinicopathologic features of OSCC patients.
Table 1.
Baseline clinical and pathologic characteristics of patients with oral squamous cell carcinoma
| Variable | N (%) | |
|---|---|---|
| Age (years) | < 65/≥ 65 | 124/36 |
| Gender | Male/Female | 122/38 |
| Personal Habits | Alcohol consumption | 92 (57.5) |
| Cigarettes smoking | 131 (81.9) | |
| Betel nut chewing | 125 (78.1) | |
| Tumor site | Buccal mucosa | 68 (42.5) |
| Tongue | 52 (32.5) | |
| Others | 40 (25.0) | |
| Overall stage | I-II | 67 (41.9) |
| III-IV | 93 (58.1) | |
| T1 | 35 (21.9) | |
| pT classification | T2 | 52 (32.5) |
| T3 | 16 (10.0) | |
| T4 | 57 (35.6) | |
| pN classification | N0 | 96 (60.0) |
| N1-N3 | 64 (40.0) | |
| PNI | 57 (35.6) | |
| ENE | 34 (21.2) | |
| LVI | 69 (43.13) | |
| DOI (mm) | < 10 | 82 (51.25) |
| ≥ 10 | 78 (48.75) | |
Abbreviations: pT, pathological T; pN, pathological N; PNI, perineural invasion; ENE, extranodal extension; LVI, lymphovascular invasion; DOI, depth of invasion. P < 0.05, that was statistically significant differences.
Relationship between HIF-1α, VEGF, uPA and clinicopathologic features of patients
ROC analysis distinguished survivors from non-survivors (Figure 3A) and Youden J statistics classified OSCC patients into high/low HIF-1α, VEGF, and uPA groups. The optimal cutoff values of HIF-1α, VEGF and uPA were 99.8 pg/mL, 130.4 pg/mL and 142.9 pg/mL, respectively. The clinicopathologic factors are shown in Table 2. In addition to age and gender (all P < 0.05), there were significant differences in drinking, smoking, chewing betel nut, overall pathological status (TNM stage), pT status, pN status, nerve invasion, extranodal extension, lymphatic vessel invasion, invasion depth, and cell differentiation among patients with higher and lower levels of serum factors (all P < 0.05).
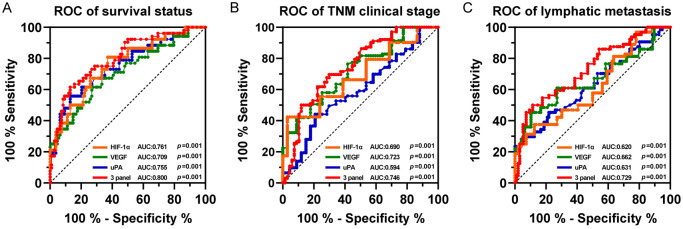
Figure 3.
ROC evaluation of serum HIF-1α, VEGF, uPA, and combined measurements of the three factors. A. Survival status. B. TNM clinical stage. C. Lymphatic metastasis. 3 panel indicates the combination of the above three factors. P < 0.05 indicates significant differences.
Table 2.
