Abstract
During lytic infections, the virion host shutoff (Vhs) protein of herpes simplex virus accelerates the degradation of both host and viral mRNAs. In so doing, it helps redirect the cell from host to viral protein synthesis and facilitates the sequential expression of different viral genes. Vhs interacts with the cellular translation initiation factor eIF4H, and several point mutations that abolish its mRNA degradative activity also abrogate its ability to bind eIF4H. In addition, a complex containing bacterially expressed Vhs and a glutathione S-transferase (GST)-eIF4H fusion protein has RNase activity. eIF4H shares a region of sequence homology with eIF4B, and it appears to be functionally similar in that both stimulate the RNA helicase activity of eIF4A, a component of the mRNA cap-binding complex eIF4F. We show that eIF4H interacts physically with eIF4A in the yeast two-hybrid system and in GST pull-down assays and that the two proteins can be coimmunoprecipitated from mammalian cells. Vhs also interacts with eIF4A in GST pull-down and coimmunoprecipitation assays. Site-directed mutagenesis of Vhs and eIF4H revealed residues of each that are important for their mutual interaction, but not for their interaction with eIF4A. Thus, Vhs, eIF4H, and eIF4A comprise a group of proteins, each of which is able to interact directly with the other two. Whether they interact simultaneously as a tripartite complex or sequentially is unclear. The data suggest a mechanism for linking the degradation of an mRNA to its translation and for targeting Vhs to mRNAs and to regions of translation initiation.
During lytic infections with herpes simplex virus (HSV), viral and cellular gene expression is regulated through a complex interplay of transcriptional and posttranscriptional controls (68). Of the posttranscriptional mechanisms, one of the best characterized is the degradation of host and viral mRNAs by the HSV virion host shutoff (Vhs) protein (UL41) (15, 59). Vhs is an endoribonuclease that is a minor structural component of HSV virions (12, 15, 61, 72, 92). Following uncoating, copies of Vhs from the infecting virions destabilize host mRNAs in the cytoplasm (19, 70, 81). This, together with the inhibition of pre-mRNA splicing by the HSV immediate-early protein ICP27 (24, 25), contributes to an overall decrease in host protein synthesis. Following the onset of viral transcription, Vhs accelerates the turnover of viral mRNAs from all kinetic classes (38, 53, 54, 81). In this role, it helps determine viral mRNA levels and facilitates sequential expression of different classes of viral genes (38, 53, 54, 59).
While mutations that inactivate Vhs have a modest effect upon virus growth in cell culture (39, 60, 61), they have a striking impact upon HSV virulence in animals (3, 22, 31, 32, 40, 52, 74, 75, 78-80). The mechanisms by which Vhs affects pathogenesis are unclear, but they appear to involve effects upon both the adaptive and innate immune responses to HSV infection (73). Vhs impedes antigen presentation by major histocompatibility complex class I (85) and class II (87) molecules; inhibits the secretion of cytokines which would otherwise recruit lymphocytes, neutrophils, or other gamma-ray-sensitive cells to the site of infection (82); and blocks the activation of infected dendritic cells (69). vhs mutations of HSV type 2 (HSV-2) are severely attenuated in normal mice (52, 75) yet are almost as virulent as wild-type virus in alpha/beta interferon receptor knockout animals, suggesting that Vhs impedes the alpha/beta interferon-mediated response to infection (52).
Recent studies have shown that Vhs interacts with the cellular translation initiation factor eIF4H in the yeast two-hybrid system, in vitro, and in mammalian cells (15, 18). eIF4H shares a domain of sequence homology with eIF4B and, like eIF4B, stimulates the ATP-dependent RNA helicase activity of eIF4A, as well as the in vitro translation activity of the cap-binding complex eIF4F (27, 62-67). eIF4A, in turn, is a DEAD-box helicase that is a component of eIF4F and acts at an early stage of ribosome scanning (23, 27, 66, 76, 83).
To date, every Vhs mutant we have examined that fails to bind eIF4H also fails to degrade mRNAs that are translated by cap-dependent ribosome scanning (15, 18). While this suggests the interaction is biologically significant, the precise role, if any, of eIF4H in Vhs-mediated decay is unclear. One possibility is that binding to eIF4H is important for determining the target specificity of Vhs. Several studies suggest that, in the absence of key cellular factors, the Vhs endonuclease lacks the target specificity seen in vivo (15, 92). Extracts of detergent-solubilized virions contain a Vhs-dependent endonuclease that is not restricted to mRNAs and that cleaves target RNAs at numerous sites throughout the molecule (92). Similarly, a complex of bacterially expressed Vhs and GST-eIF4H degrades target RNAs to fragments that can no longer be seen on an agarose gel, implying that it cuts RNAs at many sites (15). In contrast, within infected cells Vhs exhibits two important kinds of selectivity. First, it shows a strong preference for mRNAs, as opposed to non-mRNAs. This is true in vivo (53, 54, 70, 81), as well as in in vitro decay reactions containing cytoplasmic extracts from infected cells (37, 77) or in in vitro-translated Vhs (92). Second, within mRNAs, Vhs initiates cleavage at preferred sites (12-14, 30, 84). For a number of mRNAs, these preferred sites are near regions of translation initiation (12, 13, 30). Thus, in infected cells, sequences near the 5′ end of the HSV thymidine kinase mRNA are degraded prior to those at the 3′ end of the transcript (30), while in vitro-translated Vhs cleaves SRPα mRNA predominantly at sites in the 5′ quadrant of the mRNA, including a cluster in the 5′ untranslated region (5′ UTR) and near the start codon (12). Similarly, in vitro-translated Vhs cleaves mRNAs containing an encephalomyocarditis virus (EMCV) internal ribosome entry site (IRES) at sites downstream from the IRES (13, 44).
The observation that Vhs binds a cellular translation factor suggests obvious possibilities for how a sequence nonspecific endonuclease is targeted to mRNAs, as opposed to non-mRNAs, and to preferred sites within mRNAs (15, 18). However, even if binding to eIF4H is required for Vhs targeting, it cannot be sufficient, since a complex of recombinant Vhs and GST-eIF4H is still untargeted (15). This suggests that, besides eIF4H, one or more additional factors are required for Vhs targeting in vivo. In this light, we further examined protein-protein interactions involving Vhs and eIF4H. eIF4H was found to interact with eIF4AII, one of the three isoforms of eIF4A, in the yeast two-hybrid system, in in vitro-binding studies, and in mammalian cells. Vhs was also found to bind eIF4AII. Site-directed mutagenesis of Vhs identified mutations that abrogate binding to eIF4H but not to eIF4AII, suggesting that the interaction between Vhs and eIF4AII is direct and does not require a protein bridge provided by eIF4H. Similarly, mutagenesis of eIF4H revealed some mutations that affect its binding to Vhs but not to eIF4AII and others that affect its interaction with eIF4AII but not with Vhs. This indicates the interactions of eIF4H with Vhs and eIF4AII can be distinguished genetically. Vhs, eIF4H, and eIF4AII thus form a group of three proteins, each of which is able to interact with the other two. The data illuminate the interactions involving Vhs and cellular translation factors, interactions that may play key roles in recruiting Vhs to mRNAs, in targeting it to preferred cleavage sites, and in linking the degradation of an mRNA to its translation.
MATERIALS AND METHODS
Cells and virus.
CV-1 cells were purchased from the American Type Culture Collection and maintained in Eagle's minimum essential medium (Gibco) containing 10% (vol/vol) calf serum and antibiotics as described previously (16, 54, 61). The recombinant vaccinia virus vTF7 expresses the T7 RNA polymerase (51).
Antibodies.
A polyclonal rabbit antiserum raised against a Vhs-LacZ fusion protein has been described previously (61). Monoclonal antibody that reacts with an epitope of the influenza virus hemagglutinin (HA) was purchased from Covance. Anti-Flag monoclonal antibody and anti-Flag monoclonal antibody conjugated to M2 agarose beads were purchased from Sigma.
Plasmids. (i) Plasmids containing wild-type and mutant vhs alleles.
pKOSamp contains the Vhs open reading frame from HSV-1 (KOS) cloned into pcDNA1.1amp (Invitrogen) downstream from a promoter for T7 RNA polymerase and the cytomegalovirus immediate-early promoter (16, 17). It is suitable for expressing the Vhs polypeptide by in vitro transcription and translation or after transfection of mammalian cells. D34N, D82N, E192Q, D194N, D195N, T211S, D213N, D215N, and D261N are mutant vhs alleles constructed by site-directed mutagenesis of pKOSamp (15, 18). The R435H allele contains a spontaneous point mutation that changes arginine 435 to histidine (17). T214I contains a point mutation that changes threonine 214 to isoleucine and is the allele carried by the mutant virus Vhs 1 (16-18, 54, 55, 60). The deletion mutants K(89-489), K(1-453), K(1-382), and ΔSma have been described previously (18). All mutant vhs alleles were cloned into the vector pcDNA1.1amp.
(ii) Plasmids for yeast two-hybrid interactions.
pAS2-Vhs contains the Vhs open reading frame cloned into pAS2-1 (Clontech) and encodes a fusion protein of the Gal4 DNA-binding domain and the entire Vhs polypeptide (18). pAS2-4H contains the entire eIF4H open reading frame inserted into pAS2-1 and encodes a Gal4 DNA binding domain/eIF4H fusion protein (18). Similarly, pAS2-4HI encodes a fusion protein with the Gal 4 DNA-binding domain fused to an eIF4H isoform with a 20-amino-acid insertion after residue 137 (18).
The plasmids pACT2-Vhs, pACT2-4H, and pACT2-4Hi contain the Vhs, eIF4H, and eIF4Hi open reading frames cloned into the Gal4 activation domain vector pACT2 (Clontech). They encode fusions proteins of the Gal4 activation domain and the Vhs, eIF4H, and eIF4Hi polypeptides (18). pAS2-4Hi was used to screen a commercially available library of HeLa cell cDNAs cloned into pACT2. pACT2-4AII is a plasmid isolated from that library.
(iii) Plasmids for GST pull-down experiments.
pGST-4H and pGST-4Hi contain the eIF4H and eIF4Hi open reading frames cloned into the vector pGEX-5-3 (Amersham-Pharmacia) (18). They encode GST-eIF4H and GST-eIF4Hi fusion proteins that can be expressed in bacteria. To construct pGST-4AII, the eIF4AII open reading frame was PCR amplified from pACT2-4AII and inserted between the BamHI and SalI sites of pGEX-5-3. It encodes a fusion protein of GST and eIF4AII. To produce 35S-labeled target protein for GST pull-down experiments, the open reading frame for eIF4AII was PCR amplified from pACT2-4AII and cloned into pcDNA1.1amp downstream from a promoter recognized by T7 RNA polymerase to yield pcDNA-4AII. This plasmid was used to produce eIF4AII by in vitro transcription and translation. A similar procedure was used to construct pcDNA-4AII38-407, which encodes a form of eIF4AII lacking amino acids 1 through 37 of the wild-type polypeptide.
(iv) Plasmids for transient expression of Vhs, eIF4H, and eIF4AII.
pTM1-4H and PTM1-4Hi express HA-tagged eIF4H and eIF4Hi upon transfection of mammalian cells previously infected with vaccinia virus encoding the T7 RNA polymerase (18). Similarly, pTM1-Vhs allows transient expression of untagged Vhs (18). To express FLAG-tagged eIF4AII, the eIF4AII open reading frame was PCR amplified from pACT2-4AII using an upstream primer encoding the FLAG epitope. The resulting amplified FLAG-eIF4AII sequences were cloned between the NcoI and SacI sites of pTM1, to yield pTM1-4AII.
