Abstract
Uterine fibroids and endometriosis may be associated with an increased risk of ovarian cancer. Less is known about the role of hysterectomy in these associations. We estimated the independent and joint associations of hysterectomy, fibroids, and endometriosis with ovarian cancer incidence in the prospective Sister Study cohort (2003-2009). We used time-varying Cox proportional hazards models to estimate covariate-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs). By the end of follow-up, 34% of 40 928 eligible participants had fibroids, 13% had endometriosis, and 7% had both. A total of 274 women developed ovarian cancer during follow-up (median = 12.3 years). In mutually adjusted models, fibroids (HR, 1.65; 95% CI, 1.28-2.12) and possibly endometriosis (HR, 1.16; 95% CI, 0.82-1.63) were positively associated with ovarian cancer. Hysterectomies (20% of participants) were also positively associated with ovarian cancer (HR, 1.29; 95% CI, 0.95-1.74). There was some evidence that hysterectomies may mitigate ovarian cancer risk among women with fibroids (HR, 0.83; 95% CI, 0.56-1.24) but not among women with endometriosis (HR, 1.20; 95% CI, 0.65-2.22). Identifying these joint associations adds to our understanding of ovarian cancer etiology and may help inform decisions about how women with fibroids, endometriosis, and hysterectomies are treated and surveilled for ovarian cancer.
This article is part of a Special Collection on Gynecological Cancer.
Keywords: hysterectomy, endometriosis, fibroids, ovarian cancer
Introduction
Although rare, ovarian cancer is one of the most fatal cancers of the reproductive system. Ranked fifth in cancer deaths among women in the United States, there were an estimated 19 880 new cases with 12 810 deaths in 2022, and among those diagnosed, the 5-year survival rate was 50%.1 Fibroids and endometriosis are 2 benign (ie noncancerous) gynecological diseases potentially associated with elevated risk of ovarian cancer.2-7 The relationship between hysterectomies, which can prevent or treat both conditions, and ovarian cancer risk remains uncertain.4,5,8-10
Endometriosis and fibroids can co-occur and may share some environmental and genetic risk factors.11-14 Endometriosis occurs when the inner lining of the uterus, the endometrium, is present outside of the uterus.15,16 It can affect women of all ages but is most frequently observed during the reproductive years. Fibroids are benign tumors made up of smooth muscle cells and connective tissues within the uterus, reportedly most common among women between 30 and 40 years of age, with Black women having greater risk than White women.11,17-19 Women suffering from endometriosis or fibroids may experience common symptoms, including menstrual irregularities, abdominal pain during menses, and infertility. Endometriosis requires surgical diagnosis, while fibroids can be detected on ultrasound.12,20,21 Depending on the symptoms, treatment for both conditions can include pain medication, hormones, or surgery. Surgeries can be done with uterine conservation (removal of endometrial implants or myomectomy to remove one or more fibroids) or without (hysterectomy with or without oophorectomy).15,20,21
The biological mechanisms underlying the putative association between uterine fibroids, endometriosis, and ovarian cancer are not fully understood, though previous studies have provided evidence that hormone and immune-related processes are key drivers of all 3 conditions.22-27 The removal of the uterus has hormonal implications, even in women with retained ovaries,28 meaning hysterectomies could affect ovarian carcinogenesis through hormone-related pathways. Disruption of immune processes or other mechanisms underlying the association between fibroids or endometriosis and ovarian tumor development are also plausible.
As reported in a recent meta-analysis9 and pooled analysis,5 previous epidemiologic studies of the independent association between hysterectomies and ovarian cancer have reported null or inverse effect estimates, with inverse associations more consistently observed for nonserous ovarian cancers. Only a few studies have considered the joint effects of hysterectomies, fibroids, and endometriosis. An Australian population-based registry study conducted among women with endometriosis or fibroids reported that hysterectomies were inversely associated with ovarian cancer across all subtypes.8 Additional evidence for the protective effect of hysterectomy among women with benign conditions was shown in Tseng et al,7 a Taiwanese population-based case–control study reporting that among women diagnosed with fibroids, those who had undergone a hysterectomy had a lower risk of ovarian cancer than a comparable group of women without hysterectomy or myomectomy. Finally, in a recent pooled analysis, Harris et al2 reported that while fibroids and endometriosis were both positively associated with ovarian cancer overall, their estimated effects were attenuated within strata of women who had a hysterectomy.
We wished to advance our understanding of how endometriosis, fibroids, and hysterectomy without bilateral oophorectomy may be related to ovarian cancer etiology by conducting in-depth investigations of their individual and combined associations. We were particularly interested in investigating the potential protective effect of hysterectomy among women diagnosed with endometriosis or fibroids. We assessed these relationships in the Sister Study, a prospective cohort of women living in the United States.
Methods
Study sample
The Sister Study is a prospective cohort of 50 884 self-identified women residing in all 50 states plus Puerto Rico. Women aged 35-74 with half or full sisters diagnosed with breast cancer and no history of breast cancer themselves were enrolled between 2003 and 2009. More than 1 sister could participate in the study. Demographic, lifestyle, family, and reproductive histories were collected via computer-assisted telephone interviews at enrollment. Basic health information is collected annually, with more extensive follow-up questionnaires administered every 2-3 years to collect detailed data on changes in health, demographic factors, and potential exposures.
After exclusions, 41 641 women were eligible for these analyses, including 277 incident ovarian cancer cases. Participants were excluded if they withdrew from the study (n = 4), had ovarian cancer prior to enrollment (n = 204), had unknown ovarian cancer status at enrollment (n = 43), or had a bilateral oophorectomy prior to enrollment (n = 8992). The Sister Study was approved by the Institutional Review Board of the National Institutes of Health. Written informed consent was provided by all participants. Data are complete through October 2020 (data release 10.1).
Exposure and outcome assessment
Our main exposures of interest were history of a hysterectomy (without bilateral oophorectomy) and history of physician-diagnosed endometriosis or fibroids. Exposure status for each of the 3 factors was time-varying, with status reevaluated at the age each follow-up questionnaire was administered. Incident ovarian cancer cases, which included primary cancers of the ovary, fallopian tubes, or peritoneum, were identified via self-report on follow-up questionnaires or via direct contact from the participant or her next of kin. Women with ovarian cancer were asked to provide access to their pathology reports to confirm the diagnosis and obtain histology information. Cases were also considered medically confirmed if ovarian cancer was listed as a cause of death (obtained via linkage to the National Death Index). Approximately 87% of cases were medically confirmed, with a positive predictive value of 80% among those who both self-reported a diagnosis and provided medical confirmation.
