Abstract
In 2003, H5N1 avian influenza virus infections were diagnosed in two Hong Kong residents who had visited the Fujian province in mainland China, affording us the opportunity to characterize one of the viral isolates, A/Hong Kong/213/03 (HK213; H5N1). In contrast to H5N1 viruses isolated from humans during the 1997 outbreak in Hong Kong, HK213 retained several features of aquatic bird viruses, including the lack of a deletion in the neuraminidase stalk and the absence of additional oligosaccharide chains at the globular head of the hemagglutinin molecule. It demonstrated weak pathogenicity in mice and ferrets but caused lethal infection in chickens. The original isolate failed to produce disease in ducks but became more pathogenic after five passages. Taken together, these findings portray the HK213 isolate as an aquatic avian influenza A virus without the molecular changes associated with the replication of H5N1 avian viruses in land-based poultry such as chickens. This case challenges the view that adaptation to land-based poultry is a prerequisite for the replication of aquatic avian influenza A viruses in humans.
In 1997, avian H5N1 influenza A viruses were transmitted to 18 Hong Kong residents, 6 of whom died (6). These viruses were characterized by a shortened neuraminidase (NA) stalk, and several isolates contained an additional glycosylation site at the globular head of their hemagglutinin (HA) molecule (2). Both of these changes are often found in highly pathogenic H5 or H7 viruses isolated from chickens (1, 7, 18) and are thought to occur when viruses from wild aquatic birds adapt to grow in land-based poultry such as chickens. Hence, chickens are thought to be able to serve as an intermediate host required for the introduction of aquatic bird influenza A viruses into the human population.
Since 1997, direct transmission of avian viruses to humans has occurred in countries with outbreaks of avian influenza in their poultry stocks, for example, in Hong Kong (H5N1, H9N2), Vietnam (H5N1), Thailand (H5N1), The Netherlands (H7N7), and Canada (H7N3) (3-5, 8, 15, 19, 29). Two cases of H5N1 avian influenza disease were reported in 2003 (19). One developed in a boy who had close contact with live chickens during a visit to Fujian province in mainland China; an H5N1 virus (A/Hong Kong/213/03; HK213) was subsequently isolated from his nasopharyngeal aspirate. His father died of an H5N1 influenza virus infection (A/Hong Kong/212/03; HK212). Both the HK212 and HK213 isolates, whose gene sequences are almost identical, share many features with viruses isolated from aquatic birds but lack the changes typically associated with adaptation to land-based poultry, such as a deletion in the NA stalk (10, 18), suggesting that such adaptation may not be required for replication in mammals. To address this issue, we studied the biological properties of HK213.
MATERIALS AND METHODS
Viruses and cells.
A/Hong Kong/213/03 (H5N1, HK213) viruses from a 9-year-old male were isolated in parallel in either embryonated chicken eggs (HK213Egg) or Madin-Darby canine kidney (MDCK) cells (HK213CK). Virus stocks were grown either in 11-day-old embryonated chicken eggs (HK213Egg) or on MDCK cells (HK213CK) in minimal essential medium (MEM) supplemented with 0.3% bovine serum albumin. A/duck/Hong Kong/836/80, A/New Caledonia/20/99, A/Duck/Hong Kong/200/01, and A/Memphis/1/71 were propagated in MDCK cells in MEM supplemented with 0.3% bovine serum albumin. MDCK cells were maintained in MEM supplemented with 5% newborn calf serum (Sigma, St. Louis, MO) and antibiotics at 37°C in 5% CO2. All experiments with live Hong Kong H5N1 virus were performed in a biosafety level 3+ containment laboratory approved for such use by the U.S. Department of Agriculture and the Centers for Disease Control and Prevention.
Sequence analysis.
RNA was extracted from viruses either in infected allantoic fluid or in culture fluid of MDCK cells by using an RNeasy Mini Kit (QIAGEN, Valencia, CA). We performed reverse transcription-PCRs using primers specific for each gene segment. The reverse transcription-PCR products were cloned into vector pT7Blue (Novagen, Madison, WI), and at least three independent clones were sequenced per segment. Automated sequencing was performed at the University of Wisconsin—Madison Biotechnology Center.
Receptor specificity of HK213Egg and HK213CK viruses.
The solid-phase binding assay with competing glycopolymers was performed as described in a previous report (14). Briefly, viruses were allowed to attach overnight at 4°C in wells of 96-well plates. A glycopolymer containing N-acetylneuraminic acid linked to galactose through either an α-2,3 or an α-2,6 bond (Neu5Acα2,3LacNAcb-pAP and Neu5Acα2,6LacNAcb-pAP) was added to wells containing the immobilized viruses together with horseradish peroxidase (HRP)-conjugated bovine fetuin. The extent of the inhibitory effect of the polymers was determined by measuring HRP activity.