Yeast two-hybrid screen.
A search for cellular cDNAs encoding eIF4H-interacting proteins was performed using the Matchmaker Two-Hybrid System 2 (Clontech) according to standard techniques (21), as described previously for the identification of Vhs-interacting proteins (18). Yeast strain Y190, which contains the his3 and lacZ genes driven by Gal4-responsive promoters, was transformed with pAS2-4Hi. Cells receiving the bait plasmid were selected by growth on synthetic dropout (SD) medium lacking tryptophan (Trp), and transformed with plasmids from a commercial library of HeLa cell cDNAs in the Gal4 activation domain vector pACT2 (Clontech). Cells that grew on SD medium containing 35 mM 3-aminotriazole (3-AT) and lacking tryptophan, leucine, and histidine potentially did so because of activation of the Gal4-responsive his3 gene. These were replica plated onto filters and screened for lacZ expression. cDNA-containing plasmids were isolated from blue colonies, amplified in Escherichia coli strain KC8 (Clontech), sequenced, and compared to known DNA and protein sequences with the BLAST network service and software of the National Center for Biotechnology Information.
Recombination-based two-hybrid assay.
A recombination-based yeast two-hybrid assay (58) was performed as described previously (18). To investigate the interaction of mutant eIF4H polypeptides with Vhs, yeast strain 190 was transformed with a DNA cocktail containing pACT2-Vhs, the large SacII-BamHI fragment of pAS2-4H, and a SalI-linearized pGEX-eIF4H plasmid containing a mutant eIF4H allele. The large SacII-BamHI fragment of pAS2-4H contains the vector sequences from pAS2-4H, plus sequences encoding all of eIF4H except amino acids 22 through 171. SalI cuts pGEX-eIF4H plasmids within the vector sequences. Transformants were plated on SD medium containing 25 mM 3-AT and lacking tryptophan, leucine, and histidine to select for yeast that grew because transcription of the Gal4-responsive his3 gene had been activated. Colonies were replica plated onto filters and tested for expression of the lacZ gene from a Gal4-responsive promoter. Within the yeast, recombination between the SacII-BamHI fragment of pAS2-4H and the mutant eIF4H gene (on the linearized pGEX-4H plasmid) resulted in reconstitution of a pAS2-4H plasmid containing the eIF4H mutation. Transformations that contained a linearized pGEX-eIF4H plasmid carrying the wild-type eIF4H allele gave rise to at least 100 times more LacZ+ colonies on selective medium than did control transformations that received pACT2-Vhs and the large SacII-BamHI fragment of pAS2-4H, but no linearized pGEX-eIF4H plasmid. Mutant eIF4H alleles that gave rise to a comparable number of colonies as wild-type eIF4H were judged to encode proteins that retained Vhs-binding activity. In contrast, several mutant alleles gave rise to significantly fewer lacZ-expressing colonies on selective medium and were judged to encode proteins with diminished ability to bind Vhs. Experiments to test the interaction of eIF4H mutants with eIF4AII were performed using an identical recombination-based two-hybrid protocol, except that pACT2-4AII replaced pACT2-Vhs in the transformation DNA cocktail.
In vitro transcription and translation.
Vhs, eIF4AII, or eIF4AII(38-407) were produced by in vitro transcription and translation from pKOSamp (17), pcDNA-4AII, or pcDNA-4AII(38-407) using the TnT T7 Quick Coupled Transcription/Translation system from Promega (18). Following completion, the reactions were adjusted to 50 mM Tris-HCl (pH 8.0), 100 mM NaCl, 1% (vol/vol) NP-40, and 1 mM EDTA and used in GST pull-down assays.
GST pull-down assays.
For in vitro binding assays, GST, GST-eIF4H, or GST-eIF4AII was expressed in E. coli strain BL21 and isolated by binding to glutathione-Sepharose 4B as described previously (18). Beads with bound GST, GST-eIF4H, or GST-eIF4AII were resuspended in 0.4-ml portions of rabbit reticulocyte lysates containing in vitro-translated target proteins. Thirty minutes later, the beads were pelleted and washed. Complexes containing the GST fusion proteins and any bound polypeptides were eluted with 10 mM glutathione and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography.
Preparation of His-tagged eIF4AII.
To express His-tagged eIF4AII, the eIF4AII open reading frame was PCR amplified from pACT2-4AII and inserted between the XhoI and NdeI sites of pET-19b (Novagen) to yield pET19b-4AII. His-eIF4AII was expressed in E. coli BL21(DE3) and isolated by metal-chelate affinity chromatography (43, 56, 57). Aliquots of His-eIF4AII were added to GST pull-down reaction mixtures to see if it competed with [35S]methionine-labeled in vitro-translated Vhs or eIF4AII for binding GST-eIF4H.
Coprecipitation of eIF4H and eIF4AII and of Vhs and eIF4AII.
CV-1 cells were grown to 90% confluence in 100-mm-diameter petri dishes and infected with vaccinia virus vTF7 (5 PFU/cell) as described previously (18, 41). Thirty minutes later, they were transfected with 5 μg (each) of pTM1-4H and pTM1-4AII or pTM1-4AII and pTM1-Vhs. Twenty hours after transfection, the cells were harvested and lysed, and immunoprecipitates were prepared (18). To analyze interactions between HA-eIF4H and FLAG-eIF4AII, complexes containing HA-eIF4H were precipitated using HA-specific monoclonal antibody and then analyzed by Western blotting using a FLAG-specific monoclonal antibody. To examine interactions between FLAG-eIF4AII and Vhs, complexes containing FLAG-eIF4AII were immunoprecipitated with a FLAG-specific monoclonal antibody and analyzed by Western blotting using a Vhs-specific antiserum.
RESULTS
Two-hybrid screen for eIF4H-interacting proteins.
Like eIF4B, eIF4H stimulates the ATP-dependent RNA helicase activities of eIF4A and eIF4F and stimulates β-globin synthesis in a reconstituted rabbit reticulocyte lysate system (62-67). However, eIF4H cannot completely substitute for eIF4B in the assembly of 48S translation initiation complexes on mRNAs containing moderate secondary structures in the 5′ UTR (10). Clearly, much remains to be learned about the in vivo activities of eIF4H, as well as its interactions with other cellular proteins. With regard to Vhs, a chain of interactions involving Vhs, eIF4H, and perhaps other cellular proteins may be required for targeting the Vhs endonuclease to its preferred cleavage sites. Therefore, to better understand eIF4H and its role in Vhs activity, we utilized it as bait in a yeast two-hybrid screen of a human cDNA library to search for eIF4H-interacting proteins.
Earlier studies showed that Vhs interacts with two isoforms of eIF4H (15, 18). One, termed eIF4H, is a 228-amino-acid polypeptide originally purified by Merrick and colleagues (62-67). The other, designated eIF4Hi, is identical to eIF4H, except for a 20-amino-acid insertion after residue 137 (18, 46), and is encoded by an alternatively spliced mRNA (46). For the two-hybrid screen, a bait plasmid encoding full-length eIF4Hi fused to the Gal4 DNA-binding domain, was used to screen a library of HeLa cell cDNAs cloned downstream from the Gal4 activation domain. cDNA-containing plasmids were judged to encode potential eIF4H-interacting proteins if they induced expression of his3 and lacZ genes from two different Gal4-responsive promoters when used in combination with the eIF4H-encoding bait plasmid, but not in combination with an unrelated bait plasmid containing a lamin C cDNA or the empty Gal4 DNA-binding domain vector. cDNAs that met the above criteria were sequenced and compared to sequences in available protein and nucleic acid databases. All of the cDNAs encoding putative eIF4H-interacting proteins encoded one of two polypeptides. One was eIF4AII, one of the three isoforms of eIF4A. The other was U2AF35, the 35-kDa subunit of the heterodimeric splicing factor U2AF (34, 91). While the interaction between eIF4H and U2AF35 is potentially interesting, its significance to Vhs-induced decay is difficult to access. Therefore, for the remainder of this study, we focused on the interaction between eIF4H and eIF4AII.
eIF4H interacts with eIF4AII in vitro.
To corroborate the results of the two-hybrid screen, we tested whether eIF4AII is able to bind a GST-eIF4H fusion protein by a standard GST pull-down assay (18). [35S]methionine-labeled eIF4AII was produced by in vitro transcription and translation and incubated with glutathione-Sepharose beads charged with bacterially expressed GST, GST-eIF4H, or GST-eIF4Hi. Bound proteins were eluted with 10 mM glutathione and analyzed by SDS-PAGE and autoradiography to detect labeled eIF4AII.
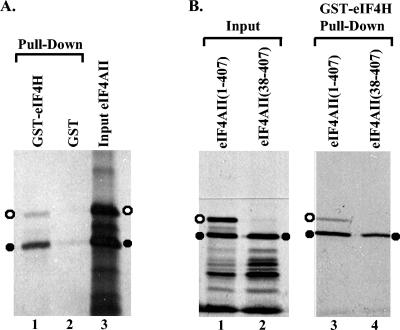
In vitro translation of eIF4AII mRNA resulted in two major products, a 45-kDa polypeptide of the size predicted for full-length eIF4AII and a 41-kDa polypeptide (Fig. 1A and B). Both proteins bound to beads charged with GST-eIF4H but not to beads carrying GST, indicating a specific interaction between eIF4H and the two eIF4AII translation products. While the two proteins were produced in roughly equal amounts (Fig. 1A, lane 3, and B, lane 1), substantially more of the 41-kDa product bound to GST-eIF4H (Fig. 1A, lane 1, and B, lane 3). Similar results were obtained for binding of the 45-kDa and 41-kDa polypeptides to GST-eIF4Hi (data not shown).
FIG. 1.
Binding of eIF4AII to a GST-eIF4H fusion protein. (A) GST or a GST-eIF4H fusion protein was produced in E. coli, bound to glutathione-Sepharose 4B, and incubated with rabbit reticulocyte lysates containing the [35S]methionine-labeled in vitro translation products of full-length eIF4AII mRNA. Bound proteins were eluted with 10 mM glutathione and analyzed by SDS-PAGE and autoradiography. Lane 1 contains proteins that bound to GST-eIF4H, lane 2 contains material that bound to GST, and lane 3 contains an aliquot of the starting material. The 45-kDa and 41-kDa products of in vitro translation of full-length eIF4AII mRNA are designated by the open and filled circles, respectively. (B) GST or GST-eIF4H was incubated with the in vitro translation products of full-length eIF4AII mRNA (lane 3) or an mRNA encoding only amino acids 38 through 407 of eIF4AII (lane 4). Bound proteins were eluted and analyzed as described in the legend to panel A. Lanes 1 and 2 contain aliquots of the starting material. The 45-kDa and 41-kDa eIF4AII polypeptides are designated by open and filled circles, respectively.