Other covariates
Potential confounders of the associations between ovarian cancer and the 3 exposures of interest were selected based on a priori knowledge of shared risk factors. The following covariates were adjusted for in all models: attained education (high school equivalent or less, some college/technical degree, completed college, graduate degree), alcohol use (nondrinker, drinker <7 drinks/week, drinker >7 drinks/week), smoking status (never, former, current), parity (0, 1, 2, 3+ children), menopause status (pre- or postmenopausal), self-reported body mass index (BMI, continuous, kg/m2), age at menarche (continuous, years), and ever hormonal birth control use (yes/no). In addition, we adjusted for race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic/Latina, other), as the distributions of ovarian cancer and all 3 exposures vary across racially and ethnically defined groups. Confounders that potentially changed over time (alcohol use, smoking status, parity, menopause status, BMI, and hormonal birth control use) were modeled as time-varying, with status updated at the age each detailed follow-up questionnaire was completed. We limited multivariable analyses to participants with complete covariate data at baseline (n = 40 928, >98% of eligible) but carried forward data from prior responses when follow-up data were missing.
In addition to the previously mentioned covariates, we additionally considered mutually adjusted models. Endometriosis and fibroids were considered as possible time-varying confounders for one another, and both were considered possible confounders of the association between hysterectomies and ovarian cancer. We did not adjust for hysterectomy in models assessing fibroids or endometriosis, as hysterectomies may be a mediator on the pathway between these gynecological conditions and ovarian cancer occurrence.
Statistical analysis
To assess the association between ovarian cancer incidence and endometriosis, fibroids, or hysterectomy status throughout follow-up, we computed hazard ratios (HRs) with 95% confidence intervals (CIs) using Cox proportional hazard models. The data were modeled prospectively, with all estimates corresponding to the association between previously occurring fibroid or endometriosis diagnosis or hysterectomy in relation to incident ovarian cancer. We used age as the primary time scale and stratified by follow-up period, allowing the baseline hazard to change with each follow-up cycle. Women were followed from age at baseline until ovarian cancer diagnosis, with censoring at age of bilateral oophorectomy, death, loss to follow-up, or end of follow-up (October 2020).
In addition to evaluating the independent associations between hysterectomy, fibroids, and endometriosis with ovarian cancer risk, we also assessed the joint association of hysterectomy and fibroids or endometriosis. To explore possible effect modification, we performed a series of stratified analyses, considering race (non-Hispanic White vs non-Hispanic Black/African American), BMI (>30 kg/m2 “obese” vs <30 kg/m2), and parity (nonparous vs parous), where BMI and parity were allowed to change over time. In addition, we considered analyses limited to medically confirmed or serous ovarian cancer cases.
Finally, in sensitivity analyses, we reevaluated our main findings after separately: (1) defining exposure status based on what the participant reported experiencing prior to enrollment (ie non-time-varying); (2) conducting a lagged analysis by excluding the first year of follow-up and only allowing time-varying fibroid, endometriosis, or hysterectomy status to be updated if more than a year had elapsed since diagnosis or surgery; (3) excluding women with prevalent uterine cancer (n = 12) and censoring women at uterine cancer diagnosis; and (4) excluding women who provided ambiguous or inconsistent information about their fibroid or endometriosis diagnosis (n = 3524). All models were adjusted for the potential confounders described previously .
Results
Women were followed for a median of 12.3 years (range 0.1-16.9). Compared to the full cohort, ovarian cancer cases were, on average, more likely to be older (mean enrollment age of 57.6 vs 54.9 years), non-Hispanic White (86% vs 84%), have BMI higher than 30 kg/m2 (33% vs 26%), and postmenopausal (68% vs 59%; Table 1). Additionally, cases were less likely to hold a graduate degree and more likely to have used hormone therapy.
Table 1.
Baseline characteristics of sister study (2003-2009) participants considering ovarian cancer status, n = 41 641.a
| Full cohort | Ovarian cancer cases | |
|---|---|---|
| n = 41 641 | n = 277 | |
| Age (years): Mean (SD) | 54.9 (9.0) | 57.6 (8.7) |
| Self-identified race | ||
| Non-Hispanic White | 34 868 (84) | 239 (86) |
| Non-Hispanic Black | 3607 (9) | 23 (8) |
| Hispanic/Latina | 2081 (5) | 10 (4) |
| Other | 1072 (3) | 5 (2) |
| Attained education; N (%) | ||
| High school or less | 6019 (14) | 52 (19) |
| Some college/technical degree | 13 613 (33) | 95 (34) |
| Bachelor’s degree | 11 620 (28) | 72 (26) |
| Graduate degree | 10 379 (25) | 58 (21) |
| Self-reported BMI (kg/m2); Mean (SD) | 27.3 (6.0) | 27.9 (6.1) |
| Self-reported BMI (kg/m2); N (%) | ||
| <25 kg/m2 | 17 542 (42) | 107 (39) |
| 25 kg/m2 - < 30 kg/m2 | 13 049 (31) | 79 (29) |
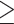 30 kg/m2
30 kg/m2
|
10 937 (26) | 90 (33) |
| Alcohol consumption; N (%) | ||
| Non-drinker | 7437 (18) | 63 (23) |
| Drinker, < 7 drinks/week | 28 362 (68) | 182 (66) |
Drinker, 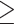 7 drinks/week 7 drinks/week |
5773 (14) | 32 (12) |
| Smoking status; N (%) | ||
| Never smoker | 23 579 (57) | 142 (51) |
| Former smoker | 14 662 (35) | 113 (41) |
| Current smoker | 3387 (8) | 22 (8) |
| Ever used hormonal birth control; N (%) | 35 481 (86) | 234 (85) |
| Age at menarche (years); N (%) | ||
 11
11 |
8185 (20) | 61 (22) |
| 12-13 | 23 490 (56) | 148 (53) |
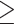 14
14 |
9932 (24) | 68 (25) |
| Parity; N (%) | ||
| Nulliparous | 7692 (18) | 61 (22) |
| 1 birth | 6013 (14) | 39 (14) |
| 2 births | 15 365 (37) | 92 (33) |
| >3 births | 12 543 (30) | 84 (30) |
| Postmenopausal; N (%) | 24 652 (59) | 188 (68) |
| Hormone therapy; N (%) | ||
| Never user | 27 348 (66) | 144 (52) |
| Estrogen only user | 4544 (11) | 55 (20) |
| Estrogen plus progestin user | 9638 (23) | 78 (28) |
Abbreviation: BMI, body mass index.
aExcluded women who withdrew from the study (n = 4), women with ovarian cancer prior to enrollment (n = 204) or unclear ovarian cancer status (n = 43), or who had a bilateral oophorectomy prior to enrollment (n = 8992).