The direct binding activity of viruses to gangliosides was determined by a previously published method (28). Briefly, IV3(Neu5Ac)nLc4B30 [Neu5Acα2-3Galβ1-4GlcNAcβ1-3Gallβ1-4Glcβ1-OCH(C14H29)2] (α2,3 ganglioside) or IV6(Neu5Ac)nLc4B30 [Neu5Acα2-6Galβ1-4GlcNAcβ1-3Gallβ1-4Glcβ1-OCH(C14H29)2] (α2,6 ganglioside) was added to wells of microtiter plates, followed by the addition of virus. Virus binding to gangliosides was then measured by adding virus-specific antibodies (12), followed by incubation with HRP-conjugated anti-mouse immunoglobulin G plus immunoglobulin M antiserum (Bio-Rad, Hercules, CA). The amount of bound virions was determined by measuring HRP activity.
Animal experiments. (i) Chickens.
The dose required to kill 50% of infected animals (LD50) was determined by intranasally inoculating 2-week-old specific-pathogen-free chickens with 50 μl of serial 10-fold dilutions of HK213Egg virus; the infected animals were observed daily for 2 weeks. Twelve chickens were also intranasally inoculated with 105 PFU of HK213Egg for virologic and histologic examinations.
(ii) Ducks.
For determination of the pathogenicity of the human HK213 virus for waterfowl, we inoculated 1-week-old or 3- to 4-month-old mallard ducks (Ridgeway Hatcheries Inc., La Rue, OH) with 300 μl or 1.0 ml, respectively, of serial 10-fold dilutions of either HK213Egg or HK213CK virus by the nasal, tracheal, or oral route and observed them for 2 weeks. For virologic and histologic examinations, ducklings inoculated with 105 PFU of HK213Egg were euthanized and examined on day 3, 6, or 12 postinfection (p.i.) (three birds per time point), while six adult ducks inoculated with the same dose were studied after necropsy on day 3 or 6 p.i.
(iii) Mice.
To determine the LD50 for mice, we intranasally infected 4-week-old female BALB/c mice (Jackson Laboratories, Harbor, ME) with 50 μl of serial 10-fold dilutions of HK213Egg or HK213CK virus (three animals per dilution) and observed them for 2 weeks. The pathogenicity of HK213 virus was investigated further in groups of mice intranasally infected with HK213Egg or HK213CK (six per group; 7 × 102 PFU/50 μl/mouse) and euthanized on day 1, 3, or 6 p.i. for virologic and pathologic examinations. Because the growth of HK213 virus was poor in MDCK cells compared with in eggs, we compared the effect of sequence variations observed between the two viruses only in mouse experiments.
(iv) Ferrets.
Seven- to 8-month-old ferrets (Marshall Farms, North Rose, NY) were intranasally infected with 1 ml (106 PFU) of HK213Egg virus under anesthesia (isoflurane, followed by ketamine and xylazine, 2 mg/kg). On day 1, 3, 6, or 12 p.i., three animals were euthanized for virologic and histologic examination.
Virus titers in the organs of the animals used in each of these animal experiments were determined by use of 11-day-old embryonated chicken eggs.
Pathologic examination.
Removed tissues were fixed in 10% phosphate-buffered formalin. They were then dehydrated, embedded in paraffin, and cut into 5-μm-thick sections that were stained with standard hematoxylin and eosin. Immunohistochemical analysis was performed as described previously (25), using antibodies to an H5 virus (A/whistling swan/Shimane/499/83).
Nucleotide sequence accession numbers.
The nucleotide sequences determined in this study were submitted to GenBank and assigned accession no. AB212050 to AB212059.
RESULTS AND DISCUSSION
Molecular characterization of the HK213 virus.
We first sequenced the entire genome of HK213 in either MDCK cells or embryonated chicken eggs. The HK213CK and HK213Egg viruses differed by two nucleotides in the PB2 segment, G versus A at position 1126 and C versus T at position 1572 with the associated substitution, amino acid Glu versus Arg, occurring at position 367; a single nucleotide change in the NS segment, A versus G at position 310, with an associated Lys versus Arg amino acid substitution at position 95, was also found.
