Abstract
The high prevalence of preexisting immunity to adenovirus serotype 5 (Ad5) in human populations will likely limit the immunogenicity and clinical utility of recombinant Ad5 (rAd5) vector-based vaccines for human immunodeficiency virus type 1 and other pathogens. A potential solution to this problem is to utilize rAd vaccine vectors derived from rare Ad serotypes such as Ad35 and Ad11. We have previously reported that rAd35 vectors were immunogenic in the presence of anti-Ad5 immunity, but the immunogenicity of heterologous rAd prime-boost regimens and the extent that cross-reactive anti-vector immunity may limit this approach have not been fully explored. Here we assess the immunogenicity of heterologous vaccine regimens involving rAd5, rAd35, and novel rAd11 vectors expressing simian immunodeficiency virus Gag in mice both with and without anti-Ad5 immunity. Heterologous rAd prime-boost regimens proved significantly more immunogenic than homologous regimens, as expected. Importantly, all regimens that included rAd5 were markedly suppressed by anti-Ad5 immunity. In contrast, rAd35-rAd11 and rAd11-rAd35 regimens elicited high-frequency immune responses both in the presence and in the absence of anti-Ad5 immunity, although we also detected clear cross-reactive Ad35/Ad11-specific humoral and cellular immune responses. Nevertheless, these data suggest the potential utility of heterologous rAd prime-boost vaccine regimens using vectors derived from rare human Ad serotypes.
Preexisting anti-vector immunity represents a major hurdle in the development of vector-based vaccines for human immunodeficiency virus type 1 (HIV-1) and other pathogens. Recombinant adenovirus serotype 5 (rAd5) vectors have been shown to elicit high-frequency immune responses and to afford protective efficacy in a variety of animal models (24, 30, 31). These vectors are therefore being advanced into large-scale clinical trials (14, 25). However, the high prevalence of anti-Ad5 immunity in human populations will likely limit the immunogenicity and clinical utility of rAd5 vector-based vaccines, particularly in the developing world (13, 25, 32). Anti-Ad5 immunity has already been shown to suppress substantially the immunogenicity of rAd5 vaccines for HIV-1 in studies in mice (2, 3, 6, 29, 34), rhesus monkeys (4), and humans in phase 1 clinical trials (26).
A potential solution to this problem is to develop rAd vectors from alternative Ad serotypes. One approach is to develop rAd vectors from species other than humans. For example, ovine (10), porcine (21), bovine (22), and chimpanzee (5, 33) Ads have been constructed. Of these vector systems, chimpanzee rAd vaccine vectors in particular have been shown to be immunogenic and only marginally affected by anti-Ad5 immunity in preclinical studies (6, 20). However, a potential hurdle for the use of nonhuman rAd vaccine vectors is their unknown clinical disease associations in humans, which may raise substantial safety and regulatory concerns.
Another approach is to develop rAd vectors from rare human Ad serotypes (12, 16, 17). A recent seroprevalence study of the 51 known human Ad serotypes demonstrated that Ad35 and Ad11 are particularly rare and may therefore offer substantial advantages over Ad5 as candidate vaccine vectors (32). We have recently reported that rAd35 vectors expressing simian immunodeficiency virus (SIV) Gag were immunogenic in mice and were not significantly suppressed by anti-Ad5 immunity, suggesting the feasibility of this approach (3). We have also constructed rAd11 vectors (11), thus allowing the evaluation of heterologous rAd prime-boost vaccine regimens involving vectors derived from two rare human Ad serotypes. However, the immunogenicity of such regimens and the extent of cross-reactive anti-vector immunity have not previously been investigated in detail.
In this study, we assess the immunogenicity of heterologous rAd prime-boost regimens involving rAd5, rAd35, and rAd11 vectors expressing SIV Gag in mice both with and without anti-Ad5 immunity. In mice with anti-Ad5 immunity, rAd35-rAd11 and rAd11-rAd35 regimens proved more immunogenic than all regimens containing rAd5, although we detected clear cross-reactive Ad35/Ad11-specific humoral and cellular immune responses. These data demonstrate the potential utility of heterologous rAd prime-boost vaccine regimens but suggest that additional vectors that are immunologically distinct from rAd11 and rAd35 should be generated.
MATERIALS AND METHODS
Vector construction, production, and purification.