Full-length eIF4AII has a methionine at residue 38. In rabbit reticulocyte lysates, an appreciable amount of translation often initiates at AUG codons downstream from the 5′ proximal one, suggesting that the 41-kDa eIF4AII product might initiate at the AUG that encodes amino acid 38 of the full-length protein. To test this hypothesis, sequences encoding residues 38 through 407 of eIF4AII were PCR amplified and cloned into pcDNA1.1amp downstream from a promoter for T7 RNA polymerase. The protein produced by in vitro transcription and translation of this plasmid comigrated on SDS-PAGE with the 41-kDa product of in vitro translation of full-length eIF4AII mRNA (Fig. 1B, lanes 1 and 2) and bound to GST-eIF4Hi equally well (Fig. 1B, lanes 3 and 4). The results show that eIF4AII binds eIF4H and eIF4Hi in vitro and that residues 1 through 37 are dispensable for the interaction.
Coimmunoprecipitation of eIF4H and eIF4AII.
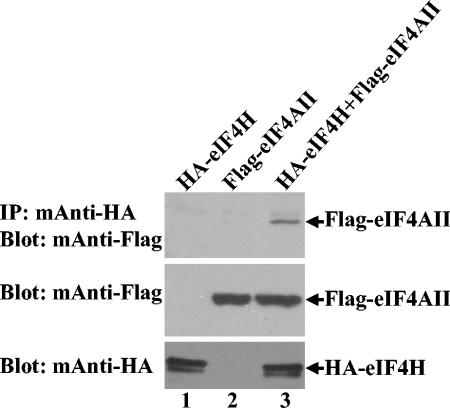
To determine whether eIF4H and eIF4AII interact in vivo, coimmunoprecipitation experiments were performed using lysates of CV-1 cells transfected with plasmids encoding HA-tagged eIF4H and FLAG-tagged eIF4AII, either individually or in combination. Complexes containing HA-eIF4H were precipitated using HA-specific monoclonal antibody and examined for coprecipitated FLAG-eIF4AII by Western blotting using a FLAG-specific monoclonal antibody (Fig. 2, top). Unfractionated cytoplasmic extracts were tested for HA-eIF4H and FLAG-eIF4AII by Western blotting using the appropriate antibody. Similar amounts of FLAG-eIF4AII were produced whether or not the cells also expressed HA-eIF4H (Fig. 2, middle), while the expression of HA-eIF4H was relatively unaffected by whether or not the cells also expressed FLAG-eIF4AII (Fig. 2, bottom). Although full-length FLAG-eIF4AII was readily detected in transfected cells, no shorter polypeptide was observed corresponding to the 41-kDa in vitro translation product. Most importantly, FLAG-eIF4AII was precipitated by the HA-specific monoclonal antibody from cells expressing both HA-eIF4H and FLAG-eIF4AII (Fig. 2, top, lane 3) but not from cells expressing either protein alone (Fig. 2, top, lanes 1 and 2). The results show that HA-eIF4H interacts with FLAG-eIF4AII in transfected cells.
FIG. 2.
Coimmunoprecipitation of eIF4H and eIF4AII from mammalian cells. CV-1 cells were infected with 5 PFU/cell of the recombinant vaccinia virus vTF7. Thirty minutes later, they were transfected with 5 μg each of plasmids expressing HA-tagged eIF4H (lane 1), Flag-tagged eIF4AII (lane 2), or HA-eIF4H and Flag-eIF4AII (lane 3). Cell extracts were prepared 20 h after transfection and complexes containing HA-eIF4H were immunoprecipitated with an HA-specific monoclonal antibody (top). Immunoprecipitates were analyzed for coprecipitated Flag-eIF4AII by SDS-PAGE and Western blotting using a Flag-specific monoclonal antibody. To check for protein expression, aliquots of the cell lysates were analyzed directly by Western blotting using a Flag-specific monoclonal antibody (middle) or an HA-specific monoclonal antibody (bottom).
Vhs interacts with eIF4AII in vitro and in vivo.
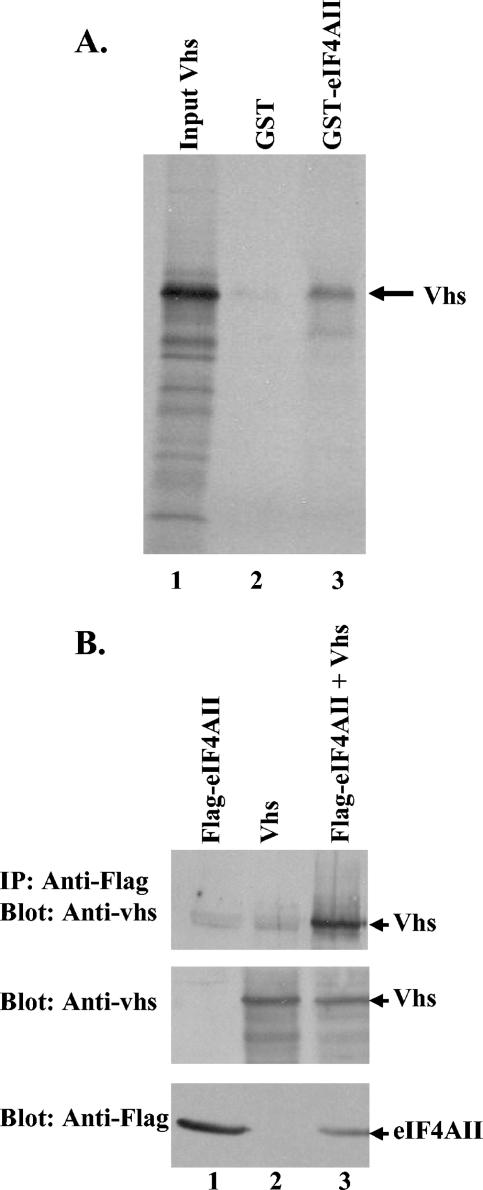
The observation that eIF4H interacts with eIF4AII, as well as with Vhs (15, 18), raised the question of whether Vhs also interacts with eIF4AII. eIF4AII was not identified as a Vhs-interacting protein in the original yeast two-hybrid screen of a HeLa cell cDNA library (18). However, there are numerous examples of protein pairs that fail to drive transcription of a Gal4-responsive promoter in the yeast two-hybrid system, even though other evidence shows that the proteins interact. To explore whether Vhs indeed interacts with eIF4AII, the eIF4AII open reading frame was cloned into pGEX-5S-3 to produce a plasmid encoding a GST-eIF4AII fusion protein. GST and GST-eIF4AII were expressed in E. coli, bound to glutathione-Sepharose beads, and incubated with [35S]methionine-labeled Vhs produced by in vitro transcription and translation. Vhs bound to beads charged with GST-eIF4AII (Fig. 3A, lane 3) but not to beads charged with GST (Fig. 3A, lane 2), indicating a specific in vitro interaction between Vhs and GST-eIF4AII.
FIG. 3.
In vitro and in vivo binding of Vhs to eIF4AII. (A) Binding of Vhs to a GST-eIF4AII fusion protein. GST or a GST-eIF4AII fusion protein was produced in E. coli, bound to glutathione-Sepharose 4B, and incubated with rabbit reticulocyte lysates containing [35S]methionine-labeled in vitro-translated Vhs. Bound proteins were eluted with 10 mM glutathione and analyzed by SDS-PAGE and autoradiography. Lanes 2 and 3 contain proteins that bound to GST-eIF4AII and GST, respectively, while lane 1 contains an aliquot of the starting material. The Vhs protein is indicated by an arrow. (B) Coimmunoprecipitation of Vhs and eIF4AII from mammalian cells. CV-1 cells were infected with 5 PFU/cell of the recombinant vaccinia virus vTF7. Thirty minutes later, they were transfected with 5 μg (each) of plasmids expressing Flag-tagged eIF4AII (lane 1), the D194N allele of Vhs (lane 2), or Flag-eIF4AII and D194N Vhs (lane 3). Cell extracts were prepared 20 h after transfection, and complexes containing Flag-eIF4AII were immunoprecipitated using a Flag-specific monoclonal antibody (top). Immunoprecipitates were analyzed for coprecipitated Vhs by SDS-PAGE and Western blotting with a Vhs-specific rabbit antiserum. To check for protein expression, aliquots of the cell lysates were analyzed directly by Western blotting using a Vhs-specific antiserum (middle) or a Flag-specific monoclonal antibody (bottom).
Next, coimmunoprecipitation experiments were performed using cytoplasmic extracts from cells transfected with plasmids encoding the D194N allele of Vhs and FLAG-eIF4AII, individually or in combination. Complexes containing FLAG-eIF4AII were precipitated with a FLAG-specific monoclonal antibody and analyzed for coprecipitated Vhs by SDS-PAGE and Western blotting using Vhs-specific antiserum (Fig. 3B, top). Unfractionated cytoplasmic extracts were also tested for Vhs or FLAG-eIF4AII by Western blotting using the appropriate antibody (Fig. 3B, middle and bottom). The D194N allele was used in these studies to maximize expression of Vhs. Previous studies have shown that significantly less Vhs is expressed in cells transfected with a wild-type Vhs allele than in cells transfected with inactive Vhs alleles, presumably because functional Vhs degrades its own mRNA (16, 17, 55). D194N has a point mutation that changes aspartate 194 of the nuclease motif to asparagine. It lacks mRNA degradative activity in transfected cells but is still able to bind eIF4H (15). Similar amounts of D194N Vhs were expressed whether or not the cells were cotransfected with a plasmid encoding FLAG-eIF4AII (Fig. 3B, middle). Simlarly, FLAG-eIF4AII was readily detected in the unfractionated extracts, whether or not the cells also expressed Vhs (Fig. 3B, bottom). Most importantly, Vhs was precipitated by the FLAG-specific antibody from cells expressing both Vhs and FLAG-eIF4AII (Fig. 3B, top, lane 3), but very little was precipitated from cells expressing Vhs alone (Fig. 3B, top, lane 2). The data indicate that Vhs binds FLAG-eIF4AII in transfected cells.
Mutant Vhs polypeptides that interact with eIF4AII, but not with eIF4H.
In the above experiments, Vhs interacted with eIF4AII in the absence of added eIF4H. However, the interaction was observed in rabbit reticulocyte lysates and in transfected cells, conditions in which endogenous eIF4H was present. Vhs binds eIF4H (18), which, in turn, is able to bind eIF4AII. This raised the possibility that the interaction between Vhs and eIF4AII is indirect, mediated by a molecule of eIF4H that binds Vhs and eIF4AII simultaneously and acts as a protein bridge. To explore this possibility, we examined whether mutant Vhs polypeptides that no longer bind eIF4H interact with eIF4AII. The identification of Vhs mutants that bind eIF4AII, even though they no longer bind eIF4H, would indicate that the Vhs-eIF4AII interaction is not mediated by an eIF4H bridge and suggest that it is direct.