These sequencing results revealed a feature that is critical for the strong pathogenicity of influenza A viruses in chickens: multiple basic amino acid residues at the HA cleavage site. HK213 did not contain a Lys amino acid known to correlate with pathogenicity in certain mammalian species, namely, Lys at position 627 of polymerase subunit PB2 (11). Notably, it also lacked both an additional glycosylation site on the globular head of the HA and a deletion in the NA stalk that likely occurs during the adaptation of highly pathogenic avian H5 or H7 influenza viruses to land-based poultry (e.g., chickens and quail) (7, 10, 18). Thus, the HK213 virus was more closely related to aquatic bird viruses than to human H5N1 viruses isolated in 1997 or to isolates from land-based poultry.
Receptor specificity of the HK213 virus.
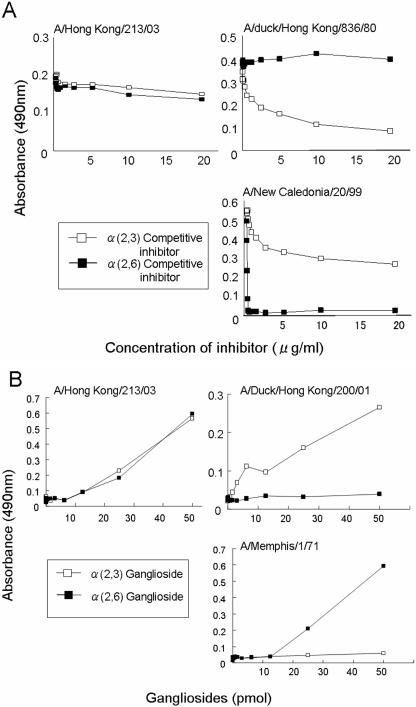
The HAs of authentic avian influenza viruses preferentially bind to oligosaccharides that terminate in an α(2,3)-linked sialic acid, whereas authentic human viruses preferentially recognize those with an α(2,6)-linked sialic acid (17, 21, 22, 32). We first assessed the receptor specificity of HK213 in an assay in which the binding of virus to fetuin (possessing both types of oligosaccharides) is inhibited with a polymer possessing either α(2,3)- or α(2,6)-linked sialic acid. In this assay, authentic avian (A/duck/Hong Kong/836/80) and human (A/New Caledonia/20/99) influenza viruses demonstrated the anticipated receptor specificity, whereas the binding of HK213 was not inhibited by either polymer (Fig. 1A). We next tested the ability of HK213 to bind directly to gangliosides possessing either Neu5Acα2-3Gal or Neu5Acα2-6Gal (12, 16, 28). The virus bound to both the α(2,3)- and α(2,6)-linked sialic acids (Fig. 1B), suggesting that after its transmission from a bird to a human, it may have acquired the ability to recognize Neu5Acα2-6Gal. This concept is consistent with the current knowledge in that although the HAs of the first strains available from all pandemics of the 20th century were transmitted from birds to humans (20, 24), they all preferentially recognized α2,6 sialyloligosaccharides (14, 21). Thus, the alteration in receptor specificity from α2,3 to α2,6 sialyloligosaccharide recognition must have occurred in the HAs upon transmission from birds to humans. A similar finding of dual recognition capacity was reported for a human H9N2 isolate (23), suggesting the possibility that this property was acquired during adaptation of the avian virus to humans.
FIG. 1.
Receptor specificity of the HK213 virus. (A) The solid-phase binding assay with competing glycopolymers was performed as described in a previous report (14). The binding of HK213 virus to fetuin was not inhibited by a polymer possessing either an α(2,3)- or an α(2,6)-linked sialic acid, unlike the human and avian viruses used as controls for α2,3 or α2,6 binder, respectively. (B) The direct binding activity of viruses to gangliosides was determined by a previously published method (28). The HK213 virus has the ability to bind to both the α(2,3)- and α(2,6)-linked sialic acids.
Pathogenicity of HK213 in animal models. (i) Chickens.
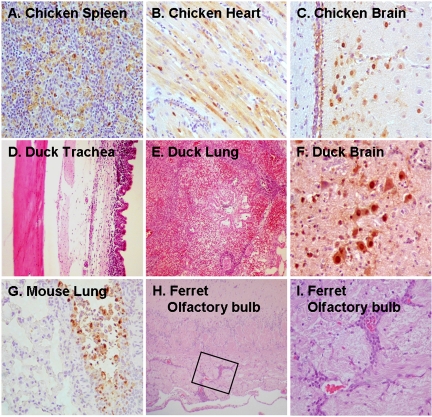
HK213Egg virus was highly virulent in chickens, with an LD50 of 3.2 × 102 PFU, similar to that of the 1997 H5N1 human strains (9). Chickens were also intranasally inoculated with 105 PFU of HK213Egg for virologic and histologic examinations. At 24 h p.i., the virus titers in representative organs of three chickens indicated systemic replication of HK213Egg virus (Table 1). The remaining six chickens succumbed to infection by day 2 p.i., with pathologic examinations confirming systemic virus replication in the spleens, cardiac myocytes, and brain tissues of these birds (Fig. 2A to C). Thus, the 2003 H5N1 human virus isolate, like the 1997 H5N1 human viruses, retained pathogenicity in chickens after replication in a mammalian system.