Based on serum HIF-1α, clinical and pathologic characteristics of the critical values of VEGF and uPA levels
| Variable | HIF-1α | p value | VEGF | p value | uPA | p value | |||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| < 99.80 (n = 76) | ≥ 99.80 (n = 84) | < 130.4 (n = 89) | ≥ 130.4 (n = 71) | < 142.9 (n = 94) | ≥ 142.9 (n = 66) | ||||
| Age (years) | |||||||||
| < 65/≥ 65 | 60/16 | 64/20 | 0.667 | 66/23 | 58/13 | 0.257 | 76/18 | 48/18 | 0.226 |
| Gender | |||||||||
| Male/Female | 58/18 | 64/20 | 0.985 | 65/24 | 57/14 | 0.284 | 70/24 | 52/14 | 0.464 |
| Personal Habits | |||||||||
| Alcohol consumption | 35 (46.0) | 57 (67.9) | 0.005 | 30 (33.7) | 62 (87.3) | < 0.001 | 40 (42.6) | 52 (78.8) | < 0.001 |
| Cigarettes smoking | 58 (76.3) | 73 (86.9) | 0.083 | 69 (77.5) | 62 (87.3) | 0.110 | 73 (77.7) | 58 (87.9) | 0.099 |
| Betel nut chewing | 50 (65.8) | 75 (89.3) | < 0.001 | 60 (67.4) | 65 (91.6) | < 0.001 | 66 (70.2) | 59 (89.4) | 0.004 |
| Overall stage | |||||||||
| I-II | 40 (52.6) | 27 (32.1) | 0.009 | 50 (56.2) | 17 (23.9) | < 0.001 | 46 (48.9) | 21 (31.8) | 0.031 |
| III-IV | 36 (47.4) | 57 (67.9) | 39 (43.8) | 54 (76.1) | 48 (51.1) | 45 (68.2) | |||
| pT classification | |||||||||
| T1-T2 | 49 (64.5) | 38 (45.3) | 0.015 | 59 (66.3) | 28 (39.4) | 0.001 | 59 (62.8) | 30 (45.5) | 0.030 |
| T3-T4 | 27 (35.5) | 46 (54.7) | 30 (33.7) | 43 (60.6) | 35 (37.2) | 36 (54.5) | |||
| pN classification | |||||||||
| N0 | 53 (69.7) | 43 (51.2) | 0.017 | 60 (71.4) | 36 (50.7) | 0.032 | 63 (67.0) | 33 (50.0) | 0.031 |
| N1-N3 | 23 (30.3) | 41 (48.8) | 29 (34.5) | 35 (49.3) | 31 (33.0) | 33 (50.0) | |||
| PNI | |||||||||
| Absent | 56 (73.7) | 47 (56.0) | 0.019 | 66 (78.6) | 37 (52.1) | 0.004 | 68 (72.3) | 35 (53.0) | 0.012 |
| Present | 20 (26.3) | 37 (44.0) | 23 (27.4) | 34 (47.9) | 26 (27.7) | 31 (47.0) | |||
| ENE | |||||||||
| Absent | 66 (86.8) | 60 (71.4) | 0.017 | 76 (85.4) | 50 (70.4) | 0.021 | 80 (85.1) | 46 (69.7) | 0.019 |
| Present | 10 (13.2) | 24 (28.6) | 13 (14.6) | 21 (29.6) | 14 (14.9) | 20 (30.3) | |||
| LVI | |||||||||
| Absent | 51 (67.1) | 40 (47.6) | 0.013 | 61 (68.5) | 30 (42.3) | 0.001 | 61 (64.9) | 30 (45.5) | 0.015 |
| Present | 25 (32.9) | 44 (52.4) | 28 (31.5) | 41 (57.7) | 33 (35.1) | 36 (54.5) | |||
| DOI (mm) | |||||||||
| < 10 | 46 (60.5) | 36 (42.9) | 0.026 | 53 (59.6) | 29 (40.8) | 0.019 | 55 (58.5) | 27 (40.9) | 0.028 |
| ≥ 10 | 30 (39.5) | 48 (57.1) | 36 (40.4) | 42 (59.2) | 39 (41.5) | 39 (49.1) | |||
| Cell differentiation | |||||||||
| W-D/M-D | 60 (78.9) | 78 (92.9) | 0.011 | 71 (79.8) | 67 (94.4) | 0.008 | 76 (80.9) | 62 (93.9) | 0.018 |
| P-D | 16 (21.1) | 6 (7.1) | 18 (20.2) | 4 (5.6) | 18 (19.1) | 4 (6.1) | |||
Abbreviations: pT, pathological T; pN, pathological N; PNI, perineural invasion; ENE, extranodal extension; LVI, lymphovascular invasion; DOI, depth of invasion; W-D, well-differentiated; M-D, moderately differentiated; P-D, poorly differentiated. P < 0.05, that was statistically significant differences.