At the time of enrollment, 11% of eligible women reported an endometriosis diagnosis, 27% reported a fibroids diagnosis, and 17% reported having had a hysterectomy without bilateral oophorectomy (Table S1). Endometriosis diagnoses occurred at a younger age, on average, than fibroids diagnoses (33.0 vs 39.8 years). Cross-tabulations showed that the exposures of interest frequently co-occurred: among women with endometriosis 45% also reported fibroids and 36% reported hysterectomy; among women with fibroids 18% reported endometriosis and 34% hysterectomy; and among women with hysterectomy, 23% reported endometriosis and 53% reported fibroids.
Among 40 928 women with complete covariate information, 34% reported ever having fibroids prior to the end of follow-up, 13% ever had endometriosis, and 20% ever had a hysterectomy. Of the 274 women with incident ovarian cancer, 118 (43%) reported ever having fibroids, 41 (15%) reported ever having endometriosis, and 76 (28%) reported having had a hysterectomy (Table 2). We observed a positive association between incident ovarian cancer and history of either hysterectomy (multivariable-adjusted HR, 1.49; 95% CI, 1.13-1.96) or fibroids (HR, 1.67; 95% CI, 1.31-2.14). The HR for fibroids was similar after additional adjustment for endometriosis (HR, 1.65; 95% CI, 1.28-2.12), but the HR for hysterectomy decreased after adjusting for both fibroids and endometriosis (HR, 1.29; 95% CI, 0.95-1.74). Although we also observed a possible positive association between endometriosis and ovarian cancer in initial assessments (HR, 1.29; 95% CI, 0.92-1.80), the effect estimate was attenuated after further adjustment for fibroids (HR, 1.16; 95% CI, 0.82-1.63). Relative to having one or neither condition, having both fibroids and endometriosis (7% overall, 9% of cases) was positively associated with ovarian cancer incidence (HR, 1.49; 95% CI, 0.98-2.28).
Table 2.
Hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between uterine conditions and ovarian cancer, n = 40 928.a
|
Exposed non-cases (% of total)
b
Total N = 40 654 |
Exposed cases
(% of total) b Total N = 274 |
Person-time (years) b | Multivariable-adjusted HR d (95% CI) | Mutually-adjusted HR e | |
|---|---|---|---|---|---|
| Overall | |||||
| Hysterectomy | 8047 (20) | 76 (28) | 87 133/391,115c | 1.49 (1.13-1.96) | 1.29 (0.95-1.74) |
| Fibroids | 13 828 (34) | 118 (43) | 146 165/332,083c | 1.67 (1.31-2.14) | 1.65 (1.28-2.12) |
| Endometriosis | 5218 (13) | 41 (15) | 56 160/422,088c | 1.29 (0.92-1.80) | 1.16 (0.82-1.63) |
| Fibroids and Endometriosis | 2784 (7) | 24 (9) | 27 731/450,517c | 1.49 (0.98-2.28) | |
| Fibroids and Hysterectomy Status | |||||
| No Fibroids, No Hysterectomy | 23 306 (57) | 117 (43) | 293 006 | 1.00 | 1.00 |
| Fibroids, No Hysterectomy | 9301 (23) | 81 (30) | 98 109 | 2.04 (1.53-2.72) | 2.03 (1.52-2.71) |
| No Fibroids, Hysterectomy | 3520 (9) | 39 (14) | 39 078 | 2.10 (1.44-3.05) | 2.07 (1.42-3.03) |
| Fibroids, Hysterectomy | 4527 (11) | 37 (14) | 48 055 | 1.72 (1.17-2.52) | 1.69 (1.14-2.50) |
| Fibroids, Hysterectomy vs. no Hysterectomyf | 0.84 (0.57-1.25) | 0.83 (0.56-1.24) | |||
| Endometriosis and Hysterectomy Status | |||||
| No Endometriosis, No Hysterectomy | 29 352 (72) | 176 (64) | 355 832 | 1.00 | 1.00 |
| Endometriosis, No Hysterectomy | 3255 (8) | 22 (8) | 35 284 | 1.25 (0.80-1.95) | 1.14 (0.73-1.79) |
| No Endometriosis, Hysterectomy | 6084 (15) | 57 (21) | 66 256 | 1.49 (1.09-2.03) | 1.31 (0.94-1.82) |
| Endometriosis, Hysterectomy | 1963 (5) | 19 (7) | 20 877 | 1.61 (1.00-2.59) | 1.37 (0.84-2.25) |
| Endometriosis, Hysterectomy vs. no Hysterectomyf | 1.29 (0.70-2.38) | 1.20 (0.65-2.22) |
aComplete case analysis with non-zero person-time
bExposure status at end of follow-up; person-time based on time-varying exposure status
cPerson-time in exposed/unexposed
dAdjusted for self-identified race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic/Latina, other), attained education (high school equivalent or less, some college/technical degree, completed college, graduate degree), alcohol use (nondrinker, drinker <7 drinks/week, drinker >7 drinks/week), smoking status (never, former, current), parity (0, 1, 2, 3+ children), menopause status at enrollment (pre- or postmenopausal), self-reported body mass index at enrollment (continuous, kg/m2), age at menarche (continuous, years), and ever hormonal birth control use (yes/no).
eIn addition to other covariates, hysterectomy estimates adjusted for fibroids and endometriosis; fibroid estimates adjusted for endometriosis, and endometriosis estimates adjusted for fibroids. The combination of fibroids and endometriosis did not require mutual adjustment.
fFrom the same model as above, estimates for direct comparison of 2 nonreferent groups
To better understand the joint effects of fibroids and hysterectomies, we categorized women as having had no fibroids or hysterectomy, fibroids but no hysterectomy, hysterectomy but no fibroids, or both fibroids and hysterectomy (Table 2). Compared to those with no fibroids and no hysterectomy, individuals with fibroids but no hysterectomy had higher rates of ovarian cancer (endometriosis-adjusted HR, 2.03 (95% CI, 1.52-2.71). Results were similar for those with hysterectomy but no fibroids (HR, 2.07; 95% CI, 1.42-3.03). Rates were also higher for women with both fibroids and hysterectomy (HR, 1.69; 95% CI, 1.14-2.50) compared to women who had neither. When we directly estimated the effect of hysterectomy among women with fibroids using the same model, we observed a possible inverse association between hysterectomy and ovarian cancer (HR, 0.83; 95% CI, 0.56-1.24).