TABLE 1.
Virus titers in organs of chickens infected with HK213Egg virusa
| Days p.i. | Virus titer (mean log10 EID50 ± SD/g)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nasal turbinates | Lungs | Brain | Liver | Spleen | Kidneys | Heart | Pancreas | Colon | Bursa of Fabricius | |
| 1 | 5.2 ± 1.9 | 5.7 ± 2.5 | 5.0, 5.5 | 5.0 ± 2.1 | 5.9 ± 2.3 | 5.0 ± 2.2 | 4.3 ± 2.2 | 6.0, 6.3 | 6.3, 6.8 | 6.6, 6.1 |
Two-week-old specific-pathogen-free chickens were infected intranasally and orally with 50 μl of virus (a total of 105 PFU). Three chickens were sacrificed on day 1 p.i., and viral titers were determined in embryonated chicken eggs. When virus was not recovered from all three chickens, individual titers were recorded. By day 2, the remaining chickens had died.
FIG. 2.
Representative histologic and immunohistologic findings on animals infected with HK213Egg virus. Chickens: detection of viral antigens (brown) in macrophages in the spleen (A), cardiac myocytes (B), and brain tissue (C) on day 2 p.i. Ducks: severe infiltration of mononuclear cells into the lamina propria mucosae of the trachea (D) and focal pneumonia with prominent infiltration of mononuclear cells (E) in asymptomatic animals and brain lesions with prominent viral antigen expression in the dead duckling (F). Mice: antigen-positive cells along the bronchial epithelium on day 3 p.i. (G). Ferrets: perivascular infiltrations of mononuclear cells in the olfactory bulb (H). The boxed area in panel H is shown at a higher magnification in panel I.
(ii) Ducks.
Phylogenetic analysis of the HA and NA segments showed that HK213 is closely related to egret (A/egret/Hong Kong/757.3/02) and goose (A/goose/Hong Kong/739.2/02) isolates, both collected during an outbreak of H5N1 viruses among waterfowl in Hong Kong in 2002 (10). These isolates were unusually pathogenic in waterfowl species, including ducks, geese, swans, and egrets (10). To determine if the pathogenicity of the human HK213 virus extended to waterfowl, we inoculated ducks with either HK213Egg or HK213CK virus by the nasal, tracheal, or oral route and observed them for 2 weeks. The infected birds showed no significant symptoms, with the exception of one duckling that died 8 days p.i. with HK213CK virus. Systemic spread of virus was detected at different intervals p.i. in asymptomatic ducks inoculated with HK213Egg virus (Table 2). Similar findings on viral replication in respiratory organs and viral shedding in feces were reported for a 1997 H5N1 human isolate (26). Pathologic examination revealed focal pneumonia in asymptomatic ducks, in contrast to rhinitis, pneumonia, and brain lesions in the dead duckling (Fig. 2D to F). Thus, although the MDCK isolate of HK213 did kill one duckling, overall, HK213 was not as pathogenic as some of the recent H5N1 isolates tested in ducks (30).
TABLE 2.