Serum HIF-1α, VEGF and uPA in OSCC patients
Given that the majority of OSCC patients demonstrate smoking and betel nut chewing habits, we aimed to mitigate the impact of these factors by selecting healthy individuals with similar habits (Cigarette smoking, 40/51, 78.43%, Betel nut chewing, 36/51, 70.59%) and matching their gender and age to those of the OSCC patients. Our analysis revealed no significant differences in age (55.4 ± 12.6 years vs 52.6 ± 15.8 years, P > 0.05) and gender distribution (Male/Female 39/12 vs 122/38) between the control group and OSCC patients. Subsequently, we conducted a comparative analysis of serum levels of HIF-1 α, VEGF, and uPA between OSCC patients and controls. The results show that, serum HIF-1α, VEGF and uPA levels in OSCC patients were 100.05 [83.2-116.98], 126.7 [114.8-142.3] and 124.5 [92.8-144.6], respectively. Serum HIF-1α, VEGF and uPA in OSCC patients were higher versus control subjects (Figure 2A). According to the UICC/AJCC guidelines, stage I and II patients were a subgroup, and stage III and IV patients were a subgroup, and patients in stage III-IV patients had higher serum levels compared with patients in stage I-II (Figure 2B). High serum HIF-1α, VEGF and uPA were associated with tumor lymphatic metastasis (Figure 2C). Spearman correlation analysis reported that serum HIF-1α, VEGF, and uPA in OSCC patients were significantly positively correlated (Figure 2D).
Figure 2.
Serum HIF-1α, VEGF, uPA levels and correlation analysis. A. Control population (n = 51) and OSCC patients (n = 160). B. OSCC patients with different clinical stages (I-II, n = 67; III-IV, n = 93). C. OSCC patients with non-lymphatic metastases (n = 96) and metastases (n = 64). D. Correlation analysis of serum HIF-1α, VEGF and uPA. The data are expressed as the median, and the Mann-Whitney U or Kruskal-Wallis H test compare the differences, and r is the correlation coefficient. ***P < 0.001; **P < 0.01; *P < 0.05.
Relationship between serum HIF-1α, VEGF, uPA and survival status in OSCC patients
To further observe the diagnostic value of serum HIF-1α, VEGF, and uPA on survival status and clinicopathologic features (TNM staging and lymphatic metastasis) of patients, ROC curve and AUC analysis were performed (Figure 3). HIF-1α, VEGF and uPA were all effective in differentiating survival status (surviving and non-surviving) (AUC = 0.761, 0.709, 0.755, respectively), clinical stage (I-II and III-IV) (AUC = 0.690, 0.723, 0.594, 0.746, respectively) (Figure 3B) and lymphatic metastasis (non-metastatic and metastatic) (AUC = 0.620, 0.662, 0.631, respectively) (Figure 3C). In addition, the prediction probability of the combined analysis of the three factors was considered, and it was observed that the combination of HIF-1α, VEGF, and uPA was more effective in diagnosing survival status, clinical stage, and lymphatic metastasis than one factor alone (AUC = 0.800, 0.746, and 0.729, respectively).
Next, patients with higher serum levels of the factor were compared using Kaplan-Meier curves, using the cutoff for the best cut-off to distinguish survival. Patients were followed for a median of 10 months (1-18 months) (Figure 4). Patients with higher serum levels had poorer OS and shorter PFS compared to patients with lower serum levels. The mean PFS (months) in patients with high HIF-1α, VEGF, and UPA were 5.9 (95% CI = 5.2-7.6), 6.1 (95% CI = 5.5-8.3), and 3.0 (95% CI = 2.1-5.3). Mean OS was 13.2 (95% CI = 12.6-16.9), 12.3 (95% CI = 10.6-15.3), and 14.0 (95% CI = 12.5-17.3) in patients with lower levels. There were significant differences in OS and PFS between patients with high and low serum levels of each factor (log-rank, P < 0.001). Through the Cox proportional hazards model, univariate analysis showed that overall pathologic staging, neural invasion, extranodal extension, lymphatic vessel invasion, DOI, degree of cell differentiation, and serum levels above critical values of HIF-1α, VEGF, and uPA were risk factors for overall survival and progression-free survival. Moreover, subsequent to controlling for variables such as age, gender, and univariate analysis, it was determined that elevated levels of serum HIF-1, VEGF, and uPA above a certain threshold were identified as autonomous risk factors for both overall survival and progression-free survival (Figure 5A, 5B).