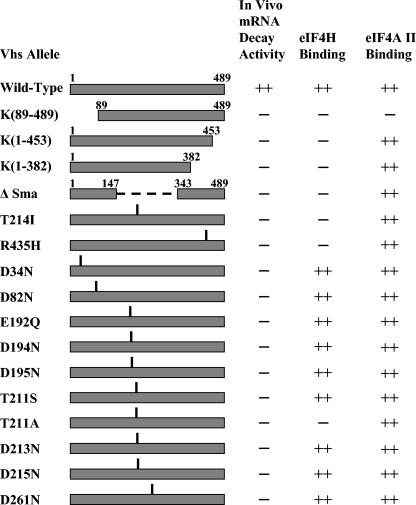
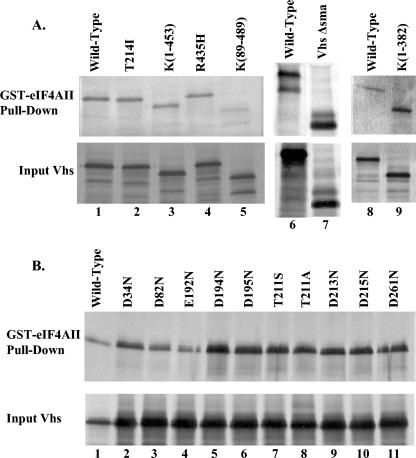
A GST-eIF4AII fusion protein was produced in bacteria and tested for its ability to bind a collection of mutant Vhs polypeptides, previously analyzed for their ability to bind eIF4H (15, 18). These included two spontaneous point mutants (T214I and R435H), four deletion/truncation mutants [K(89-489), K(1-453), K(1-382), and ΔSma], and 10 site-directed mutants (D34N, D82N, E192Q, D194N, D195N, T211S, T211A, D213N, D215N, and D261N) containing alterations of key residues in the Vhs nuclease motif (15). For a diagram of the structures of the mutant Vhs polypeptides, along with a summary of their abilities to bind eIF4AII and eIF4H, see Fig. 5. The results of the GST pull-down experiments are shown in Fig. 4A and B. All of the mutants fail to induce mRNA degradation in infected or transfected cells (Fig. 5) (53-55, 60, 61). Seven failed to bind eIF4H (Fig. 5) (15, 18). These included four deletion/truncation mutants [K(89-489), K(1-453), K(1-382), and ΔSma] and three point mutants (T211A, T214I, and R435H). Of the seven mutants that failed to bind eIF4H, six continued to interact with eIF4AII in the GST pull-down assay (Fig. 4A, lanes 2 to 5, 7, and 9, and 4B, lane 8, and Fig. 5). Clearly, the binding of Vhs to eIF4AII is not dependent upon its binding to eIF4H.
FIG. 5.
Summary of the in vivo mRNA-degradative activities of wild-type and mutant Vhs polypeptides and their abilities to bind eIF4H and eIF4AII. The structures of the wild-type and mutant Vhs polypeptides are diagrammed on the left. For deletion mutants, the Vhs residues included in the mutant proteins are indicated. For each point mutant, the location of the altered residue is indicated by the vertical line above the bar representing the protein. The in vivo mRNA-degradative activity of each Vhs protein is shown in the column immediately to the right of the diagram. This was assayed in transfected cells for all of the alleles and during virus infections for wild-type Vhs and the mutants K(1-382), Vhs ΔSma, and T214I. ++, wild-type activity; −, no detectable mRNA-degradative activity. The middle column indicates whether a Vhs protein binds (++) or does not bind (−) eIF4H in the yeast two-hybrid system and in GST pull-down assays. These data were reported previously (18). The right column indicates whether a Vhs protein binds (++) or does not bind (−) eIF4AII and summarizes the data shown in Fig. 4.
FIG. 4.
eIF4AII binding by wild-type and mutant Vhs. [35S]methionine-labeled wild-type and mutant Vhs polypeptides were produced by in vitro transcription and translation and analyzed for the ability to bind a GST-eIF4AII fusion protein, as described in the legend for Fig. 1. Material that bound to GST-eIF4AII and was eluted with 10 mM glutathione is shown in the upper gels (GST-eIF4AII pull-down) of panels A and B. Aliquots of the input in vitro-translated material are shown in the lower gels (Input Vhs). The mutant and wild-type Vhs polypeptides are indicated at the top of each lane. Their structures are diagrammed in Fig. 5.
The results also begin to delineate the regions of Vhs required for its interaction with eIF4AII and indicate that they are different from those required for binding eIF4H. While deletion of as few as 36 amino acids from the C terminus of Vhs is sufficient to impede its binding to eIF4H [Fig. 5, mutant K(1-453)] (18), removal of the last 106 amino acids had no detectable effect upon its interaction with eIF4AII (Fig. 4A, lane 9). Similarly, the deletion of Vhs residues 148 through 342 abolished its interaction with eIF4H (mutant ΔSma) (Fig. 5), yet if anything slightly enhanced its interaction with eIF4AII (Fig. 4A, lanes 6 and 7). Together, the results narrow the residues of Vhs required for binding eIF4AII to two regions of the primary sequence, from residue 1 to 147 and 343 to 382 (Fig. 5). Consistent with this, deletion of residues 1 to 87 abrogates the binding of Vhs to eIF4AII (Fig. 4A, lane 5). However, this deletion also abolished binding to eIF4H (Fig. 5) (18), raising the possibility that it causes at least partial misfolding of the protein.
Mutations in eIF4H that reduce binding to Vhs but not to eIF4AII.
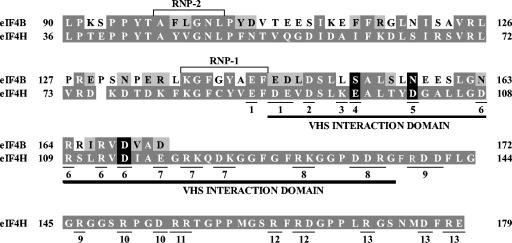
Previous studies identified a region of eIF4H (residues 90 to 137) that is sufficient for binding Vhs in the yeast two-hybrid system and in GST pull-down assays (18). To further characterize this domain, site-directed mutagenesis was used to change clusters of charged amino acids to alanine. Full-length eIF4H polypeptides containing clustered charged-to-alanine substitutions were then assayed for their ability to bind Vhs and eIF4AII in the recombination-based yeast two-hybrid system and in GST pull-down assays (see Fig. 7 and 8). The rationale behind this approach was that clusters of charged amino acids are likely to be found on the surface of a protein, where they may be involved in interactions with other molecules (9, 28, 90).
FIG. 7.
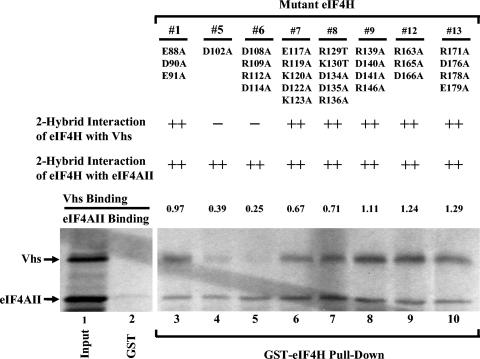
Binding of mutant eIF4H polypeptides to Vhs and eIF4AII. Eight of the mutant eIF4H polypeptides diagrammed in Fig. 6 were tested for binding to Vhs and full-length eIF4AII using the recombination-based yeast two-hybrid system and GST pull-down assays. The number of each mutant is shown at the top of the figure, just above the amino acid substitutions that each mutant contains. The second and third lines contain the results of recombination based yeast two-hybrid assays to test the interaction of the mutant eIF4H polypeptides with Vhs and eIF4AII, respectively. ++ indicates that the mutant eIF4H allele yielded a number of colonies similar to that of the wild-type eIF4H in the recombination-based two-hybrid assay; − indicates that the number of colonies was reduced at least 10 fold relative to those of wild-type eIF4H. For GST pull-down assays, full-length mutant eIF4H polypeptides were expressed as GST-eIF4H fusion proteins in E. coli, bound to glutathione-Sepharose 4B, and incubated with rabbit reticulocyte lysates containing a mixture of [35S]methionine-labeled Vhs and eIF4AII produced by in vitro transcription and translation. Bound proteins were eluted with 10 mM glutathione and analyzed by SDS-PAGE and autoradiography as described in the legends to Fig. 1 and 3. The positions of Vhs and full-length eIF4AII are indicated by the arrows to the left of the gel. For each mutant, the relative amounts of bound Vhs and eIF4AII were determined by densitometric scanning of the autoradiogram and expressed as a ratio normalized to the ratio determined for wild-type eIF4H (data not shown). These ratios are indicated in line 4. Lane 1 contains an aliquot of the input mixture of Vhs and full-length eIF4AII; lane 2 contains material that bound to GST alone; lanes 3 through 10 contain material that bound to each of the mutant GST-eIF4H fusion proteins.
FIG. 8.
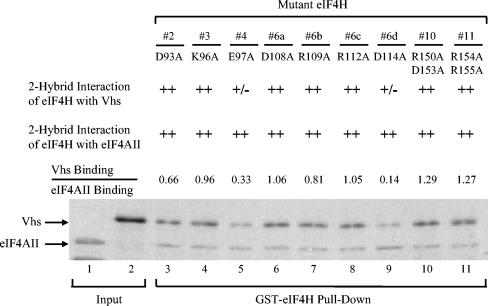
Binding of mutant eIF4H polypeptides to Vhs and eIF4AII. Five of the eIF4H mutants diagrammed in Fig. 6 and four additional mutants (mutants 6a, 6b, 6c, and 6d), were tested for binding to Vhs and full-length eIF4AII by using the recombination-based yeast two-hybrid system and GST pull-down assays. The number of each mutant is shown at the top of the figure, just above the amino acid substitutions that each mutant contains. The second and third lines contain the results of recombination based yeast two-hybrid assays to test the interaction of the mutant eIF4H polypeptides with Vhs and eIF4AII, respectively. ++, mutant eIF4H allele yielded a number of colonies similar to that of wild-type eIF4H by the recombination-based two-hybrid assay; +/−, the number of colonies was reduced at least fourfold but <10 fold relative to wild-type eIF4H. For GST pull-down assays, full-length mutant eIF4H polypeptides were expressed as GST-eIF4H fusion proteins in E. coli, bound to glutathione-Sepharose 4B, and incubated with rabbit reticulocyte lysates containing a mixture of [35S]methionine-labeled Vhs and eIF4AII produced by in vitro transcription and translation. Bound proteins were eluted with 10 mM glutathione and analyzed by SDS-PAGE and autoradiography as described in the legends to Fig. 1 and 3. The positions of Vhs and full-length eIF4AII are indicated by the arrows to the left of the gel. For each mutant, the relative amounts of bound Vhs and eIF4AII were determined by densitometric scanning of the autoradiogram and expressed as a ratio normalized to the ratio that was determined for wild-type eIF4H (data not shown). These ratios are indicated in line 4. Lane 1 contains an aliquot of the input full-length eIF4AII, lane 2 contains an aliquot of the input Vhs, and lanes 3 through 11 contain material that bound to each of the mutant GST-eIF4H fusion proteins.
Thirteen eIF4H alleles were constructed encoding proteins with clusters of charged residue-to-alanine substitutions between amino acids 88 and 179 (Fig. 6 to 8). Two contained mutations that reduced the level of Vhs binding in the recombination-based yeast two-hybrid system to that of control transformations lacking an intact eIF4H allele. These alleles encoded a polypeptide with the single substitution D102A and a protein with the cluster of four substitutions (D108A, R109A, R112A, and D114A) (Fig. 7, lanes 4 and 5). A third polypeptide, with the single substitution E97A, showed reduced Vhs binding but not to control levels (Fig. 8, lane 5). Significantly, the two-hybrid interaction of each of the three proteins with eIF4AII was indistinguishable from that of wild-type eIF4H.
FIG. 6.