Virus titers in organs of ducks infected with HK213Egg virus
| Age of duck and days p.i. | Virus titer (mean log10 EID50 ± SD/g)
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nasal turbinates | Lungs | Trachea | Brain | Liver | Spleen | Kidney | Heart | Pancreas | S.I.d | L.I.e | Muscle | Feces | |
| 3-4 moa | |||||||||||||
| 3 | 2.3 ± 0.3 | 4.8 ± 0.9 | 4.4 ± 1.6 | 1.8 | 3.5 ± 0.6 | 4.3, 4.3 | 3.7 ± 2.0 | 2.2 ± 0.8 | 3.2 ± 1.8 | 4.4, 4.4 | 3.8 ± 2.6 | 1.5, 1.8 | 3.0, 5.6 |
| 6 | 2.1 | —c | — | — | — | — | — | 1.4 | — | — | 1.4 | — | — |
| 1 wkb | |||||||||||||
| 3 | 4.8 ± 0.4 | 3.0, 5.6 | 2.2 ± 0.8 | — | 2.7 ± 0.8 | 3.1 | 3.8 | 1.2, 2.7 | 1.8, 4.3 | — | 2.1 | 1.6 | — |
| 6 | 3.1, 4.4 | 4.6 | — | — | 1.5 | 4.4 | 2.1 | 1.3 | 1.6 | 1.8 | 2.4 | 2.3 | 1.2, 2.3 |
| 12 | 2.2 | — | — | — | 1.2 | — | — | — | — | — | — | — | — |
Adult mallard ducks were inoculated with 1 ml of virus by the intranasal, intraocular, intratracheal, and intracloacal routes (a total of 105 PFU). Three animals were euthanized on day 3 or 6 p.i. for virus titration. When virus was not recovered from all three animals, individual titers were recorded.
One-week-old mallard ducks were infected intranasally and orally with 300 μl of HK213Egg virus (a total of 105 PFU). Three ducks from each infected group were sacrificed on day 3, 6, or 12 p.i. for virus titration. When virus was not recovered from all three ducks, individual titers were recorded.
—, virus was not isolated.
S.I., small intestine.
L.I., large intestine.
We considered that serial passage of virus isolated from the dead duckling described above might lead to increased pathogenicity in ducks. Thus, brain homogenates from the dead bird were used to inoculate 10 ducklings by the oral-respiratory route. After the fifth passage, the virus had become more pathogenic in ducklings, causing nine deaths among 10 infected birds. Viruses obtained after the sixth passage produced nervous system signs in one of four infected adult ducks and caused the death of one of seven uninfected ducklings caged with the infected adult ducks. Further passages in either ducklings or adult ducks did not enhance the virulence of this virus. To the contrary, the percentages of ducklings dying after infection with viruses obtained from the seventh or eighth passage decreased to 25% and 0%, respectively, and none of the adult ducks died. Thus, although the HK213 virus has the potential to be pathogenic in ducks, it failed to produce the consistently high rates of morbidity and mortality seen with some of the recent H5N1 viruses (27).
(iii) Mice.
Mice infected with either HK213CK or HK213Egg virus did not succumb to infection, regardless of the test dose. High titers of HK213Egg virus were detected in the lungs throughout the experimental period (Table 3; Fig. 2G), reaching a peak on day 3 p.i.; lung titers remained high even on day 6 p.i. Although HK213CK virus replicated efficiently in lungs as well, its titers were slightly lower than those obtained with HK213Egg. HK213 virus isolates were also evident in extrarespiratory organs (Table 3), but the extent of replication in these organs did not permit detection by pathologic examination. These findings, together with the report by Guan et al. (10) that HK213 was only lethal to mice at a high virus dose (0.5 × 106.5 50% egg infective doses [EID50]), suggest a virulence profile similar to that described for a group of relatively benign H5N1 viruses isolated from humans in 1997 (9, 11, 13).
TABLE 3.
Virus titers in organs of mice infected with HK213Egg or HK213CK virusa
| Virus and days p.i. | Virus titer (mean log10 EID50 ± SD/g)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Nasal turbinates | Lungs | Spleen | Kidneys | Heart | Brain | Pancreas | Colon | |
| HK213Egg | ||||||||
| 1 | 2.2 ± 0.04 | 6.3 ± 0.2 | —b | 1.7 | — | — | 2.0 | — |
| 3 | 3.9 ± 0.5 | 7.8 ± 0.4 | 2.6, 3.5 | 1.9 ± 0.5 | — | — | 2.6 ± 0.6 | 2.6, 3.1 |
| 6 | 4.4 ± 1.0 | 7.6 ± 0.4 | 1.6, 1.6 | — | 1.7 | 1.9, 2.1 | — | — |
| HK213CK | ||||||||
| 1 | — | 4.3 ± 0.02 | — | — | — | — | — | — |
| 3 | 1.6, 2.5 | 6.8 ± 0.4 | — | — | — | — | 1.5 | 2.8 |
| 6 | — | 6.5 ± 0.3 | — | — | — | — | 2.0 | — |
BALB/c mice, anesthetized with isoflurane, were inoculated intranasally with virus (7 × 102 PFU/50 μl). Three animals from each infected group were euthanized on day 1, 3, or 6 p.i. for virus titration. When virus was not recovered from all three mice, individual titers were recorded.
—, Virus was not isolated.
(iv) Ferrets.