Compared to those with no endometriosis and no hysterectomy, those with both endometriosis and hysterectomy had an elevated ovarian cancer rate (fibroid-adjusted HR, 1.37; 95% CI, 0.84-2.25; Table 2), with a similar HR observed for women who had undergone a hysterectomy but did not have endometriosis (HR, 1.31; 95% CI, 0.94-1.82). Women who had endometriosis without hysterectomy had only marginally higher rates (HR, 1.14; 95% CI, 0.73-1.79) compared to women who had neither. Among women with endometriosis, having a hysterectomy was positively, if imprecisely, associated with ovarian cancer (HR, 1.20; 95% CI, 0.65-2.22).
Positive associations between fibroids and ovarian cancer, regardless of hysterectomy status, were seen among both non-Hispanic White women (HR, 1.64; 95% CI, 1.26-2.14) and non-Hispanic Black women (HR, 1.57; 95% CI, 0.60-4.12, Table 3), with P-for-heterogeneity = 0.96 overall and 0.68 for fibroid-hysterectomy combinations. We also did not observe any clear differences by BMI or parity. The overall positive association between fibroids and ovarian cancer incidence was consistent for medically confirmed cases (HR, 1.63; 95% CI, 1.22-2.18) and serous cases (HR, 1.80; 95% CI, 1.22-2.65).
Table 3.
Subgroup-specific associations between fibroids (with or without hysterectomy) and incident ovarian cancer (n = 40 928, including 274 cases).
| Fibroids |
Fibroids, no hysterectomy
a
(N = 9382; 81 cases)
HR b , c (95% CI) |
No fibroids, hysterectomy
a
(N = 3559; 39 cases)
HR b , c (95% CI) |
Fibroids, hysterectomy
a
(N = 4564, 37 cases)
HR b , c (95% CI) |
||
|---|---|---|---|---|---|
| Cases (fibroids/no fibroids) a | HR b (95% CI) | ||||
| Overall | 118/156 | 1.65 (1.28-2.12) | 2.03 (1.52-2.71) | 2.07 (1.42-3.03) | 1.69 (1.14-2.50) |
| Race/Ethnicity | |||||
| Non-Hispanic White | 96/140 | 1.64 (1.26-2.14) | 1.97 (1.44-2.68) | 2.08 (1.38-3.13) | 1.76 (1.15-2.69) |
| Non-Hispanic Black | 16/7 | 1.57 (0.60-4.12) | 2.21 (0.68-7.21) | 1.62 (0.31-8.53) | 1.24 (0.34-4.50) |
| P-for-heterogeneity | 0.96 | 0.90 | |||
| BMI (kg/m2) | |||||
| <30 kg/m2 | 85/107 | 1.84 (1.38-2.46) | 2.20 (1.57-3.07) | 2.26 (1.40-3.67) | 1.97 (1.24-3.13) |
 30 kg/m2
30 kg/m2
|
33/49 | 1.28 (0.79-2.08) | 1.64 (0.93-2.90) | 1.65 (0.89-3.08) | 1.23 (0.60-2.53) |
| P-for-heterogeneity | 0.25 | 0.83 | |||
| Parous | |||||
| No | 29/30 | 2.17 (1.27-3.68) | 2.38 (1.36-4.15) | 1.39 (0.32-6.07) | 1.70 (0.64-4.51) |
| Yes | 89/126 | 1.53 (1.15-2.04) | 1.93 (1.37-2.70) | 2.07 (1.39-3.07) | 1.67 (1.09-2.56) |
| P-for-heterogeneity | 0.77 | 0.82 | |||
| Medically confirmed ovarian cancer | 91/123 | 1.63 (1.22-2.18) | 1.94 (1.39-2.71) | 1.94 (1.27-2.96) | 1.73 (1.11-2.68) |
| Serous ovarian cancer | 52/64 | 1.80 (1.22-2.65) | 2.16 (1.39-3.35) | 1.94 (1.07-3.51) | 1.81 (1.00-3.27) |
Abbreviation: BMI, Body mass index.
Among participants with fibroids and no hysterectomy, average age of fibroid diagnosis was 41.3 years (standard deviation, 9.6); among participants with hysterectomy and no fibroids, average age of surgery was 37.6 (SD, 8.4); among participants with both fibroids and hysterectomy, average age of fibroids was 37.5 (SD = 7.7), with an average gap of 3.0 years (SD, 5.7) between fibroid diagnosis and surgery.
aExposure status at end of follow-up.
bAdjusted for self-identified race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic/Latina, other), attained education (high school equivalent or less, some college/technical degree, completed college, graduate degree), alcohol use (nondrinker, drinker <7 drinks/week, drinker >7 drinks/week), smoking status (never, former, current), parity (0, 1, 2, 3+ children), menopause status at enrollment (pre- or postmenopausal), self-reported body mass index at enrollment (continuous, kg/m2), age at menarche (continuous, years), ever hormonal birth control use (yes/no), and endometriosis status (ever, never).
cCompared to those with no fibroids, no hysterectomy (n = 23 423; 117 cases).
The HR for ovarian cancer associated with endometriosis was higher among non-Hispanic Black women (HR, 2.21; 95% CI, 0.75-6.41) than among non–Hispanic White women (HR, 1.07, CI, 0.73-1.56, Table 4). Though the estimates for ever vs never having endometriosis were not statistically heterogenous (P = .26), there was possible racial heterogeneity for some of the endometriosis-hysterectomy combinations (P = .07). For example, the HR for hysterectomy, no endometriosis (vs neither) was 1.48 (95% CI, 1.04-2.11) among White women and 0.38 (95% CI, 0.11-1.26) among Black women. For obesity-stratified analyses, the association between endometriosis and ovarian cancer incidence was positive (HR, 1.81; 95% CI, 1.04-3.16) among women with BMI >30 kg/m2, but close to null (HR, 0.92; 95% CI, 0.59-1.42, Table 4) among women with BMI <30 kg/m2 (P-for-heterogeneity = 0.06). There were no clear differences by parity or for endometriosis-hysterectomy combinations across any of the strata of interest. The estimated HRs for analyses limited to medically confirmed cases were nearly identical to the overall findings, but endometriosis was not associated with serous ovarian cancer (HR, 1.00; 95% CI, 0.58-1.73).
Table 4.