Figure 4.
OS and PFS of OSCC patients grouped according to serum (A) HIF-1α, (B) VEGF, and (C) uPA cutoff values (differentiating patient survival status) according to ROC analysis are shown as Kaplan-Meier curves. p value was calculated using the log-rank test. P < 0.05 indicates significant differences.
Figure 5.
Univariate and Cox multivariate analyses of prognostic factors in OSCC patients. P < 0.05 indicates significant differences.
Further, after adjusting for age, gender, and variance in univariate analysis, serum HIF-1α, VEGF, and uPA were prognostic factors for OS (P < 0.001, P < 0.001, P = 0.006) and PFS (P < 0.012, P < 0.001, P = 0.010) (Figure 4A-C).
Discussion
Clinical or pathologic staging is valuable in predicting the prognosis of patients with OSCC. Tumor microenvironment (TME) hinders the effectiveness of clinical practice [24]. The TME provides the basis for tumorigenesis, progression, invasion and metastasis [25]. We sought to reveal the role of serum markers in predicting survival in OSCC patients. The HIF-1α/VEGF pathway is able to promote peripheral angiogenesis and cell proliferation under hypoxic conditions [26]. VEGF triggers uPA/uPAR activation and degradation of extracellular matrix proteins confirmed as pathways for tumor migration and invasion [27-29]. In our current study, we investigated for the first time the relationship between HIF-1α, VEGF and uPA and survival. The results showed that high serum levels of HIF-1α, VEGF, and uPA were associated with poorer survival in OSCC patients.
We first compared HIF-1α, VEGF, and uPA between OSCC patients and controls and found that the levels of these serum markers were higher in OSCC patients than in controls. The upregulation of HIF-1α, VEGF, and uPA may reflect their regulation of the TME to promote tumor microangiogenesis, tumor metastasis, and invasion in hypoxic environments. The depth of lymphatic metastasis and invasion of tumors is known to promote dramatic cancer progression [24,25]. Hypoxic tumor-associated macrophages are able to secrete VEGF in a HIF-1α-dependent manner in synergy with tumor biologic mechanisms leading to increased tumor angiogenesis [26]. Although the progression of some of these cancers may be due to a combination of hypoxia and genetic variation [24-26]. Previous evidence suggests that hypoxic tumor-associated macrophages are able to secrete VEGF in a HIF-1α-dependent manner and induce MMP-9 expression in synergy with tumor biological mechanisms leading to increased tumor angiogenesis [30]. In addition, uPAR-VEGFR2 interaction also promotes VEGF/VEGFR signaling in vascular endothelial cells [31,32]. Activation of VEGF/VEGFR signaling promotes tumor microangiogenesis to provide nutrients and oxygen, thereby promoting tumor cell growth [29]. The results of this study showed that HIF-1α, VEGF, and uPA were higher in the serum of OSCC patients with stage III-IV clinical stage and lymphatic metastases, and HIF-1α was strongly correlated with VEGF and uPA. This suggests that in OSCC, HIF-1α can promote VEGF- and uPA-dependent cellular signals at least in part by contributing to alterations in the hypoxic environment of tumor cells affecting tumor cell cycle, metabolism, angiogenesis and invasion.
How cancer cells interact and adapt to the hypoxic tumor microenvironment remains an important question. When the metabolic demands of rapid tumor growth exceed the limits of vascular supply, a hypoxic microenvironment is generated in and around the tumor cell mass, leading to HIF-1α activation. HIF-1α activation has also been shown to lead to metabolic reprogramming, which indirectly promotes cancer progression [33]. In general, higher HIF-1α expression predicts a higher risk of death and disease progression than lower HIF-1α, an outcome that was predictable. In this research, higher circulating levels of HIF-1α were associated with shorter OS and PFS, predicting adverse outcomes. More than 100 HIF-1α transcription target genes have been identified, including factors associated with angiogenesis [34]. VEGF overexpression predicts uncontrolled angiogenesis and is generally considered to be a marker of poor prognosis and aggressive tumor phenotypes [35,36]. VEGF expression and serum levels have been shown to be reliable markers to distinguish patients with OSCC from normal subjects [37]. These all suggest an interaction of the three serum markers in cancer by promoting microvessel formation in the TME, which mediates cellular junctions and adhesion, and the adherent tumour cells metastasize with lymphatic fluids, further leading to tumour progression. In our study, poorer survival may be observed in patients with lymph node metastasis and higher levels of serum markers.