HK213Egg replicated mainly in the upper respiratory organs, including the nasal turbinates and trachea (Table 4). Notably, the inflammation in the nasal mucosa was so severe that the inflammatory change extended to the olfactory bulb (Fig. 2H and I). However, virus was not isolated from the brain, in contrast to the 1997 H5N1 human isolates (33). Our results with HK213 in ferrets differ from those of Webby et al. (31); however, there are multiple variations in the experimental conditions between the two studies, including the age of the ferrets, the inoculum viral dose, the number of nasal washings, and the anesthetic dose per animal.
TABLE 4.
Virus titers in organs of ferrets infected with HK213Egg virusa
| Days p.i. | Virus titer (mean log10 EID50 ± SD/g)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nasal turbinates | Lungs | Trachea | Brain | Liver | Spleen | Kidneys | Heart | Pancreas | Spinal cord | |
| 1 | 7.0 ± 1.0 | 5.2 | 5.3, 5.3 | —b | — | — | — | — | 1.1 | — |
| 3 | 4.4 ± 0.7 | 3.7 | — | — | — | — | — | — | — | — |
| 6 | 0.6 | — | — | — | — | — | — | — | — | — |
| 12 | — | — | — | — | — | — | — | — | — | — |
Ferrets, anesthetized with isoflurane and xylazine, were intranasally infected with virus (106 PFU/ml). Three animals were euthanized on day 1, 3, 6, or 12 p.i. for virus titration. When virus was not recovered from all of the three animals, individual titers were reported.
—, Virus was not isolated.
The potential of avian H5N1 influenza A viruses to infect humans continues to elicit global concern. Here we characterized biologic properties of one of two H5N1 avian viruses isolated from humans in 2003. The virus was not lethal in mice and ferrets, although it retained pathogenicity in chickens and acquired this property in ducks after several passages. Most striking, it does not appear to have undergone the molecular changes that defined the avian H5N1 viruses transmitted from chickens to Hong Kong residents in 1997. This case challenges the view that adaptation to land-based poultry is a prerequisite for the replication of aquatic avian influenza A viruses in humans and underscores the hazard posed by these viruses.
Acknowledgments
We thank Krisna Wells and Martha McGregor for excellent technical assistance and John Gilbert for editing the manuscript. Glycopolymers containing N-acetylneuraminic acid linked to galactose through either an α-2,3 or an α-2,6 bond were kindly provided by Taichi Usui and Takeomi Murata, Shizuoka University.
This work was supported by National Institute of Allergy and Infectious Diseases, Public Health Service, research grants; by CREST (Japan Science and Technology Corporation); by Grants-in-Aid by the Ministry of Education, Culture, Sports, Science and Technology; by the Ministry of Health, Labor and Welfare, Japan; and by research fellowships of the Japan Society for the Promotion of Science for Young Scientists.
REFERENCES
- 1.Banks, J., E. S. Speidel, E. Moore, L. Plowright, A. Piccirillo, I. Capua, P. Cordioli, A. Fioretti, and D. J. Alexander. 2001. Changes in the haemagglutinin and the neuraminidase genes prior to the emergence of highly pathogenic H7N1 avian influenza viruses in Italy. Arch. Virol. 146:963-973. [DOI] [PubMed] [Google Scholar]
- 2.Bender, C., H. Hall, J. Huang, A. Klimov, N. Cox, A. Hay, V. Gregory, K. Cameron, W. Lim, and K. Subbarao. 1999. Characterization of the surface proteins of influenza A (H5N1) viruses isolated from humans in 1997-1998. Virology 254:115-123. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2004. Cases of influenza A (H5N1)—Thailand, 2004. Morb. Mortal. Wkly. Rep. 53:100-103. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2004. Outbreak of Asian influenza A (H5N1) in Asia and interim recommendations for evaluation and reporting of suspected cases—United States, 2004. Morb. Mortal. Wkly. Rep. 53:97-100. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2004. Update: influenza activity—United States, 2003-04 season. Morb. Mortal. Wkly. Rep. 53:284-287. [PubMed] [Google Scholar]