Subgroup-specific associations between endometriosis (with or without hysterectomy) and incident ovarian cancer (n = 40 928, including 274 cases).
| Endometriosis | Endometriosis, no hysterectomy a (N = 3277; 22 cases) HR b , c (95% CI) | No endometriosis, hysterectomy a (N = 6141; 57 cases) HR b , c (95% CI) |
Endometriosis, hysterectomy
a
(N = 1982; 19 cases)
HR b , c (95% CI) |
||
|---|---|---|---|---|---|
|
Cases (endo/
no endo) a |
HR (95% CI) b | ||||
| Overall | 41/233 | 1.16 (0.82-1.63) | 1.14 (0.73-1.79) | 1.31 (0.94-1.82) | 1.37 (0.84-2.25) |
| Race/Ethnicity | |||||
| Non-Hispanic White | 33/203 | 1.07 (0.73-1.56) | 1.20 (0.75-1.93) | 1.48 (1.04-2.11) | 1.14 (0.63-2.04) |
| Non-Hispanic Black | 5/18 | 2.21 (0.76-6.41) | 0.65 (0.08-5.32) | 0.37 (0.11-1.26) | 2.80 (0.83-9.46) |
| p-for-heterogeneity | 0.26 | 0.07 | |||
| BMI (kg/m2) | |||||
| <30 kg/m2 | 24/168 | 0.92 (0.59-1.43) | 0.85 (0.48-1.51) | 1.35 (0.89-2.03) | 1.20 (0.63-2.29) |
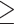 30 kg/m2
30 kg/m2
|
17/65 | 1.81 (1.04-3.16) | 2.16 (1.05-4.44) | 1.28 (0.73-2.25) | 1.76 (0.81-3.83) |
| p-for-heterogeneity | 0.06 | 0.38 | |||
| Parous | |||||
| No | 11/48 | 0.98 (0.50-1.92) | 1.27 (0.63-2.59) | 1.18 (0.49-2.84) | 0.32 (0.04-2.40) |
| Yes | 30/185 | 1.24 (0.83-1.83) | 1.02 (0.57-1.85) | 1.31 (0.92-1.88) | 1.68 (1.00-2.81) |
| p-for-heterogeneity | 0.60 | 0.32 | |||
| Medically confirmed ovarian cancer (n = 214) | 33/181 | 1.20 (0.81-1.76) | 1.14 (0.68-1.89) | 1.29 (0.89-1.88) | 1.47 (0.85-2.52) |
| Serous ovarian cancer (n = 116) | 16/100 | 1.00 (0.58-1.73) | 1.02 (0.50-2.06) | 1.26 (0.77-2.06) | 1.12 (0.50-2.51) |
Abbreviation: BMI, Body mass index.
Among participants with endometriosis and no hysterectomy, average age of endometriosis diagnosis was 32.8 (SD, 9.0), among participants with hysterectomy and no endometriosis, average age of surgery was 39.2 (SD, 7.9), among participants with both endometriosis and hysterectomy, average age of endometriosis was 33.8 (SD, 8.1), with an average gap of 4.4 years (SD, 7.2) between endometriosis diagnosis and surgery.
aExposure status at end of follow-up.
bAdjusted for self-identified race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic/Latina, other), attained education (high school equivalent or less, some college/technical degree, completed college, graduate degree), alcohol use (nondrinker, drinker <7 drinks/week, drinker >7 drinks/week), smoking status (never, former, current), parity (0, 1, 2, 3+ children), menopause status at enrollment (pre- or post-menopausal), self-reported body mass index at enrollment (continuous, kg/m2), age at menarche (continuous, years), ever hormonal birth control use (yes/no), and fibroid status (ever, never).
cCompared to those with no endometriosis, no hysterectomy (n = 29 528; 176 cases).
Lastly, when considering joint exposure of hysterectomy and either of the benign uterine conditions, the association between hysterectomy and ovarian cancer was elevated for all groups, but it was most pronounced among women with hysterectomy but no endometriosis or fibroids compared to women without hysterectomy or either condition (HR, 2.04; 95% CI, 1.35-3.09; Table 5). The positive association between hysterectomies and ovarian cancer was apparent among non-Hispanic White women (HR, 1.37; 95% CI, 0.98-1.90) but not among non-Hispanic Black women (HR, 0.72; 95% CI, 0.29-1.77, P-for-heterogeneity = 0.23). Obesity and parity did not modify the association. HRs were similar for medically confirmed (HR, 1.29; 95% CI, 0.9-1.80) and serous (HR, 1.23; 95% CI, 0.78-1.94) ovarian cancer.
Table 5.
Subgroup-specific associations between hysterectomy and incident ovarian cancer (n = 40 928, including 274 cases).
| Hysterectomy |
No hysterectomy,
with fibroids or endometriosis a (N = 11 113; 90 cases) HR b , c (95% CI) |
Hysterectomy, no endometriosis or fibroids a (N = 3029; 31 cases) HR b , c (95% CI) |
Hysterectomy, with endometriosis or fibroids
a
(N = 5094; 45 cases) HR b , c (95% CI) |
||
|---|---|---|---|---|---|
|
Cases (hyst/
no hyst) a |
HR b (95% CI) | ||||
| Overall | 76/198 | 1.29 (0.95-1.74) | 1.51 (0.80-2.84) | 2.04 (1.35-3.09) | 1.42 (0.69-2.93) |
| Race/Ethnicity | |||||
| Non-Hispanic White | 64/172 | 1.37 (0.98-1.90) | 1.47 (0.73-2.96) | 2.20 (1.44-3.38) | 1.40 (0.63-3.10) |
| Non-Hispanic Black | 7/16 | 0.72 (0.29-1.77) | 1.31 (0.12-13.8) | 1.13 (0.13-10.2) | 0.86 (0.08-9.97) |
| p-for-heterogeneity | 0.23 | 0.89 | |||
| BMI (kg/m 2 ) | |||||
| <30 kg/m2 | 49/143 | 1.36 (0.93-1.97) | 0.94 (0.42-2.10) | 2.20 (1.38-3.79) | 0.90 (0.37-2.21) |
| 30 kg/m2 | 27/55 | 1.14 (0.69-1.90) | 2.83 (0.96-8.33) | 1.65 (0.81-3.39) | 2.50 (0.72-8.65) |
| p-for-heterogeneity | 1.00 | 0.35 | |||
| Parous | |||||
| No | 8/51 | 0.83 (0.36-1.93) | 1.25 (0.32-4.81) | 2.28 (0.53-9.74) | 0.82 (0.15-4.37) |
| Yes | 68/147 | 1.36 (0.99-1.88) | 1.58 (0.76-3.28) | 1.97 (1.28-3.04) | 1.61 (0.72-3.59) |
| p-for-heterogeneity | 0.18 | 0.72 | |||
| Medically confirmed ovarian cancer (n = 214) | 60/154 | 1.29 (0.93-1.80) | 1.68 (0.82-3.46) | 1.88 (1.17-3.01) | 1.70 (0.76-3.82) |
| Serous ovarian cancer (n = 116) | 31/85 | 1.23 (0.78-1.94) | 3.04 (1.08-8.57) | 1.80 (0.92-3.50) | 3.01 (0.93-9.73) |
Abbreviation: BMI, Body mass index.