However, until now, the clinicopathologic and prognostic effects of HIF-1α, VEGF and uPA circulating levels in peripheral blood on OSCC have not been well established. Therefore, we designed this prospective study and analyzed the short-term survival of 160 patients with OSCC. We confirmed the relationship between serum levels of HIF-1α, VEGF, and uPA and clinicopathological characteristics of OSCC patients. For objective stratification of HIF-1α, VEGF, and uPA levels, ROC analyses distinguished patient survival states (surviving versus non-surviving, within 18 months of follow-up) and selected the best cutoff value. This study found that higher serum levels of the factor were associated with alcohol consumption, smoking, and betel nut chewing, which is consistent with some previous reports [38,39].
Characteristics of OSCC (including overall pathologic status, pT status, pN status, nerve invasion, extranodal extension, lymphatic vessel invasion, invasion depth, and cell differentiation) reflect the metastasis and aggressiveness of OSCC tumors. Higher serum levels of HIF-1α, VEGF, and uPA were associated with these features. The combination of the three factors had a high diagnostic value for the clinicopathology and survival status of OSCC patients. Many studies have also shown that multifactor combination can improve the prognostic value of patients [40,41]. Increased VEGF in the circulation or microenvironment is a sign of neovascularization and may indicate the possible presence of tumor metastasis in patients. Tumor metastasis and the degree of metastasis are the main factors for patient’ survival 40. Kaplan-Meier curves showed that patients with higher serum levels of HIF-1α, VEGF, and uPA had shorter OS and PFS. Finally, we elucidated the results of overall and progression-free survival after surgery by univariate and multivariate survival analyses. Above threshold values of serum HIF-1α, VEGF, and uPA were found to be independent risk factors for predicting OS and PFS. This may point to more aggressive treatment of OSCC patients with high serum biomarkers or lymph node metastases.
According to our findings, preoperative testing for HIF-1α, VEGF, and uPA provides an easy way to get information about a patient’s overall condition, which may help make better preoperative assessments and personalized treatment decisions.
Limitations
A first limitation is the relatively small cohort size that may affect data validity. Second, it is necessary to confirm the relationship between serum HIF-1α, VEGF, uPA levels and OSCC in other cohorts. In addition, follow-up time is limited and longer studies are needed to confirm our findings.
Conclusion
Higher HIF-1α, VEGF, and uPA in OSCC patients were associated with adverse clinicopathologic factors, such as global pathologic status, pT status, pN status, nerve invasion, extranodal extension, lymphatic vessel invasion, invasion depth, and cell differentiation. In addition, HIF-1α, VEGF, and uPA were shown to be independent prognostic factors for OS and PFS, and could therefore also be used pre-treatment to predict survival.
Disclosure of conflict of interest
None.