- 6.Claas, E. C. J., A. D. M. E. Osterhaus, R. Van Beek, J. C. De Jong, G. F. Rimmelzwaan, D. A. Senne, S. Krauss, K. F. Shortridge, and R. G. Webster. 1998. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 351:472-477. [DOI] [PubMed] [Google Scholar]
- 7.Deshpande, K. L., C. W. Naeve, and R. G. Webster. 1985. The neuraminidases of the virulent and avirulent A/Chicken/Pennsylvania/83 (H5N2) influenza A viruses: sequence and antigenic analyses. Virology 147:49-60. [DOI] [PubMed] [Google Scholar]
- 8.Fouchier, R. A., P. M. Schneeberger, F. W. Rozendaal, J. M. Broekman, S. A. Kemink, V. Munster, T. Kuiken, G. F. Rimmelzwaan, M. Schutten, G. J. Van Doornum, G. Koch, A. Bosman, M. Koopmans, and A. D. Osterhaus. 2004. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc. Natl. Acad. Sci. USA 101:1356-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao, P., S. Watanabe, T. Ito, H. Goto, K. Wells, M. McGregor, A. J. Cooley, and Y. Kawaoka. 1999. Biological heterogeneity, including systemic replication in mice, of H5N1 influenza A virus isolates from humans in Hong Kong. J. Virol. 73:3184-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan, Y., L. L. Poon, C. Y. Cheung, T. M. Ellis, W. Lim, A. S. Lipatov, K. H. Chan, K. M. Sturm-Ramirez, C. L. Cheung, Y. H. Leung, K. Y. Yuen, R. G. Webster, and J. S. Peiris. 2004. H5N1 influenza: A protean pandemic threat. Proc. Natl. Acad. Sci. USA 101:8156-8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatta, M., P. Gao, P. Halfmann, and Y. Kawaoka. 2001. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science 293:1840-1842. [DOI] [PubMed] [Google Scholar]
- 12.Horimoto, T., N. Fukuda, K. Iwatsuki-Horimoto, Y. Guan, W. Lim, M. Peiris, S. Sugii, T. Odagiri, M. Tashiro, and Y. Kawaoka. 2004. Antigenic differences between H5N1 human influenza viruses isolated in 1997 and 2003. J. Vet. Med. Sci. 66:303-305. [DOI] [PubMed] [Google Scholar]
- 13.Katz, J. M., X. Lu, T. M. Tumpey, C. B. Smith, M. W. Shaw, and K. Subbarao. 2000. Molecular correlates of influenza A H5N1 virus pathogenesis in mice. J. Virol. 74:10807-10810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobasa, D., A. Takada, K. Shinya, M. Hatta, P. Halfmann, S. Theriault, H. Suzuki, H. Nishimura, K. Mitamura, N. Sugaya, T. Usui, T. Murata, Y. Maeda, S. Watanabe, M. Suresh, T. Suzuki, Y. Suzuki, H. Feldmann, and Y. Kawaoka. 2004. Enhanced virulence of influenza A viruses with the haemagglutinin of the 1918 pandemic virus. Nature 431:703-707. [DOI] [PubMed] [Google Scholar]
- 15.Koopmans, M., B. Wilbrink, M. Conyn, G. Natrop, H. van der Nat, H. Vennema, A. Meijer, J. van Steenbergen, R. Fouchier, A. Osterhaus, and A. Bosman. 2004. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in The Netherlands. Lancet 363:587-593. [DOI] [PubMed] [Google Scholar]
- 16.Masuda, H., T. Suzuki, Y. Sugiyama, G. Horiike, K. Murakami, D. Miyamoto, K. Hirari, T. Ito, H. Kida, M. Kiso, K. Fukunaga, M. Ochiai, T. Toyada, A. Ishihara, Y. Kawaoka, and Y. Suzuki. 1999. Substitution of amino acid residue in influenza A virus hemagglutinin affects recognition of sialyl-oligosaccharides containing N-glycolylneuraminic acid. FEBS Lett. 464:71-74. [DOI] [PubMed] [Google Scholar]
- 17.Matrosovich, M., A. Tuzikov, N. Bovin, A. Gambaryan, A. Klimov, M. R. Castrucci, I. Donatelli, and Y. Kawaoka. 2000. Early alterations of the receptor-binding properties of H1, H2, and H3 avian influenza virus hemagglutinins after their introduction into mammals. J. Virol. 74:8502-8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matrosovich, M., N. Zhou, Y. Kawaoka, and R. Webster. 1999. The surface glycoproteins of H5 influenza viruses isolated from humans, chickens, and wild aquatic birds have distinguishable properties. J. Virol. 73:1146-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peiris, J. S., W. C. Yu, C. W. Leung, C. Y. Cheung, W. F. Ng, J. M. Nicholls, T. K. Ng, K. H. Chan, S. T. Lai, W. L. Lim, K. Y. Yuen, and Y. Guan. 2004. Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet 363:617-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reid, A. H., and J. K. Taubenberger. 2003. The origin of the 1918 pandemic influenza virus: a continuing enigma. J. Gen. Virol. 84:2285-2292. [DOI] [PubMed] [Google Scholar]