Among participants with endometriosis or fibroids and no hysterectomy, average age of first diagnosis of either condition was 38.8 (SD, 10.4), among participants with hysterectomy and no endometriosis or fibroids, average age of surgery was 37.9 (SD, 8.7), among participants with hysterectomy and either fibroids or endometriosis, average age of first diagnosis of either condition was 36.1 (SD, 8.3), with an average gap of 1.7 years (SD, 8.4) between last diagnosis and surgery.
aExposure status at end of follow-up.
bAdjusted for self-identified race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic/Latina, other), attained education (high school equivalent or less, some college/technical degree, completed college, graduate degree), alcohol use (nondrinker, drinker <7 drinks/week, drinker >7 drinks/week), smoking status (never, former, current), parity (0, 1, 2, 3+ children), menopause status at enrollment (pre- or postmenopausal), self-reported body mass index at enrollment (continuous, kg/m2), age at menarche (continuous, years), ever hormonal birth control use (yes/no), fibroid status (ever, never), and endometriosis status (ever, never).
cCompared to those with no hysterectomy, no fibroids or endometriosis (n = 21 692; 108 cases).
None of the sensitivity analyses materially altered the reported findings (Table S2). Based on concerns that women who received hysterectomies were inherently different from women who did not, we compared frequencies of medical conditions and reasons for hysterectomy among women with and without hysterectomy and among women with and without ovarian cancer (Table S3). In addition to being more likely to have had fibroids or endometriosis, women with hysterectomies were more likely to have had polycystic ovarian syndrome, pelvic inflammatory disease, and ovarian cysts. The most common reason for hysterectomy was abnormal bleeding (71%) followed by pelvic pain (57%) and fibroids (53%).
Discussion
Our findings from this large, prospective cohort study provide evidence that both fibroids and hysterectomies (without bilateral oophorectomies) are independently, positively associated with ovarian cancer risk. The association between history of endometriosis and ovarian cancer incidence was weaker, though also positive. In assessments of joint exposures, relative hazards were consistently higher for women who had either fibroids or hysterectomy, relative to neither. The positive relationship between fibroids and ovarian cancer was modestly attenuated among women who also had a hysterectomy, but our data did not support the hypothesis that having a hysterectomy may mitigate ovarian cancer risk for those with endometriosis.
Although both fibroids and hysterectomies were more common among non-Hispanic Black women than other racial/ethnic groups, effect estimates for those factors were generally consistent across strata defined by race. As an exception, we observed some possible modification by race when assessing the joint effects of endometriosis and hysterectomy. The HRs for the estimated effects of fibroids and/or hysterectomy remained elevated in analyses limited to serous ovarian cancer cases, but endometriosis was not associated with the serous histotype.
Our finding of a positive association between fibroids and ovarian cancer risk are consistent with prior studies of this topic.2,7 Though the positive association between endometriosis and ovarian cancer was largely attenuated after adjustment for fibroids, the observed HR is in the same direction as that reported by several other studies.2-6
However, our finding of a positive association between hysterectomies and ovarian cancer is somewhat in contrast with prior reports, including a 2019 meta-analysis,9 a 2016 pooled cohort analysis that included the Sister Study,5 and 2 recent registry-based studies,8,10 all of which reported no independent association between hysterectomy and overall ovarian cancer risk. Although many of these studies reported inverse associations between hysterectomies and endometrioid and clear cell histotypes,5,9,10 due to sample size constraints we were only able to independently consider serous ovarian cancers. Notably, a review and meta-analysis published in 201329 noted a temporal shift in the direction of the association, from inverse prior to 2000, to positive in studies conducted after that time. The current findings are consistent with the latter time frame. The authors suggest that the change could be driven by trends discouraging hysterectomies in young women and the use of unopposed estrogen hormone therapy in women who do have their uteri removed.
The mechanisms underlying the association between hysterectomy and ovarian cancer in the absence of fibroids or endometriosis is uncertain but may be entwined with hysterectomy indications. For example, women with hysterectomies were more likely to have polycystic ovarian syndrome, pelvic inflammatory disease, and ovarian cysts, all of which are inflammatory conditions with potential links to ovarian cancer.24,30-32 In our sample and others,17,18 the most common reasons for hysterectomy include abnormal bleeding, pelvic pain, and fibroids. Based on concerns that these symptoms, particularly pelvic pain, could be early symptoms of undiagnosed ovarian cancer, we ran sensitivity analyses that considered hysterectomy status to be a time-fixed exposure, based on status at the time of enrollment, and examined its association with incident (ie postenrollment) ovarian cancer diagnoses. This meant that among the 17% of participants who had prebaseline hysterectomies, those who experienced such symptoms in the time leading up to their surgery typically had them several years, if not decades, prior to their ovarian cancer diagnosis. Similarly, we considered lagged time-varying analyses, where women were not considered exposed to a hysterectomy (or fibroid or endometriosis) until at least a year after the surgery or diagnosis occurred. The similarity between the results of these sensitivity analyses and our main time-varying results implies undiagnosed ovarian cancer was not a major driver of the positive association we observed between hysterectomy and incident ovarian cancer.
Unmeasured confounding remains a plausible explanation for the difference between our study and prior work. Additionally, our cohort is unique in that all participants had a full or half-sister with breast cancer and are thus inherently more likely to be BRCA1/2 mutation carriers. Because of this and other shared genetic and environmental factors, Sister Study participants have a higher risk of developing both breast and ovarian cancer compared to women without a family history of breast cancer. Broadly, this may mean that associations observed in the Sister Study are not generalizable to other populations.