E1/E3-deleted, replication-incompetent rAd5, rAd35, or rAd11 vectors were generated in PER.C6 or PER.C6/55K cells using pBR322-based adaptor plasmids pAdApt, pAdApt535, or pAdApt11 together with cosmids pWE.Ad.AflII-rITR.dE3, pWE.Ad35.pIX-rITR.dE3, or pWE.Ad11.dE3, respectively, as previously described (8, 11, 32). The SIVmac239 gag gene optimized for high levels of expression in mammalian cells (GeneART, Regensburg, Germany) was cloned into the adaptor plasmids under control of an immediate-early cytomegalovirus (CMV) promoter and an SV40 polyadenylation signal. These plasmids were linearized and transfected into PER.C6 or PER.C6/55K cells together with the linearized cosmids using lipofectamine (Invitrogen, Breda, The Netherlands). Homologous recombination led to the generation of rAd5-Gag, rAd35-Gag, or rAd11-Gag. These vectors were then plaque-purified, analyzed for transgene expression, amplified in 24 to 48 triple-layer 175-cm2 flasks, purified by double CsCl gradient ultracentrifugation, and dialyzed into phosphate-buffered saline (PBS) containing 5% sucrose. Purified rAd vectors were stored at −80°C. Virus particle (vp) titers were determined by high-performance liquid chromatography (HPLC). Infectivity was assessed by PFU assays. SIV Gag expression was confirmed by infection of A549 cells followed by intracellular staining for Gag p27 and analysis by flow cytometry. Replication-incompetent rAd vectors expressing luciferase (rAd-Luciferase) or no transgene (rAd-Empty) were produced using similar methods.
Mice and immunizations.
Six- to 8-week-old C57/BL6 or Balb/c mice were purchased from Charles River Laboratories (Wilmington, MA). Mice were injected intramuscularly (i.m.) with various doses of E1/E3-deleted, replication-incompetent rAd5, rAd35, or rAd11 vector expressing SIVmac239 Gag in 100 μl sterile PBS in the quadriceps muscles. For DNA immunizations, mice were injected i.m. with 50 μg plasmid VRC-4307 expressing SIVmac239 Gag-Pol-Nef (Vaccine Research Center, National Institutes of Health, Bethesda, MD) in 100 μl sterile saline. Prime-boost studies involved priming at week 0 and boosting at week 4. To induce active anti-vector immunity, mice were preimmunized twice, separated by a 4-week interval, i.m. with 1010 vp rAd5-Empty, rAd35-Empty, or rAd11-Empty in 100 μl sterile PBS.
AL11-specific tetramer binding assays.
Tetrameric H-2Db complexes folded around the immunodominant SIV Gag AL11 epitope (AAVKNWMTQTL) (3) were prepared and utilized to stain peptide-specific CD8+ T lymphocytes as described previously (1, 2). Mouse blood was collected in RPMI 1640 containing 40 U/ml heparin. Following lysis of the red blood cells, 0.1 μg of phycoerythrin (PE)-labeled Db/AL11 tetramer in conjunction with allophycocyanin labeled anti-CD8α monoclonal antibody (MAb) (Ly-2; Caltag, San Francisco, CA) was utilized to stain AL11-specific CD8+ T lymphocytes. The cells were washed in PBS containing 2% fetal bovine serum (FBS) and fixed in 0.5 ml PBS containing 1.5% paraformaldehyde. Samples were analyzed by two-color flow cytometry on a fluorescence-activated cell sorter (FACS) Calibur (Becton Dickinson Pharmingen, Mountain View, CA). Gated CD8+ T lymphocytes were examined for staining with the Db/AL11 tetramer. CD8+ T lymphocytes from naïve mice were utilized as negative controls and exhibited <0.1% tetramer staining.
Gag-specific ELISPOT.
Gag-specific cellular immune responses were assessed by gamma-interferon (IFN-γ) ELISPOT assays as described previously (3, 31). Murine splenocytes were assessed for responses to individual Gag epitope peptides or a pool of overlapping 15-amino-acid peptides covering the entire SIVmac239 Gag protein. Ninety-six-well multiscreen plates (Millipore, Bedford, MA) coated overnight with 100 μl/well of 10 μg/ml rat anti-mouse IFN-γ (Pharmingen, San Diego, CA) were washed three times with endotoxin-free Dulbecco's PBS (Life Technologies, Gaithersberg, MD) containing 0.25% Tween-20 and blocked with PBS containing 5% FBS for 2 h at 37°C. The plates were washed three times with Dulbecco's PBS containing 0.25% Tween-20, rinsed with RPMI 1640 containing 10% FBS, and incubated in triplicate with 5 × 105 splenocytes per well in a 100-μl reaction volume containing 1 μg/ml peptide. Following an 18-h incubation, the plates were washed nine times with Dulbecco's PBS containing 0.25% Tween-20 and once with distilled water. The plates were then incubated for 2 h with 75 μl/well of 5-μg/ml biotinylated rat anti-mouse IFN-γ (Pharmingen, San Diego, CA), washed six times with Coulter Wash (Coulter Corporation, Miami, FL), and incubated for 2 h with a 1:500 dilution of streptavidin-alkaline phosphatase (Southern Biotechnology Associates, Birmingham, AL). Following five washes with Coulter Wash and one with PBS, the plates were developed with nitro blue tetrazolium-5-bromo-4-chloro-3-indolyl-phosphate chromogen (Pierce, Rockford, IL), stopped by washing with tap water, air dried, and read using an ELISPOT reader (HiTech Instruments, Edgement, PA).
Virus neutralization assay.