- 21.Rogers, G. N., and J. C. Paulson. 1983. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology 127:361-373. [DOI] [PubMed] [Google Scholar]
- 22.Rogers, G. N., T. J. Pritchett, J. L. Lane, and J. C. Paulson. 1983. Differential sensitivity of human, avian, and equine influenza A viruses to a glycoprotein inhibitor of infection: selection of receptor specific variants. Virology 131:394-408. [DOI] [PubMed] [Google Scholar]
- 23.Saito, T., W. Lim, T. Suzuki, Y. Suzuki, H. Kida, S. I. Nishimura, and M. Tashiro. 2001. Characterization of a human H9N2 influenza virus isolated in Hong Kong. Vaccine 20:125-133. [DOI] [PubMed] [Google Scholar]
- 24.Scholtissek, C., W. Rohde, V. Von Hoyningen, and R. Rott. 1978. On the origin of the human influenza virus subtypes H2N2 and H3N2. Virology 87:13-20.664248 [Google Scholar]
- 25.Shinya, K., S. Hamm, M. Hatta, H. Ito, T. Ito, and Y. Kawaoka. 2004. PB2 amino acid at position 627 affects replicative efficiency, but not cell tropism, of Hong Kong H5N1 influenza A viruses in mice. Virology 320:258-266. [DOI] [PubMed] [Google Scholar]
- 26.Shortridge, K. F., N. N. Zhou, Y. Guan, P. Gao, T. Ito, Y. Kawaoka, S. Kodihalli, S. Krauss, D. Markwell, K. G. Murti, M. Norwood, D. Senne, L. Sims, A. Takada, and R. G. Webster. 1998. Characterization of avian H5N1 influenza viruses from poultry in Hong Kong. Virology 252:331-342. [DOI] [PubMed] [Google Scholar]
- 27.Sturm-Ramirez, K. M., T. Ellis, B. Bousfield, L. Bissett, K. Dyrting, J. E. Rehg, L. Poon, Y. Guan, M. Peiris, and R. G. Webster. 2004. Reemerging H5N1 influenza viruses in Hong Kong in 2002 are highly pathogenic to ducks. J. Virol. 78:4892-4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki, T., A. Portner, R. A. Scroggs, M. Uchikawa, N. Koyama, K. Matsuo, Y. Suzuki, and T. Takimoto. 2001. Receptor specificities of human respiroviruses. J. Virol. 75:4604-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tran, T. H., T. L. Nguyen, T. D. Nguyen, T. S. Luong, P. M. Pham, V. C. Nguyen, T. S. Pham, C. D. Vo, T. Q. Le, T. T. Ngo, B. K. Dao, P. P. Le, T. T. Nguyen, T. L. Hoang, V. T. Cao, T. G. Le, D. T. Nguyen, H. N. Le, K. T. Nguyen, H. S. Le, V. T. Le, D. Christiane, T. T. Tran, J. de Menno, C. Schultsz, P. Cheng, W. Lim, P. Horby, J. Farrar, and the World Health Organization International Avian Influenza Investigative Team. 2004. Avian influenza A (H5N1) in 10 patients in Vietnam. N. Engl. J. Med. 350:1179-1188. [DOI] [PubMed] [Google Scholar]
- 30.Tumpey, T. M., D. L. Suarez, L. E. Perkins, D. A. Senne, J. G. Lee, Y. J. Lee, I. P. Mo, H. W. Sung, and D. E. Swayne. 2002. Characterization of a highly pathogenic H5N1 avian influenza A virus isolated from duck meat. J. Virol. 76:6344-6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webby, R. J., D. R. Perez, J. S. Coleman, Y. Guan, J. H. Knight, E. A. Govorkova, L. R. McClain-Moss, J. S. Peiris, J. E. Rehg, E. I. Tuomanen, and R. G. Webster. 2004. Responsiveness to a pandemic alert: use of reverse genetics for rapid development of influenza vaccines. Lancet 363:1099-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zambon, M. C. 2001. The pathogenesis of influenza in humans. Rev. Med. Virol. 11:227-241. [DOI] [PubMed] [Google Scholar]
- 33.Zitzow, L. A., T. Rowe, T. Morken, W. J. Shieh, S. Zaki, and J. M. Katz. 2002. Pathogenesis of avian influenza A (H5N1) viruses in ferrets. J. Virol. 76:4420-4429. [DOI] [PMC free article] [PubMed] [Google Scholar]