References
- 1.Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020;6:92. doi: 10.1038/s41572-020-00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kao HK, Löfstrand J, Loh CY, Lao WW, Yi JS, Chang YL, Chang KP. Nomogram based on albumin and neutrophil-to-lymphocyte ratio for predicting the prognosis of patients with oral cavity squamous cell carcinoma. Sci Rep. 2018;8:13081. doi: 10.1038/s41598-018-31498-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen TC, Chang HL, Yang TL, Lou PJ, Chang YL, Ko JY, Wang CP. Impact of dysplastic surgical margins for patients with oral squamous cell carcinoma. Oral Oncol. 2019;97:1–6. doi: 10.1016/j.oraloncology.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 4.Singh P, Rai A, Verma AK, Alsahli MA, Rahmani AH, Almatroodi SA, Alrumaihi F, Dev K, Sinha A, Sankhwar S, Dohare R. Survival-based biomarker module identification associated with oral squamous cell carcinoma (OSCC) Biology (Basel) 2021;10:760. doi: 10.3390/biology10080760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teppo S, Sundquist E, Vered M, Holappa H, Parkkisenniemi J, Rinaldi T, Lehenkari P, Grenman R, Dayan D, Risteli J, Salo T, Nyberg P. The hypoxic tumor microenvironment regulates invasion of aggressive oral carcinoma cells. Exp Cell Res. 2013;319:376–389. doi: 10.1016/j.yexcr.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Wu YY, Chang KP, Lin CY, Pai PC, Wang HM, Hsu CL, Liao CT, Yen TC, Fang TJ, Huang SF, Kang CJ, Fang KH, Lin WN, Wang YC, Hsin LJ, Tsang NM. Prognostic significance of combined pretreatment lymphocyte counts and body mass index in patients with head and neck cancer treated with radiation therapy. Cancer Med. 2018;7:2808–2815. doi: 10.1002/cam4.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ou D, Wu Y, Zhang J, Liu J, Liu Z, Shao M, Guo X, Cui S. miR-340-5p affects oral squamous cell carcinoma (OSCC) cells proliferation and invasion by targeting endoplasmic reticulum stress proteins. Eur J Pharmacol. 2022;920:174820. doi: 10.1016/j.ejphar.2022.174820. [DOI] [PubMed] [Google Scholar]
- 8.Zhang JY, Zhu WW, Wang MY, Zhai RD, Wang Q, Shen WL, Liu LK. Cancer-associated fibroblasts promote oral squamous cell carcinoma progression through LOX-mediated matrix stiffness. J Transl Med. 2021;19:513. doi: 10.1186/s12967-021-03181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valentini V, Terenzi V, Battisti A, Cassoni A, Anelli A, Priore P, Petrinca P. Management of clinically negative neck in maxillary carcinoma. J Craniofac Surg. 2010;21:759–762. doi: 10.1097/SCS.0b013e3181d878d1. [DOI] [PubMed] [Google Scholar]
- 10.Gholami S, Chamorro-Petronacci C, Pérez-Sayáns M, Suárez Peñaranda J, Longatto-Filho A, Baltazar F, Afonso J. Immunoexpression profile of hypoxia-inducible factor (HIF) targets in potentially malignant and malignant oral lesions: a pilot study. J Appl Oral Sci. 2023;31:e20220461. doi: 10.1590/1678-7757-2022-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu C, Zhang Y, Xu J, Chen W, Yu P, Wang Y, Bao Z, Zhang R, Xu Z, Cheng X. Prognostic significance of serum tumor marker normalization in the perioperative period for patients with advanced gastric cancer. Ann Transl Med. 2022;10:153. doi: 10.21037/atm-22-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Björkman K, Jalkanen S, Salmi M, Mustonen H, Kaprio T, Kekki H, Pettersson K, Böckelman C, Haglund C. A prognostic model for colorectal cancer based on CEA and a 48-multiplex serum biomarker panel. Sci Rep. 2021;11:4287. doi: 10.1038/s41598-020-80785-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu X, Prodger A, Sim J, Evans CE. Pulmonary thrombosis promotes tumorigenesis via myeloid hypoxia-inducible factors. Biomolecules. 2022;12:1354. doi: 10.3390/biom12101354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koyasu S, Kobayashi M, Goto Y, Hiraoka M, Harada H. Regulatory mechanisms of hypoxia-inducible factor 1 activity: two decades of knowledge. Cancer Sci. 2018;109:560–571. doi: 10.1111/cas.13483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouyang C, Zhang J, Lei X, Xie Z, Liu X, Li Y, Huang S, Wang Z, Tang G. Advances in antitumor research of HIF-1α inhibitor YC-1 and its derivatives. Bioorg Chem. 2023;133:106400. doi: 10.1016/j.bioorg.2023.106400. [DOI] [PubMed] [Google Scholar]