Residues of eIF4H whose alteration reduces Vhs binding. The region of sequence homology shared by eIF4H and eIF4B is shown, with the eIF4H amino acids and those eIF4B residues that are identical to amino acids in eIF4H indicated by white letters on a dark grey background. eIF4B residues that represent conservative changes relative to those in eIF4H are depicted by black letters on a light grey background. eIF4B residues that are nonconservative changes from eIF4H amino acids are indicated by black letters on a white background. eIF4H and eIF4B share an RNA recognition motif RNA-binding domain containing two RNP motifs (RNP-1 and RNP-2) that are boxed. The Vhs interaction domain of eIF4H, as defined by deletion mutagenesis (18), is indicated by the dark line under the eIF4H sequence. Thirteen eIF4H mutants were constructed in which clusters of charged amino acids were altered by site-directed mutagenesis. The amino acids that were altered in a particular mutant are underlined and indicated by the number of the mutant under the residue. For example, in mutant 1, E88, D90, and E91 were altered together. All of the indicated charged amino acids were changed to alanine except for mutant 8, in which R139 and K130 were both changed to threonine. The three charged eIF4H amino acids, whose modification to alanine significantly reduces Vhs binding, are indicated by white type on a black background, as are the corresponding residues of eIF4B.
Qualitatively similar results were obtained using a GST pull-down assay. GST-eIF4H fusion proteins containing the various charged-to-alanine substitutions were incubated with a mixture of in vitro-translated Vhs and eIF4AII, and the relative amounts of bound Vhs and eIF4AII were determined by densitometry of autoradiograms from SDS-PAGE gels of the bound proteins. None of the charged-to-alanine substitutions had a significant effect upon the binding of eIF4AII to the GST-eIF4H fusion proteins (Fig. 7 and 8). In contrast, the cluster of substitutions D108A, R109A, R112A, and D114A reduced the binding of Vhs relative to eIF4AII by fourfold (Fig. 7, lane 5). Similarly, the single substitutions E97A and D102A reduced the ratio of bound Vhs to bound eIF4AII to 33% and 39% of the ratio for wild-type eIF4H, respectively (Fig. 8, lane 5, and Fig. 7, lane 4).
To further define which substitutions in the cluster D108A, R109A, R112A, and D114A are responsible for the reduced binding to Vhs, eIF4H alleles were constructed containing each of the substitutions individually. None of the single substitutions D108A, R109A, or R112A had an appreciable effect upon binding to Vhs or eIF4AII in either the yeast two-hybrid or GST pull-down assays (Fig. 8, lanes 6 to 8). In contrast, the single-substitution D114A reduced the two-hybrid interaction with Vhs to a moderate extent and reduced the ratio of bound Vhs to bound eIF4AII to 14% of the ratio for wild-type GST-eIF4H (Fig. 8, lane 9). Taken together, the results identified three mutations in eIF4H (E97A, D102A, and D114A) that reduced its interaction with Vhs, while not affecting its binding to eIF4AII (Fig. 6).
eIF4AII does not compete with Vhs for binding eIF4H.
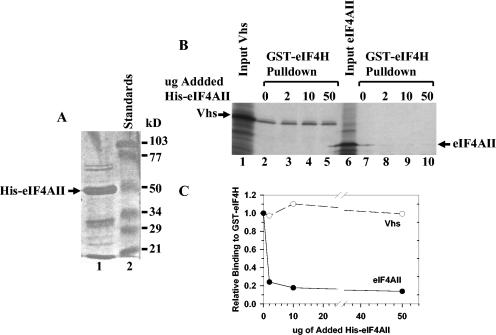
These experiments show that the binding of eIF4H to Vhs and to eIF4AII can be distinguished genetically. They do not distinguish whether the binding sites for the two proteins on eIF4H are physically separate or overlap. This question was addressed in two ways. First, recombinant His-tagged eIF4AII was expressed in E. coli and isolated by metal chelate affinity chromatography. It was then added in increasing amounts to in vitro-binding reaction mixtures containing GST-eIF4H and 35S-methionine labeled eIF4AII or Vhs to see if it competed with the radiolabeled polypeptides for binding GST-eIF4H (Fig. 9). As expected, an excess of the preparation of His-eIF4AII competed effectively with 35S-labeled eIF4AII for binding GST-eIF4H. In contrast, it had no detectable effect upon the binding of 35S-labeled Vhs, suggesting that Vhs and eIF4AII do not compete for binding to GST-eIF4H.
FIG. 9.
eIF4AII does not compete with Vhs for binding to GST-eIF4H. (A) His-tagged eIF4AII was expressed in E. coli and isolated by metal chelate affinity chromatography. An aliquot was analyzed by SDS-PAGE and detected by staining the gel with Coomassie (lane 1). His-eIF4AII is indicated by the arrow. Lane 2 contains molecular mass standards. (B) GST-eIF4H was expressed in E. coli, bound to glutathione-Sepharose 4B, and incubated with rabbit reticulocyte lysates containing [35S]methionine-labeled in vitro-translated Vhs (lanes 2 to 5) or full-length eIF4AII (lanes 7 to 10). The incubations also contained 0 μg (lanes 2 and 7), 2 μg (lanes 3 and 8), 10 μg (lanes 4 and 9), or 50 μg (lanes 5 and 10) of the His-eIF4AII preparation shown in panel A. Material that bound GST-eIF4H and was eluted with 10 mM glutathione was analyzed by SDS-PAGE and autoradiography. Aliquots of the input Vhs and eIF4AII were run in lanes 1 and 6, respectively. The positions of the Vhs and full-length eIF4AII polypeptides are shown by arrows. (C) For each of the incubations, the amount of 35S-labeled Vhs or eIF4AII that bound GST-eIF4H was determined by densitometric scanning of the lanes shown in panel B. For each concentration of added His-eIF4AII, the amount of bound 35S-labeled Vhs or eIF4AII was plotted as a fraction of the amount that bound GST-eIF4H in the absence of added His-eIF4AII.
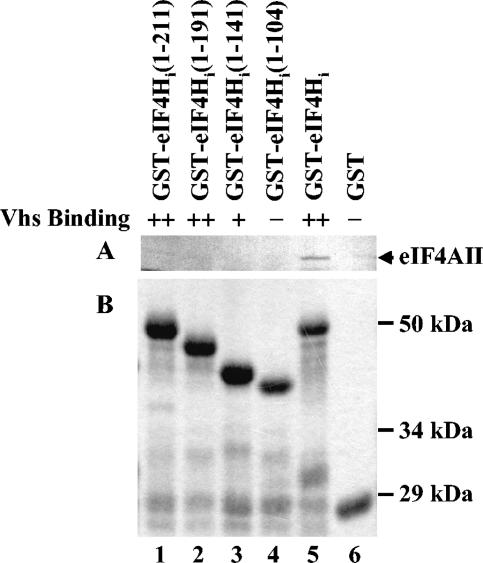
The second set of experiments examined the binding of 35S-labeled eIF4AII to fusion proteins containing GST fused to various full-length or deleted forms of eIF4Hi (Fig. 10). In previous experiments, these fusion proteins were characterized for Vhs binding in GST pull-down assays, while the truncated eIF4Hi polypeptides were analyzed for their interaction with Vhs in the yeast two-hybrid system (18). In both assays, truncated eIF4Hi polypeptides containing the first 211 or first 191 amino acids of eIF4Hi interacted with Vhs as well as the 248-amino-acid wild-type protein (Fig. 10, top). Shortening eIF4Hi to 141 amino acids did not affect its binding to Vhs in the GST pull-down assay and had only a modest effect on the yeast two-hybrid interaction. Truncating eIF4Hi to the first 104 amino acids abolished its interaction with Vhs in both assays (18). In contrast, shortening the eIF4Hi polypeptide by as few as 37 amino acids (to 211 amino acids) abolished its binding to eIF4AII in GST pull-down assays (Fig. 10,A, lane 1). These results, with those shown in Fig. 7 through 9, indicate that mutations in eIF4Hi that affect its binding to Vhs and eIF4AII map to different regions of the protein. This, together with the observation that Vhs and a preparation of His-eIF4AII do not compete for binding GST-eIF4H, suggests the Vhs- and eIF4AII-binding sites on eIF4H are physically distinct.
FIG. 10.
eIF4AII binding by deletion mutants of eIF4Hi. Deletion mutants of eIF4Hi were previously used to define the regions of eIF4Hi necessary to bind Vhs by the conventional yeast two-hybrid system and in GST pull-down experiments (18). These mutants were now used to examine the region of eIF4Hi required to bind full-length eIF4AII. Full-length wild-type [35S]methionine-labeled eIF4AII was produced by in vitro translation and analyzed for the ability to bind various fusion proteins containing GST fused to full-length eIF4Hi (lane 5) or deletion mutant forms of eIF4Hi (lanes 1 to 4). Full-length eIF4AII bound by the various GST-eIF4Hi polypeptides and eluted with 10 mM glutathione is shown in the autoradiogram in panel A. The material that bound to just GST is shown in lane 6. The eIF4Hi amino acids that were present in the wild-type and various mutant forms of eIF4Hi are shown in parentheses at the top of each lane of the gel. A Coomassie-stained gel of the mutant and wild-type GST-eIF4Hi proteins used in the GST pull-down reactions is shown in panel B. The ability of the eIF4Hi polypeptides to bind Vhs was reported previously (18) and is summarized above panel A. ++, the mutant eIF4Hi polypeptide binds Vhs as well as wild-type eIF4Hi by both the yeast two-hybrid and GST pull-down assays; +, the mutant eIF4Hi polypeptide binds Vhs as well as wild-type eIF4Hi by the yeast two-hybrid assay but binds threefold-less Vhs in GST pull-down experiments; −, there was no detectable interaction between the mutant eIF4Hi and Vhs by either the yeast two-hybrid or GST pull-down assay.
DISCUSSION
These studies are important because they extend our knowledge of protein-protein interactions involving Vhs and cellular translation factors involved in early stages of translation initiation. In earlier experiments, we showed that Vhs binds the helicase accessory factor eIF4H and identified six mutations in Vhs that abolish the interaction (15, 18). Significantly, all six abrogate the ability of Vhs to degrade mRNAs that are translated by cap-dependent scanning (15, 18), suggesting that the interaction is biologically significant. In this study, we show that eIF4H, in turn, binds the RNA helicase eIF4AII and that Vhs also binds eIF4AII. Mutations were identified in Vhs that abrogate its interaction with eIF4H but not with eIF4AII, indicating that the interaction between Vhs and eIF4AII is not mediated by a protein bridge of eIF4H and, most likely, is direct. Similarly, the interactions of eIF4H with Vhs and with eIF4AII can be distinguished genetically, and Vhs and eIF4AII do not compete with each other for binding eIF4H. Taken together, the data suggest that Vhs, eIF4H, and eIF4AII comprise a group of three proteins, each of which is able to interact directly with the other two.
These results have significant implications for understanding how a sequence-nonspecific endonuclease, such as Vhs, is targeted to mRNAs, as opposed to non-mRNAs, and to regions of translation initiation in at least some mRNAs. Our earlier observation that Vhs binds eIF4H suggested that the targeting of Vhs may occur through its interaction with one or more cellular translation factors (15, 18). However, implicit in this model was a requirement for additional cellular factors beside eIF4H, since eIF4H is a helicase accessory factor that lacks sequence specificity. Not surprisingly, a complex of recombinant Vhs and GST-eIF4H remains a nonselective nuclease that lacks the specificity to distinguish mRNAs from nontranslated RNAs and to recognize preferred sites within mRNAs (15). In this light, the observation that Vhs and eIF4H both bind eIF4AII is important because eIF4AII binds eIF4G and, together with eIF4G and eIF4E, is a component of the cap-binding complex eIF4F (23, 27). The results, thus, suggest a model in which eIF4AII is a key participant in a chain of protein-protein interactions that recruits Vhs to the 5′ end of capped mRNAs.