Although not a statistically significant finding, the inverse association we observed between hysterectomies and ovarian cancer among women with fibroids was consistent with prior literature.2,7,8 However, we did not see evidence consistent with the previously observed protective benefits of hysterectomy among women diagnosed with endometriosis.2,8,10
The co-occurrence of fibroids and endometriosis was rare overall, but the 2 uterine conditions overlapped more than would be expected by chance and having both was positively associated with ovarian cancer incidence. These 2 conditions likely have shared genetic and environmental risk factors with each other11-14 and might also share common causes with breast, ovarian, or other hormone-related cancers.33,34
African American women typically have higher incidence of fibroids and a greater burden of the disease compared to non-Hispanic White women11,17,19,33 and are also more likely to undergo a hysterectomy.33,35,36 The effect estimates for fibroids and hysterectomy were fairly consistent across the 2 racial groups we compared, though we did observe some potential racial differences in the association between the combinations of endometriosis and hysterectomy and ovarian cancer incidence. This is somewhat in contrast with the findings reported by Harris et al,2 who found that history of fibroids or endometriosis were similarly associated with greater odds of ovarian cancer among both Black and non-Hispanic White women. Although numerous studies have demonstrated that nulliparity is associated with an increased risk of both fibroids or endometriosis,12,33,34 we did not observe effect modification by parity in our analyses.
Due to the rarity of ovarian cancer, we had low power for stratified analysis. For our race-stratified estimates, we limited comparisons to non-Hispanic White and non-Hispanic Black women and could not separately consider the effects among women who identified as Latina or a different racial/ethnic group. We could only consider the most common subtype of serous ovarian cancer for this analysis, but the Sister Study contributed to a 2016 pooled analysis of histotype-specific associations.5 In these pooled analyses, hysterectomies were not associated with ovarian cancer overall but were inversely associated with clear cell and possibly mucinous ovarian cancers. The HR for endometriosis and overall ovarian cancer was positive, with the strongest associations seen for clear cell and endometrioid histotypes.
Strengths of this analysis include its prospective design, which eliminates the concern of recall bias, which can be present in retrospective studies if there is differential reporting of exposure for women with or without ovarian cancer. We had detailed information on many important confounders and confirmed most of the ovarian cancer diagnoses via medical records or linkage to death certificates. Our thorough analysis of the individual and joint association of these interconnected factors, including within subcategories of race, parity, and BMI, provides new clues to their role in ovarian cancer etiology.
Conclusions
Sister Study participants who reported fibroids, hysterectomy, or both had higher rates of ovarian cancer compared to those without those exposures. A history of endometriosis may also be positively associated with ovarian cancer incidence. Although our results were consistent with our theory that having a hysterectomy may be associated with a lower risk of ovarian cancer among women with fibroids, they did not support our parallel hypothesis that hysterectomies may mitigate the effects of endometriosis.
Understanding these joint associations may help inform clinical decisions about treatments for fibroids and endometriosis and guide surveillance of women who have received a hysterectomy or who have been diagnosed with either condition. Future investigations that included long-term follow-up of young women at risk of fibroids, endometriosis, or incident hysterectomy, coupled with detailed information on other inflammation-related conditions, hormone use, and treatments, could provide additional insights into the etiologies and long-term health impacts of these gynecological conditions and surgeries.
Supplementary Material
Contributor Information
Hana Tekle, University of Maryland, College Park (HT), College Park, Maryland, United States.
Dale P Sandler, Epidemiology Branch, National Institute of Environmental Health Sciences, Research Triangle Park, NC, United States.
Kemi Ogunsina, Epidemiology Branch, National Institute of Environmental Health Sciences, Research Triangle Park, NC, United States.
Katie M O’Brien, Epidemiology Branch, National Institute of Environmental Health Sciences, Research Triangle Park, NC, United States.
Supplementary material
Supplementary material is available at the American Journal of Epidemiology online.
Funding
This work was funded by the intramural research program of the National Institute of Environmental Health Sciences, National Institutes of Health (Z01-ES044005 to DPS).
Conflict of interest
The authors have no conflicts of interest to declare.
Data availability
Requests for deidentified Sister Study data, including the data used in this manuscript, can be made through the Sister Study website (https://www.sisterstudystars.org/).
References
- 1. Epidemiology and End Results Program NCIS . Cancer Stat Facts: Ovarian Cancer. 2023. Accessed August 1, 2023. https://seer.cancer.gov/statfacts/html/ovary.html
- 2. Harris HR, Peres LC, Johnson CE, et al. Racial differences in the Association of Endometriosis and Uterine Leiomyomas with the risk of ovarian cancer. Obstet Gynecol. 2023;141(6):1124-1138. 10.1097/AOG.0000000000005191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brinton LA, Sakoda LC, Sherman ME, et al. Relationship of benign gynecologic diseases to subsequent risk of ovarian and uterine Tumors. Cancer Epidemiol Biomarkers Prev. 2005;14(12):2929-2935. 10.1158/1055-9965.EPI-05-0394 [DOI] [PubMed] [Google Scholar]
- 4. Wang C, Liang Z, Liu X, et al. The association between endometriosis, tubal ligation, hysterectomy and epithelial ovarian cancer: meta-analyses. Int J Environ Res Public Health. 2016;13(11):1138. 10.3390/ijerph13111138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wentzensen N, Poole EM, Trabert B, et al. Ovarian cancer risk factors by histologic subtype: an analysis from the ovarian cancer cohort consortium. J Clin Oncol. 2016;34(24):2888-2898. 10.1200/JCO.2016.66.8178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park HK, Schildkraut JM, Alberg AJ, et al. Benign gynecologic conditions are associated with ovarian cancer risk in African-American women: a case-control study. Cancer Causes Control. 2018;29(11):1081-1091. 10.1007/s10552-018-1082-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tseng JJ, Huang CC, Chiang HY, et al. Prior uterine myoma and risk of ovarian cancer: a population-based case-control study. J Gynecol Oncol. 2019;30(5):e72. 10.3802/jgo.2019.30.e72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dixon-Suen SC, Webb PM, Wilson LF, et al. The association between hysterectomy and ovarian cancer risk: a population-based record-linkage study. J Natl Cancer Inst. 2019;111(10):1097-1103. 10.1093/jnci/djz015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huo X, Yao L, Han X, et al. Hysterectomy and risk of ovarian cancer: a systematic review and meta-analysis. Arch Gynecol Obstet. 2019;299(3):599-607. 10.1007/s00404-018-5020-1 [DOI] [PubMed] [Google Scholar]