- 16.Ahn GO, Seita J, Hong BJ, Kim YE, Bok S, Lee CJ, Kim KS, Lee JC, Leeper NJ, Cooke JP, Kim HJ, Kim IH, Weissman IL, Brown JM. Transcriptional activation of hypoxia-inducible factor-1 (HIF-1) in myeloid cells promotes angiogenesis through VEGF and S100A8. Proc Natl Acad Sci U S A. 2014;111:2698–2703. doi: 10.1073/pnas.1320243111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho R, Sakurai Y, Jones HS, Akita H, Hisaka A, Hatakeyama H. Silencing of VEGFR2 by RGD-modified lipid nanoparticles enhanced the efficacy of anti-PD-1 antibody by accelerating vascular normalization and infiltration of T cells in tumors. Cancers (Basel) 2020;12:3630. doi: 10.3390/cancers12123630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breuss JM, Uhrin P. VEGF-initiated angiogenesis and the uPA/uPAR system. Cell Adh Migr. 2012;6:535–615. doi: 10.4161/cam.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baart VM, Houvast RD, de Geus-Oei LF, Quax PHA, Kuppen PJK, Vahrmeijer AL, Sier CFM. Molecular imaging of the urokinase plasminogen activator receptor: opportunities beyond cancer. EJNMMI Res. 2020;10:87. doi: 10.1186/s13550-020-00673-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li M, Lin J, Liang S, Huang S, Wen Z, Mo Z. Predicted fat mass, lean body mass, and risk of hypertension: results from a Chinese male cohort study. Obes Facts. 2022;15:638–647. doi: 10.1159/000524653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan J, Liu J, Wang H, Li W, Du X, Lin Q, Zhang X, Qi D, Tu J, Ning X, Yang Q, Wang J. Association of carotid atherosclerosis with lipid components in asymptomatic low-income Chinese: a population-based cross-sectional study. Front Neurol. 2020;11:276. doi: 10.3389/fneur.2020.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeh CC, Kao HK, Huang Y, Tsai TY, Young CK, Hung SY, Lu CY, Chang KP. Discovering the clinical and prognostic role of pan-immune-inflammation values on oral cavity squamous cell carcinoma. Cancers (Basel) 2023;15:322. doi: 10.3390/cancers15010322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang SH, O’Sullivan B. Overview of the 8th edition TNM classification for head and neck cancer. Curr Treat Options Oncol. 2017;18:40. doi: 10.1007/s11864-017-0484-y. [DOI] [PubMed] [Google Scholar]
- 24.Ramos RN, Amano MT, Paes Leme AF, Fox JW, de Oliveira AK. Editorial: tumor microenvironment (TME) and tumor immune microenvironment (TIME): new perspectives for prognosis and therapy. Front Cell Dev Biol. 2022;10:971275. doi: 10.3389/fcell.2022.971275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mlecnik B, Bindea G, Pagès F, Galon J. Tumor immunosurveillance in human cancers. Cancer Metastasis Rev. 2011;30:5–12. doi: 10.1007/s10555-011-9270-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim MY, Shin JY, Kim JO, Son KH, Kim YS, Jung CK, Kang JH. Anti-tumor efficacy of CKD-516 in combination with radiation in xenograft mouse model of lung squamous cell carcinoma. BMC Cancer. 2020;20:1057. doi: 10.1186/s12885-020-07566-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang C, Zheng X. Identification of a hypoxia-related lncRNA biomarker signature for head and neck squamous cell carcinoma. J Oncol. 