While this is an attractive model, many details remain to be tested. Consistent with the model, Vhs can be isolated as a component of cap-binding complexes prepared by chromatography of cytoplasmic extracts on 7-methyl GTP Sepharose 4B (D. Agarwal, H. Garson, and G. S. Read, Abstr. 29th Int. Herpesvirus Wkshp., abstr. 1.62, 2004). While it is logical to think this association with the cap-binding complex is mediated through the binding of Vhs to eIF4AII and/or eIF4H, this remains to be shown. In addition, there is currently little genetic data indicating that the interaction with eIF4AII is required for targeted Vhs activity in vivo. At present, only one mutant Vhs polypeptide, K(89-489), has been identified that fails to bind eIF4AII (Fig. 5). It fails to degrade mRNAs in transfected cells, a finding that is consistent with a role for eIF4AII binding in Vhs activity. However, this mutant also fails to bind eIF4H, making it unclear whether its lack of mRNA degradative activity is due to a defect in binding eIF4AII, a defect in binding eIF4H, or to something else. Efforts are under way to isolate Vhs mutants that do not bind eIF4AII but retain eIF4H-binding and endonuclease activities and to test their mRNA degradative activities in transfected and infected cells.
Examination of the existing mutants reveals six that fail to degrade mRNAs that are translated by cap-dependent scanning (15, 18, 53-55, 61), even though they still bind eIF4AII (Fig. 5). All six fail to bind eIF4H (18). Although it has no effect on the vast majority of scanned mRNAs (53, 54, 60), at least one of the mutant polypeptides, T214I, still cleaves mRNAs containing an EMCV IRES in rabbit reticulocyte lysates, indicating that it is an active endonuclease (44). These results have several important implications. (i) Endonuclease activity is not the only attribute required for Vhs to degrade mRNAs in vivo. A mutant polypeptide, such as T214I, may fail to degrade mRNAs that are translated by scanning, even if it is an active endonuclease, presumably because it fails in key protein-protein interactions required to target it to preferred cleavage sites or to localize it in the cell. (ii) Since virus containing the T214I mutation is viable (60), expression of an active, but apparently untargeted, Vhs endonuclease is not so detrimental to an infected cell that it precludes virus growth. (iii) Binding to eIF4AII may prove to be required for targeting of the Vhs endonuclease and cleavage of mRNAs that are translated by scanning, but it is not sufficient. This is shown by the existence of mutant polypeptides, such as T214I, that have endonuclease activity and bind eIF4AII but do not cleave scanned mRNAs in vivo. To date, T214I and every other mutant that fails to bind eIF4H also fail to degrade mRNAs that are translated by cap-dependent scanning. The data are consistent with the possibility that binding to both eIF4AII and eIF4H is required for cleavage of scanned mRNAs but that binding to either factor alone is not sufficient.
In contrast to the situation for scanned mRNAs, cleavage of mRNAs containing an EMCV IRES may not require the interaction between Vhs and eIF4H. This is suggested by the observation that the T214I polypeptide cleaves EMCV IRES-containing mRNAs (44), even though it does not bind eIF4H (18). A key to the IRES-directed cleavage activity of the T214I protein may be its ability to bind eIF4AII (Fig. 5), since an early step in translation initiation at an EMCV IRES is the direct binding of eIF4G to the IRES, along with eIF4A (26, 35, 36, 43, 57). This raises the possibility that Vhs cleavage of mRNAs that are translated by cap-dependent scanning requires the binding of Vhs to both eIF4H and eIF4AII but that cleavage downstream from an EMCV IRES requires binding only to eIF4AII. Analysis of mutants that bind eIF4H but not eIF4AII or that bind eIF4AII but not eIF4H will be an important test of this possibility.
The binding of Vhs to eIF4AII and eIF4H reprises a theme that recurs in both eukaryotic and prokaryotic systems, that of an RNase or another protein involved in mRNA decay that interacts with an RNA helicase. In E. coli, the degradosome is a multienzyme complex involved in the degradation of numerous mRNAs (4, 5, 33, 89). It includes RNase E, a single-strand specific endonuclease that also serves as a scaffold for the other components, as well as poly(A) polymerase, enolase, polynucleotide phosphorylase, and the DEAD box RNA helicase RhlB (4, 5, 33, 89). Degradation of some structured mRNAs is initiated by RNase E cleavage, followed by polynucleotide phosphorylase-mediated degradation of the fragments through a process facilitated by the helicase activity of RhlB (5, 7, 33, 45, 89). In yeast, the DEAD box helicase Dhh1p interacts with the decapping factor Dcp1p and several other decapping proteins. dhh1Δ mutants accumulate mRNAs that are deadenylated but still capped, indicating that Dhh1p stimulates the decapping step of mRNA degradation, following mRNA deadenylation and before 5′-to-3′ exonuclease digestion (8). Similarly, the exosome is a complex of 10 or more proteins involved in RNA processing and in 3′-to-5′ exonuclease digestion of mRNAs in yeast and mammalian cells (1, 2, 50, 86, 88). Exosome components include several exonucleases, as well as the Ski2p RNA helicase. Ski2p facilitates the 3′-to-5′ degradation of yeast mRNAs, suggesting that the helicase activity aids exonuclease activity by unwinding secondary structures in the mRNA or disrupting RNA-protein interactions (2, 88). Whether the eIF4H-stimulated helicase activity of eIF4AII plays a similar role in Vhs activity remains to be determined.
Recently, Doepker and colleagues made the important observation that Vhs binds eIF4B in a Far-Western blotting assay (11). They also found that recombinant eIF4B and eIF4H both stimulated the Vhs-dependent endonuclease activity present in whole-cell extracts of yeast engineered to express Vhs (11). Previous studies of eIF4H deletion mutants had shown that a region of eIF4H spanning amino acids 90 to 137 is necessary and sufficient for binding Vhs in GST pull-down assays and in the yeast two-hybrid system (18). As shown in Fig. 6, this region partially overlaps the region of sequence homology that eIF4H shares with eIF4B (63). Furthermore, the three eIF4H amino acids (E97, D102, and D114) whose modification to alanine reduces Vhs binding fall within this region of overlap (Fig. 6). While eIF4H and eIF4B both stimulate the ATP-dependent RNA helicase activity of eIF4A and appear to have similar functions in stimulating translation initiation (27, 62-65, 67), eIF4B has properties that have not been reported for eIF4H and which may be important for understanding the details of Vhs activity. eIF4B functions as a dimer, while eIF4H functions as a monomer (62). The proteins share an RNA recognition motif RNA-binding domain, but within its carboxy-terminal half eIF4B also has an arginine-rich RNA-binding motif that eIF4H lacks (62). eIF4B can bind two different RNAs at once (47), it can bind ribosomes in vitro (29), and it also interacts with eIF3 (48). As a consequence, besides its role in stimulating the helicase activity of eIF4A, eIF4B has been postulated to facilitate ribosome binding by providing a bridge between the mRNA and ribosomes, either directly or through eIF3 (23, 27, 48, 49). In view of these differences, it is important to determine whether the in vivo activity of Vhs is dependent upon its binding to eIF4H, to eIF4B, or to both. An approach to this question may be afforded by recent studies identifying Vhs mutations that affect the binding to eIF4H and to eIF4B differently (D. Agarwal, H. Garson, and G. S. Read, Abstr. 29th Int.al Herpesvirus Wkshp., abstr. 1.62, 2004). Further characterization of these mutants may provide important insights into the mechanism of Vhs activity.
Our studies show that Vhs binds eIF4AII. Whether it binds the other eIF4A isoforms, eIF4AI and eIF4AIII, remains an important unanswered question. eIF4AI is 91% identical in amino acid sequence to eIF4AII, and the two are functionally interchangeable by most in vitro translation assays (42). Given this similarity, it is striking that Doepker and coworkers detected no interaction between Vhs and eIF4AI by a far Western blotting assay (11). The difference between their results and ours may reflect a real difference in the binding of Vhs to eIF4AII and to eIF4AI. Alternatively, it may stem from differences in the procedures used to detect the interaction. In our study, an interaction was detected between soluble Vhs and eIF4AII by GST pull-down and coimmunoprecipitation assays. In Doepker's experiments, eIF4AI was denatured, subjected to SDS-PAGE, and blotted to nitrocellulose; binding to Vhs was tested by probing the membrane with [35S]methionine-labeled in vitro-translated Vhs (11). The lack of interaction could be explained if eIF4AI did not renature to a sufficient extent to bind Vhs. The third isoform, eIF4AIII, is only 65% identical to eIF4AI; while it possesses RNA helicase activity, it cannot substitute for eIF4AI or eIF4AII in a reconstituted 40S ribosome-binding assay in vitro (42). Recently, eIF4AIII has been shown to be a component of the exon junction complex that is recruited to mRNAs as a result of splicing (6, 20, 71) and to function during nonsense-mediated mRNA decay (20, 71). A finding that Vhs binds eIF4AIII would be significant because it would suggest a potential mechanism by which Vhs could be targeted to sites on mRNAs other than regions of translation initiation.
In this regard, recent studies from Roizman and coworkers suggest that Vhs cleavage of some ARE-containing mRNAs may initiate in the 3′ UTR rather than in the 5′ UTR or near the start codon (14, 84). How many mRNAs are cleaved at sites other than in regions of translation initiation and how Vhs might be targeted to those sites are unclear. One possibility is that cleavage at sites near regions of initiation is dependent upon the binding of Vhs to cellular translation initiation factors, while cleavage at sites outside of initiation regions is not, requiring instead the binding of Vhs to other cellular RNA-binding proteins. If this is the case, the fraction of mRNAs that are cleaved outside of regions of initiation would appear to be small, since the T214I point mutation, which impedes binding to eIF4H, abrogates Vhs cleavage of the vast majority of cellular and viral mRNAs even though the Vhs polypeptide retains endonuclease activity (53, 54, 60, 61). Alternatively, the targeting of Vhs may depend upon its ability to interact with translation factors, regardless of whether the preferred cleavage sites are in regions of translation initiation or not. Vhs may be initially directed to mRNAs through its interaction with eIF4H, eIF4AII, or eIF4B and indirectly with other components of the translation apparatus. Following this initial loading, it may be targeted to regions of translation initiation in some mRNAs through a process requiring progression through one or more steps in the pathway of ribosome scanning. For other mRNAs, secondary structure, either intrinsic to the mRNA or resulting from interactions between translation factors at the 5′ end and RNA-binding proteins bound to sequences in the 3′ UTR, may bring Vhs into the vicinity of the preferred cleavage sites. These and other models are currently under investigation.
Acknowledgments
This work was supported by U.S. Public Health Service grant RO1 AI-21501 from the National Institute of Allergy and Infectious Diseases and by a grant from the University of Missouri Research Board.
We thank Lindsey Hutt-Fletcher for many helpful discussions and for providing the recombinant vaccinia virus.