- 10. Ring LL, Baandrup L, Zheng G, et al. Hysterectomy and risk of epithelial ovarian cancer by histologic type, endometriosis, and menopausal hormone therapy. Cancer Epidemiol. 2023;84:102359. 10.1016/j.canep.2023.102359 [DOI] [PubMed] [Google Scholar]
- 11. Katon JG, Plowden TC, Marsh EE. Racial disparities in uterine fibroids and endometriosis: a systematic review and application of social, structural, and political context. Fertil Steril. 2023;119(3):355-363. 10.1016/j.fertnstert.2023.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Uimari O, Järvelä I, Ryynänen M. Do symptomatic endometriosis and uterine fibroids appear together? J Hum Reprod Sci. 2011;4(1):34-38. 10.4103/0974-1208.82358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnatty SE, Stewart CJR, Smith D, et al. Co-existence of leiomyomas, adenomyosis and endometriosis in women with endometrial cancer. Sci Rep. 2020;10(1):3621. 10.1038/s41598-020-59916-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McGrath IM, Montgomery GW, Mortlock S. Insights from mendelian randomization and genetic correlation analyses into the relationship between endometriosis and its comorbidities. Hum Reprod Update. 2023;29(5):655-674. 10.1093/humupd/dmad009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kalaitzopoulos DR, Samartzis N, Kolovos GN, et al. Treatment of endometriosis: a review with comparison of 8 guidelines. BMC Womens Health. 2021;21(1):397. 10.1186/s12905-021-01545-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spaczynski RZ, Duleba AJ. Diagnosis of endometriosis. Semin Reprod Med. 2003;21(2):193-208. 10.1055/s-2003-41326 [DOI] [PubMed] [Google Scholar]
- 17. Stewart EA, Nicholson WK, Bradley L, et al. The burden of uterine fibroids for African-American women: results of a National Survey. J Womens Health. 2013;22(10):807-816. 10.1089/jwh.2013.4334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baird DD, Harmon QE, Upson K, et al. A prospective, ultrasound-based study to evaluate risk factors for uterine fibroid incidence and growth: methods and results of recruitment. J Womens Health. 2015;24(11):907-915. 10.1089/jwh.2015.5277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moorman PG, Leppert P, Myers ER, et al. Comparison of characteristics of fibroids in African American and white women undergoing premenopausal hysterectomy. Fertil Steril. 2013;99(3):768-776.e1. 10.1016/j.fertnstert.2012.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Giuliani E, As-Sanie S, Marsh EE. Epidemiology and management of uterine fibroids. Int J Gynecol Obstet. 2020;149(1):3-9. 10.1002/ijgo.13102 [DOI] [PubMed] [Google Scholar]
- 21. Samimi G, Sathyamoorthy N, Tingen CM, et al. Report of the National Cancer Institute and the Eunice Kennedy Shriver National Institute of Child Health and Human Development–sponsored workshop: gynecology and women’s health–benign conditions and cancer. Am J Obstet Gynecol. 2020;223(6):796-808. 10.1016/j.ajog.2020.08.049 [DOI] [PubMed] [Google Scholar]
- 22. King LA, Wentzensen N, Purdue MP, et al. Inflammatory markers in women with reported benign gynecologic pathology: an analysis of the prostate, lung, colorectal and ovarian cancer screening trial. Ann Epidemiol. 2022;68:1-8. 10.1016/j.annepidem.2021.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Elkafas H, Bariani MV, Ali M, et al. Pro-inflammatory and immunosuppressive environment contributes to the development and progression of uterine fibroids. Fertil Steril. 2020;114(3):e87. 10.1016/j.fertnstert.2020.08.264 [DOI] [Google Scholar]
- 24. Ness RB, Grisso JA, Cottreau C, et al. Factors related to inflammation of the ovarian epithelium and risk of ovarian cancer. Epidemiology. 2000;11(2):111-117. 10.1097/00001648-200003000-00006 [DOI] [PubMed] [Google Scholar]
- 25. Leenen S, Hermens M, Van Steenwijk DV, et al. Immunologic factors involved in the malignant transformation of endometriosis to endometriosis-associated ovarian carcinoma. Cancer Immunol Immunother. 2021;70(7):1821-1829. 10.1007/s00262-020-02831-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev. 2013;34(1):130-162. 10.1210/er.2012-1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pellegrini C, Gori I, Achtari C, et al. The expression of estrogen receptors as well as GREB1, c-MYC, and cyclin D1, estrogen-regulated genes implicated in proliferation, is increased in peritoneal endometriosis. Fertil Steril. 2012;98(5):1200-1208. 10.1016/j.fertnstert.2012.06.056 [DOI] [PubMed] [Google Scholar]
- 28. Laughlin GA, Barrett-Connor E, Kritz-Silverstein D. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2000;85(2):645, 651. 10.1210/jc.85.2.645 [DOI] [PubMed] [Google Scholar]
- 29. Jordan SJ, Nagle CM, Coory MD, et al. Has the association between hysterectomy and ovarian cancer changed over time? A systematic review and meta-analysis. Eur J Cancer. 2013;49(17):3638-3647. 10.1016/j.ejca.2013.07.005 [DOI] [PubMed] [Google Scholar]
- 30. Jonsson S, Jonsson H, Lundin E, et al. Pelvic inflammatory disease and risk of epithelial ovarian cancer: a national population-based case-control study in Sweden. Am J Obstet Gynecol. 2024;230(1):75.e1-75.e15. 10.1016/j.ajog.2023.09.094 [DOI] [PubMed] [Google Scholar]
- 31. Zhou Z, Zeng F, Yuan J, et al. Pelvic inflammatory disease and the risk of ovarian cancer: a meta-analysis. Cancer Causes Control. 2017;28(5):415-428. 10.1007/s10552-017-0873-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harris HR, Terry KL. Polycystic ovary syndrome and risk of endometrial, ovarian, and breast cancer: a systematic review. Fertil Res Pract. 2016;2(1):14. 10.1186/s40738-016-0029-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pavone D, Clemenza S, Sorbi F, et al. Epidemiology and risk factors of uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2018;46:3-11. 10.1016/j.bpobgyn.2017.09.004 [DOI] [PubMed] [Google Scholar]
- 34. Parazzini F, Esposito G, Tozzi L, et al. Epidemiology of endometriosis and its comorbidities. Eur J Obstet Gynecol Reprod Biol. 2017;209:3-7. 10.1016/j.ejogrb.2016.04.021 [DOI] [PubMed] [Google Scholar]
- 35. Eltoukhi HM, Modi MN, Weston M, et al. The health disparities of uterine fibroid tumors for African American women: a public health issue. Am J Obstet Gynecol. 2014;210(3):194-199. 10.1016/j.ajog.2013.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wechter ME, Stewart EA, Myers ER, et al. Leiomyoma-related hospitalization and surgery: prevalence and predicted growth based on population trends. Am J Obstet Gynecol. 2011;205(5):492.e1-492.e5. 10.1016/j.ajog.2011.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Requests for deidentified Sister Study data, including the data used in this manuscript, can be made through the Sister Study website (https://www.sisterstudystars.org/).