2022;2022:6775496. doi: 10.1155/2022/6775496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H, Wang W, Fan S. Emerging roles of lncRNA in nasopharyngeal carcinoma and therapeutic opportunities. Int J Biol Sci. 2022;18:2714–2728. doi: 10.7150/ijbs.70292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu HY, Hwang PA. Clinical applications of fucoidan in translational medicine for adjuvant cancer therapy. Clin Transl Med. 2019;8:15. doi: 10.1186/s40169-019-0234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang G, Zhou H, Tian L, Yan T, Han X, Chen P, Li H, Wang W, Xiao Z, Hou L, Xue X. A prognostic DNA damage repair genes signature and its impact on immune cell infiltration in glioma. Front Oncol. 2021;11:682932. doi: 10.3389/fonc.2021.682932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahmood N, Mihalcioiu C, Rabbani SA. Multifaceted role of the urokinase-type plasminogen activator (uPA) and its receptor (uPAR): diagnostic, prognostic, and therapeutic applications. Front Oncol. 2018;8:24. doi: 10.3389/fonc.2018.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Q, Lu S, Li T, Yu L, Zhang Y, Zeng H, Qian X, Bi J, Lin Y. ACE2 inhibits breast cancer angiogenesis via suppressing the VEGFa/VEGFR2/ERK pathway. J Exp Clin Cancer Res. 2019;38:173. doi: 10.1186/s13046-019-1156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elzakra N, Kim Y. HIF-1α metabolic pathways in human cancer. Adv Exp Med Biol. 2021;1280:243–260. doi: 10.1007/978-3-030-51652-9_17. [DOI] [PubMed] [Google Scholar]
- 34.Xiong Q, Liu B, Ding M, Zhou J, Yang C, Chen Y. Hypoxia and cancer related pathology. Cancer Lett. 2020;486:1–7. doi: 10.1016/j.canlet.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Mori K, Schuettfort VM, Katayama S, Laukhtina E, Pradere B, Quhal F, Sari Motlagh R, Mostafaei H, Grossmann NC, Rajwa P, König F, Aydh A, Soria F, Moschini M, Karakiewicz PI, Lotan Y, Scherr D, Haydter M, Nyirady P, Teoh JYC, Egawa S, Compérat E, Shariat SF. The value of preoperative plasma VEGF levels in urothelial carcinoma of the bladder treated with radical cystectomy. Eur Urol Focus. 2022;8:972–979. doi: 10.1016/j.euf.2021.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Takino J, Yamagishi S, Takeuchi M. Glycer-AGEs-RAGE signaling enhances the angiogenic potential of hepatocellular carcinoma by upregulating VEGF expression. World J Gastroenterol. 2012;18:1781–1788. doi: 10.3748/wjg.v18.i15.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aggarwal S, Devaraja K, Sharma SC, Das SN. Expression of vascular endothelial growth factor (VEGF) in patients with oral squamous cell carcinoma and its clinical significance. Clin Chim Acta. 2014;436:35–40. doi: 10.1016/j.cca.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 38.Lambert R, Sauvaget C, de Camargo Cancela M, Sankaranarayanan R. Epidemiology of cancer from the oral cavity and oropharynx. Eur J Gastroenterol Hepatol. 2011;23:633–641. doi: 10.1097/MEG.0b013e3283484795. [DOI] [PubMed] [Google Scholar]
- 39.Liao CT, Wallace CG, Lee LY, Hsueh C, Lin CY, Fan KH, Wang HM, Ng SH, Lin CH, Tsao CK, Chen IH, Huang SF, Kang CJ, Yen TC. Clinical evidence of field cancerization in patients with oral cavity cancer in a betel quid chewing area. Oral Oncol. 2014;50:721–731. doi: 10.1016/j.oraloncology.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 40.Xu X, Xiao Y, Hong B, Hao B, Qian Y. Combined detection of CA19-9 and B7-H4 in the diagnosis and prognosis of pancreatic cancer. Cancer Biomark. 2019;25:251–257. doi: 10.3233/CBM-190067. [DOI] [PubMed] [Google Scholar]
- 41.Hosaka K, Yang Y, Seki T, Du Q, Jing X, He X, Wu J, Zhang Y, Morikawa H, Nakamura M, Scherzer M, Sun X, Xu Y, Cheng T, Li X, Liu X, Li Q, Liu Y, Hong A, Chen Y, Cao Y. Therapeutic paradigm of dual targeting VEGF and PDGF for effectively treating FGF-2 off-target tumors. Nat Commun. 2020;11:3704. doi: 10.1038/s41467-020-17525-6. [DOI] [PMC free article] [PubMed] [Google Scholar]