REFERENCES
- 1.Allmang, C., E. Petfalski, A. Podtelejnikov, M. Mann, D. Tollervey, and P. Mitchell.c 1999. The yeast exosome and human PM-Scl are related complexes of 3′→5′ exonucleases. Genes Dev. 13:2148-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, J. S. J., and R. Parker. 1998. The 3′ to 5′ degradation of yeast mRNAs is a general mechanism for mRNA turnover that requires the SKI2 DEVH box protein and 3′ to 5′ exonucleases of the exosome complex. EMBO J. 17:1497-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker, Y., E. Tavor, Y. Asher, C. Berkowiltz, and M. Moyal. 1993. Effect of herpes simplex virus type-1 UL41 gene on the stability of mRNA from the cellular genes: beta-actin, fibronectin, glucose transporter-1, and docking protein, and on virus intraperitoneal pathogenicity of newborn mice. Virus Genes 7:133-143. [DOI] [PubMed] [Google Scholar]
- 4.Callaghan, A. J., J. P. Aurikko, L. L. Ilag, G. J. Gunter, V. Chandran, K. Kuhnel, L. Poljak, A. J. Carpousis, C. V. Robinson, M. F. Symmons, and B. F. Luisi. 2004. Studies of the RNA degradosome-organizing domain of the Escherichia coli ribonuclease RNase E. J. Mol. Biol. 340:965-979. [DOI] [PubMed] [Google Scholar]
- 5.Carpousis, A. J. 2002. The Escherichia coli RNA degradosome: structure, function and relationship in other ribonucleolytic multienzyme complexes. Biochem. Soc. Trans. 30:150-155. [PubMed] [Google Scholar]
- 6.Chan, C. C., J. Dostie, M. D. Diem, W. Feng, M. Mann, J. Rappsilber, and G. Dreyfuss. 2004. eIF4A3 is a novel component of the exon junction complex. RNA 10:200-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coburn, G. A., X. Miao, D. J. Briant, and G. A. Mackie. 1999. Reconstitution of a minimal RNA degradosome demonstrates functional coordination between a 3′ exonuclease and a DEAD-box RNA helicase. Genes Dev. 13:2594-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coller, J. M., M. Tucker, U. Sheth, M. A. Valencia-Sanchez, and R. Parker. 2001. The DEAD box helicase, Dhh1p, functions in mRNA decapping and interacts with both the decapping and deadenylase complexes. RNA 7:1717-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diamond, S. E., and K. Kirkegaard. 1994. Clustered charged-to-alanine mutagenesis of poliovirus RNA-dependent RNA polymerase yields multiple temperature-sensitive mutants defective in RNA synthesis. J. Virol. 68:863-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dmitriev, S. E., I. M. Terenin, Y. E. Dunaevsky, W. C. Merrick, and I. N. Shatsky. 2003. Assembly of 48S translation initiation complexes from purified components with mRNAs that have some base pairing within their 5′ untranslated regions. Mol. Cell. Biol. 23:8925-8933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doepker, R. C., W. L. Hsu, H. A. Saffran, and J. R. Smiley. 2004. Herpes simplex virus virion host shutoff protein is stimulated by translation initiation factors eIF4B and eIF4H. J. Virol. 78:4684-4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elgadi, M. M., C. E. Hayes, and J. R. Smiley. 1999. The herpes simplex virus vhs protein induces endoribonucleolytic cleavage of target RNAs in cell extracts. J. Virol. 73:7153-7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elgadi, M. M., and J. R. Smiley. 1999. Picornavirus internal ribosome entry site elements target RNA cleavage events induced by the herpes simplex virus virion host shutoff protein. J. Virol. 73:9222-9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esclatine, A., B. Taddeo, L. Evans, and B. Roizman. 2004. The herpes simplex virus 1 UL41 gene-dependent destabilization of cellular RNAs is selective and may be sequence-specific. Proc. Natl. Acad. Sci. USA 101:3603-3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everly, D. N., Jr., P. Feng, I. S. Mian, and G. S. Read. 2002. mRNA degradation by the virion host shutoff protein (UL41) of herpes simplex virus: genetic and biochemical evidence that UL41 is a nuclease. J. Virol. 76:8560-8571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Everly, D. N., Jr., and G. S. Read. 1997. Mutational analysis of the virion host shutoff gene (UL41) of herpes simplex virus (HSV): characterization of HSV type 1 (HSV-1)/HSV-2 chimeras. J. Virol. 71:7157-7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Everly, D. N., Jr., and G. S. Read. 1999. Site-directed mutagenesis of the virion host shutoff gene (UL41) of herpes simplex virus: analysis of functional differences between HSV-1 and HSV-2 alleles. J. Virol. 73:9117-9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng, P., D. N. Everly, Jr., and G. S. Read. 2001. mRNA decay during herpesvirus infections: interaction between a putative viral nuclease and a cellular translation factor. J. Virol. 75:10272-10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fenwick, M. L., and M. M. McMenamin. 1984. Early virion-associated suppression of cellular protein synthesis by herpes simplex virus is accompanied by inactivation of mRNA. J. Gen. Virol. 65:1225-1228. [DOI] [PubMed] [Google Scholar]
- 20.Ferraiuolo, M. A., C. S. Lee, L. W. Ler, J. L. Hsu, M. Costa-Mattioli, M. J. Luo, R. Reed, and N. Sonenberg. 2004. A nuclear translation-like factor eIF4AIII is recruited to the mRNA during splicing and functions in nonsense-mediated decay. Proc. Natl. Acad. Sci. USA 101:4118-4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fields, S., and O. Song. 1989. A novel genetic system to detect protein-protein interactions. Nature 340:245-247. [DOI] [PubMed] [Google Scholar]
- 22.Geiss, B. J., T. J. Smith, D. A. Leib, and L. A. Morrison. 2000. Disruption of virion host shutoff activity improves the immunogenicity and protective capacity of a replication-incompetent herpes simplex virus type 1 vaccine strain. J. Virol. 74:11137-11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gingras, A. C., B. Raught, and N. Sonenberg. 1999. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 68:913-963. [DOI] [PubMed] [Google Scholar]
- 24.Hardwicke, M. A., and R. M. Sandri-Goldin. 1994. The herpes simplex virus regulatory protein ICP27 contributes to the decrease in cellular mRNA levels during infection. J. Virol. 68:4797-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardy, W. R., and R. M. Sandri-Goldin. 1994. Herpes simplex virus inhibits host cell splicing, and the regulatory protein ICP27 is required for this effect. J. Virol. 68:7790-7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hellen, C. U., and P. Sarnow. 2001. Internal ribosome entry sites in eukaryotic mRNA molecules. Genes Dev. 15:1593-1612. [DOI] [PubMed] [Google Scholar]
- 27.Hershey, J. W. B., and W. C. Merrick. 2000. Pathway and mechanism of initiation of protein synthesis, p. 33-89. In N. Sonenberg, J. W. B. Hershey, and M. B. Mathews (ed.), Translational control of gene expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 28.Hope, D. A., S. E. Diamond, and K. Kirkegaard. 1997. Genetic dissection of interaction between poliovirus 3D polymerase and viral protein 3AB. J. Virol. 71:9490-9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes, D. L., T. E. Dever, and W. C. Merrick. 1993. Further biochemical characterization of rabbit reticulocyte eIF-4B. Arch. Biochem. Biophys. 301:311-319. [DOI] [PubMed] [Google Scholar]
- 30.Karr, B. M., and G. S. Read. 1999. The virion host shutoff function of herpes simplex virus degrades the 5′ end of a target mRNA before the 3′ end. Virology 264:195-204. [DOI] [PubMed] [Google Scholar]
- 31.Keadle, T. L., K. A. Laycock, J. L. Morris, D. A. Leib, L. A. Morrison, J. S. Pepose, and P. M. Stuart. 2002. Therapeutic vaccination with vhs(-) herpes simplex virus reduces the severity of recurrent herpetic stromal keratitis in mice. J. Gen. Virol. 83:2361-2365. [DOI] [PubMed] [Google Scholar]
- 32.Keadle, T. L., L. A. Morrison, J. L. Morris, J. S. Pepose, and P. M. Stuart. 2002. Therapeutic immunization with a virion host shutoff-defective, replication-incompetent herpes simplex virus type 1 strain limits recurrent herpetic ocular infection. J. Virol. 76:3615-3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khemici, V., and A. J. Carpousis. 2004. The RNA degradosome and poly(A) polymerase of Escherichia coli are required in vivo for the degradation of small mRNA decay intermediates containing REP-stabilizers. Mol. Microbiol. 51:777-790. [DOI] [PubMed] [Google Scholar]
- 34.Kielkopf, C. L., S. Lucke, and M. R. Green. 2004. U2AF homology motifs: protein recognition in the RRM world. Genes Dev. 18:1513-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolupaeva, V. G., I. B. Lomakin, T. V. Pestova, and C. U. Hellen. 2003. Eukaryotic initiation factors 4G and 4A mediate conformational changes downstream of the initiation codon of the encephalomyocarditis virus internal ribosomal entry site. Mol. Cell. Biol. 23:687-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolupaeva, V. G., T. V. Pestova, C. U. T. Hellen, and I. N. Shatsky. 1998. Translation eukaryotic initiation factor 4G recognizes a specific structural element within the internal ribosome entry site of encephalomyocarditis virus RNA. J. Biol. Chem. 273:18599-18604. [DOI] [PubMed] [Google Scholar]
- 37.Krikorian, C. R., and G. S. Read. 1991. An in vitro mRNA degradation system to study the virion host shutoff function of herpes simplex virus. J. Virol. 65:112-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwong, A. D., and N. Frenkel. 1987. Herpes simplex virus-infected cells contain a function(s) that destabilizes both host and viral mRNAs. Proc. Natl. Acad. Sci. USA 84:1926-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwong, A. D., J. A. Kruper, and N. Frenkel. 1988. Herpes simplex virus virion host shutoff function. J. Virol. 62:912-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leib, D. A., T. E. Harrison, K. M. Laslo, M. A. Machalek, N. J. Moorman, and H. W. Virgin. 1999. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J. Exp. Med. 189:663-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li, Q., S. M. Turk, and L. M. Hutt-Fletcher. 1995. The Epstein-Barr virus (EBV) BZLF2 gene product associates with the gH and gL homologs of EBV and carries an epitope critical to infection of B cells but not of epithelial cells. J. Virol. 69:3987-3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li, Q. Y., H. Imataka, S. Morino, G. W. Rogers, Jr., N. J. Richter-Cook, W. C. Merrick, and N. Sonenberg. 1999. Eukaryotic translation initiation factor 4AIII (eTF4AIII) is functionally distinct from eIF4AI and eIF4AII. Mol. Cell. Biol. 19:7336-7346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lomakin, I. B., C. U. Hellen, and T. V. Pestova. 2000. Physical association of eukaryotic initiation factor 4G (eIF4G) with eIF4A strongly enhances binding of eIF4G to the internal ribosomal entry site of encephalomyocarditis virus and is required for internal initiation of translation. Mol. Cell. Biol. 20:6019-6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu, P., H. A. Saffran, and J. R. Smiley. 2001. The vhs1 mutant form of herpes simplex virus virion host shutoff protein retains significant internal ribosome entry site-directed RNA cleavage activity. J. Virol. 75:1072-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mackie, G. A. 1998. Ribonuclease E is a 5′-end-dependent endonuclease. Nature 395:720-723. [DOI] [PubMed] [Google Scholar]
- 46.Martindale, D. W., M. D. Wilson, D. Wang, R. D. Burke, X. Chen, V. Duronio, and B. F. Koop. 2000. Comparative genomic sequence analysis of the Williams syndrome region (LIMK1-RFC2) of human chromosome 7q11.23. Mamm. Genome 11:890-898. [DOI] [PubMed] [Google Scholar]
- 47.Methot, N., G. Pickett, J. D. Keene, and N. Sonenberg. 1996. In vitro RNA selection identifies RNA ligands that specifically bind to eukaryotic translation initiation factor 4B: the role of the RNA remotif. RNA 2:38-50. [PMC free article] [PubMed] [Google Scholar]
- 48.Méthot, N., E. Rom, H. Olsen, and N. Sonenberg. 1997. The human homologue of the yeast Prt1 protein is an integral part of the eukaryotic initiation factor 3 complex and interacts with p170. J. Biol. Chem. 272:1110-1116. [DOI] [PubMed] [Google Scholar]
- 49.Méthot, N., M. S. Song, and N. Sonenberg. 1996. A region rich in aspartic acid, arginine, tyrosine, and glycine (DRYG) mediates eukaryotic initiation factor 4B (eIF4B) self-association and interaction with eIF3. Mol. Cell. Biol. 16:5328-5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mitchell, P., E. Petfalski, A. Shevchenko, M. Mann, and D. Tollervey. 1997. The exosome: a conserved eukaryotic RNA processing complex containing multiple 3′→5′ exoribonucleases. Cell 91:457-466. [DOI] [PubMed] [Google Scholar]
- 51.Moss, B., O. Elroy-Stein, T. Mizukami, W. A. Alexander, and T. R. Fuerst. 1990. New mammalian expression vectors. Nature 348:91-92. [DOI] [PubMed] [Google Scholar]
- 52.Murphy, J. A., R. J. Duerst, T. J. Smith, and L. A. Morrison. 2003. Herpes simplex virus type 2 virion host shutoff protein regulates alpha/beta interferon but not adaptive immune responses during primary infection in vivo. J. Virol. 77:9337-9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oroskar, A. A., and G. S. Read. 1987. A mutant of herpes simplex virus type 1 exhibits increased stability of immediate-early (alpha) mRNAs. J. Virol. 61:604-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oroskar, A. A., and G. S. Read. 1989. Control of mRNA stability by the virion host shutoff function of herpes simplex virus. J. Virol. 63:1897-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pak, A. S., D. N. Everly, K. Knight, and G. S. Read. 1995. The virion host shutoff protein of herpes simplex virus inhibits reporter gene expression in the absence of other viral gene products. Virology 211:491-506. [DOI] [PubMed] [Google Scholar]
- 56.Pestova, T. V., S. I. Borukhov, and C. U. T. Hellen. 1998. Eukaryotic ribosomes require initiation factors 1 and 1A to locate initiation codons. Nature 394:854-859. [DOI] [PubMed] [Google Scholar]
- 57.Pestova, T. V., C. U. T. Hellen, and I. N. Shatsky. 1996. Canonical eukaryotic initiation factors determine initiation of translation by internal ribosomal entry. Mol. Cell. Biol. 16:6859-6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petermann, R., B. M. Mossier, D. N. Aryee, and H. Kovar. 1998. A recombination based method to rapidly assess specificity of two-hybrid clones in yeast. Nucleic Acids Res. 26:2252-2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Read, G. S. 1997. Control of mRNA stability during herpes simplex virus infections, p. 311-321. In J. B. Harford and D. R. Morris (ed.), mRNA metabolism and post-transcriptional gene regulation. Wiley-Liss, Inc., New York, N.Y.
- 60.Read, G. S., and N. Frenkel. 1983. Herpes simplex virus mutants defective in the virion-associated shutoff of host polypeptide synthesis and exhibiting abnormal synthesis of alpha (immediate-early) polypeptides. J. Virol. 46:498-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Read, G. S., B. M. Karr, and K. Knight. 1993. Isolation of a herpes simplex virus type 1 mutant with a deletion in the virion host shutoff gene and identification of multiple forms of the vhs (UL41) polypeptide. J. Virol. 67:7149-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Richter, N. J., G. W. Rogers, J. O. Hensold, and W. C. Merrick. 1999. Further biochemical and kinetic characterization of human eukaryotic initiation factor 4H. J. Biol. Chem. 274:35415-35424. [DOI] [PubMed] [Google Scholar]
- 63.Richter-Cook, N. J., T. E. Dever, J. O. Hensold, and W. C. Merrick. 1998. Purification and characterization of a new eukaryotic protein translation factor—eukaryotic initiation factor 4H. J. Biol. Chem. 273:7579-7587. [DOI] [PubMed] [Google Scholar]
- 64.Rogers, G. W., W. F. Lima, and W. C. Merrick. 2001. Further characterization of the helicase activity of eIF4A. Substrate specificity. J. Biol. Chem. 276:12598-12608. [DOI] [PubMed] [Google Scholar]
- 65.Rogers, G. W., Jr., N. J. Richter, and W. C. Merrick. 1999. Biochemical and kinetic characterization of the RNA helicase activity of eukaryotic initiation factor 4A. J. Biol. Chem. 274:12236-12244. [DOI] [PubMed] [Google Scholar]
- 66.Rogers, G. W. J., A. A. Komar, and W. C. Merrick. 2002. eIF4A: the godfather of the DEAD box helicases. Prog. Nucleic Acid Res. Mol. Biol. 72:307-331. [DOI] [PubMed] [Google Scholar]
- 67.Rogers, G. W. J., N. J. Richter, W. F. Lima, and W. C. Merrick. 2001. Modulation of the helicase activity of eIF4A by eIF4B, eIF4H, and eIF4F. J. Biol. Chem. 276:30914-30922. [DOI] [PubMed] [Google Scholar]
- 68.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2459. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 69.Samady, L., E. Costigliola, L. MacCormac, Y. McGrath, S. Cleverley, C. E. Lilley, J. Smith, D. S. Latchman, B. Chain, and R. S. Coffin. 2003. Deletion of the virion host shutoff protein (vhs) from herpes simplex virus (HSV) relieves the viral block to dendritic cell activation: potential of vhs− HSV vectors for dendritic cell-mediated immunotherapy. J. Virol. 77:3768-3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schek, N., and S. L. Bachenheimer. 1985. Degradation of cellular mRNAs induced by a virion-associated factor during herpes simplex virus infection of Vero cells. J. Virol. 55:601-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shibuya, T., T. O. Tange, N. Sonenberg, and M. J. Moore. 2004. eIF4AIII binds spliced mRNA in the exon junction complex and is essential for nonsense-mediated decay. Nat. Struct. Mol. Biol. 11:346-351. [DOI] [PubMed] [Google Scholar]
- 72.Smibert, C. A., D. C. Johnson, and J. R. Smiley. 1992. Identification and characterization of the virion-induced host shutoff product of herpes simplex virus gene UL41. J. Gen. Virol. 73:467-470. [DOI] [PubMed] [Google Scholar]
- 73.Smiley, J. R. 2004. Herpes simplex virus virion host shutoff protein: immune evasion mediated by a viral RNase? J. Virol. 78:1063-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith, T. J., C. E. Ackland-Berglund, and D. A. Leib. 2000. Herpes simplex virus virion host shutoff (vhs) activity alters periocular disease in mice. J. Virol. 74:3598-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith, T. J., L. A. Morrison, and D. A. Leib. 2002. Pathogenesis of herpes simplex virus type 2 virion host shutoff (vhs) mutants. J. Virol. 76:2054-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sonenberg, N., and T. E. Dever. 2003. Eukaryotic translation initiation factors and regulators. Curr. Opin. Struct. Biol. 13:56-63. [DOI] [PubMed] [Google Scholar]
- 77.Sorenson, C. M., P. A. Hart, and J. Ross. 1991. Analysis of herpes simplex virus-induced mRNA destabilizing activity using an in vitro mRNA decay system. Nucleic Acids Res. 19:4459-4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Strelow, L., T. Smith, and D. A. Leib. 1997. The virion host shutoff function of herpes simplex virus type 1 plays a role in corneal invasion and functions independently of the cell cycle. Virology 231:28-34. [DOI] [PubMed] [Google Scholar]
- 79.Strelow, L. I., and D. A. Leib. 1995. Role of the virion host shutoff (vhs) of herpes simplex virus type 1 in latency and pathogenesis. J. Virol. 69:6779-6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Strelow, L. I., and D. A. Leib. 1996. Analysis of conserved domains of UL41 of herpes simplex virus type 1 in virion host shutoff and pathogenesis. J. Virol. 70:5665-5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Strom, T., and N. Frenkel. 1987. Effects of herpes simplex virus on mRNA stability. J. Virol. 61:2198-2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Suzutani, T., M. Nagamine, T. Shibaki, M. Ogasawara, I. Yoshida, T. Daikoku, Y. Nishiyama, and M. Azuma. 2000. The role of the UL41 gene of herpes simplex virus type 1 in evasion of non-specific host defence mechanisms during primary infection. J. Gen. Virol. 81:1763-1771. [DOI] [PubMed] [Google Scholar]
- 83.Svitkin, Y. V., A. Pause, A. Haghighat, S. Pyronnet, G. Witherell, G. J. Belsham, and N. Sonenberg. 2001. The requirement for eukaryotic initiation factor 4A (elF4A) in translation is in direct proportion to the degree of mRNA 5′ secondary structure. RNA 7:382-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Taddeo, B., A. Esclatine, W. Zhang, and B. Roizman. 2003. The stress-inducible immediate-early responsive gene IEX-1 is activated in cells infected with herpes simplex virus 1, but several viral mechanisms, including 3′ degradation of its RNA, preclude expression of the gene. J. Virol. 77:6178-6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tigges, M. A., S. Leng, D. C. Johnson, and R. L. Burke. 1996. Human herpes simplex virus (HSV)-specific CD8+ CTL clones recognize HSV-2-infected fibroblasts after treatment with IFN-γ or when virion host shutoff functions are disabled. J. Immunol. 156:3901-3910. [PubMed] [Google Scholar]
- 86.Tollervey, D., and J. F. Caceres. 2000. RNA processing marches on. Cell 103:703-709. [DOI] [PubMed] [Google Scholar]
- 87.Trgovcich, J., D. Johnson, and B. Roizman. 2002. Cell surface major histocompatibility complex class II proteins are regulated by the products of the γ134.5 and UL41 genes of herpes simplex virus 1. J. Virol. 76:6974-6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Van Hoof, A., and R. Parker. 1999. The exosome: A proteasome for RNA? Cell 99:347-350. [DOI] [PubMed] [Google Scholar]
- 89.Vanzo, N. F., Y. S. Li, B. Py, E. Blum, C. F. Higgins, L. C. Raynal, H. M. Krisch, and A. J. Carpousis. 1998. Ribonuclease E organizes the protein interactions in the Escherichia coli RNA degradosome. Genes Dev. 12:2770-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wertman, K. F., D. G. Drubin, and D. Botstein. 1992. Systematic mutational analysis of the yeast ACT1 gene. Genetics 132:337-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu, S., C. M. Romfo, T. W. Nilsen, and M. R. Green. 1999. Functional recognition of the 3′ splice site AG by the splicing factor U2AF35. Nature 402:832-835. [DOI] [PubMed] [Google Scholar]
- 92.Zelus, B. D., R. S. Stewart, and J. Ross. 1996. The virion host shutoff protein of herpes simplex virus type 1: messenger ribonucleolytic activity in vitro. J. Virol. 70:2411-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]