Ad5-, Ad35-, and Ad11-specific neutralizing antibody (NAb) responses were assessed by luciferase-based virus neutralization assays as described previously (28). A549 human lung carcinoma cells were plated at a density of 1 × 104 cells per well in 96-well plates and infected with E1/E3-deleted, replication-incompetent rAd-Luciferase reporter constructs at a multiplicity of infection of 500 with twofold serial dilutions of serum in 200-μl reaction volumes. Following a 24-h incubation, luciferase activity in the cells was measured using the Steady-Glo Luciferase Reagent System (Promega, Madison, WI). Neutralization titers were defined as the maximum serum dilution that neutralized 90% of luciferase activity.
Adoptive transfers.
To study the inhibitory effects of Ad35- and Ad11-specific NAbs and CD8+ T lymphocytes, adoptive transfer studies were performed as described previously (29). Donor mice were preimmunized twice with rAd35-Empty or rAd11-Empty to generate anti-vector immune responses. Purified immunoglobulin G (IgG) from preimmunized or control mice was prepared from 2 ml serum pooled from 8 mice using a Protein A Antibody Purification kit (Sigma, St. Louis, MO) and dialyzed into 5 ml endotoxin-free Dulbecco's PBS (Life Technologies, Gaithersberg, MD). All IgG preparations had similar total protein concentrations of 2 to 3 mg/ml (Bio-Rad, Hercules, CA). Purified CD8+ T lymphocytes were prepared by negative selection with a murine CD8+ T Cell Isolation kit (Miltenyi Biotec, Auburn, CA) and were >95% pure as assessed by flow cytometry. CD8+ T lymphocytes were resuspended at a density of 108 cells/ml in endotoxin-free Dulbecco's PBS (Life Technologies, Gaithersberg, MD). Five-hundred microliters of purified IgG or CD8+ T lymphocytes were then adoptively transferred to naïve recipient mice by tail vein injection. On the day following the adoptive transfer, recipient mice were immunized with 2 × 108 vp rAd35-Gag or rAd11-Gag, and vaccine-elicited immune response were assessed.
Statistical analyses.
Statistical analyses were performed with GraphPad Prism version 4.01 (GraphPad Software, Inc.). Comparisons of mean immune responses among groups of mice were performed by two-tailed t tests for two groups of animals or by analyses of variance (ANOVA) for more than two groups. Bonferroni adjustments were included when appropriate to account for multiple comparisons. In all cases, P values of less than 0.05 were considered significant.
RESULTS
Immunogenicity of rAd5, rAd35, and rAd11 vectors expressing SIV Gag.
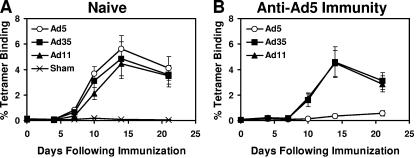
We previously reported that rAd35 vectors expressing SIV Gag elicited Gag-specific cellular immune responses that were not suppressed by anti-Ad5 immunity in mice (3). We also recently constructed novel, E1/E3-deleted, replication-incompetent rAd11 vaccine vectors (11). We therefore initiated studies to assess the immunogenicity of rAd11 vectors expressing SIV Gag in mice both with and without anti-Ad5 immunity. Groups of C57/BL6 mice (n = 12/group) were immunized once i.m. with 109 vp rAd5-Gag, rAd35-Gag, rAd11-Gag, or a sham vector control. As shown in Fig. 1A, all three vaccine vectors at this dose elicited comparable Gag-specific CD8+ T-lymphocyte responses to the immunodominant AL11 epitope (AAVKNWMTQTL) (3) as measured by Db/AL11 tetramer binding assays. Mean tetramer CD8+ T lymphocyte responses were 4.4 to 5.6% for these vaccine vectors on day 14 following immunization. These results were confirmed by pooled peptide IFN-γ ELISPOT assays (data not shown).
FIG. 1.
Immunogenicity of rAd5, rAd35, and rAd11 vectors expressing SIV Gag. (A) Naïve C57/BL6 mice and (B) mice with anti-Ad5 immunity were immunized with 109 vp rAd5-Gag, rAd35-Gag, or rAd11-Gag or a sham vector control. Gag-specific CD8+ T-lymphocyte responses were assessed by Db/AL11 tetramer binding assays at multiple time points following immunization. The rAd5-Gag responses are depicted in the open circles.
We next assessed the impact of anti-Ad5 immunity on the immunogenicity of rAd5-Gag, rAd35-Gag, and rAd11-Gag. Groups of C57/BL6 mice (n = 12/group) were preimmunized twice at 8 weeks and 4 weeks prior to vaccination with 1010 vp rAd5-Empty to generate anti-Ad5 immunity. Ad5-specific neutralizing antibody (NAb) titers in these mice were 8,192 to 16,384, comparable with Ad5-specific NAb titers observed in the developing world (13). As shown in Fig. 1B, rAd5-Gag responses were essentially ablated in these mice and exhibited mean tetramer-positive CD8+ T lymphocyte responses of only 0.3% on day 14 following immunization. In contrast, rAd35-Gag and rAd11-Gag responses were not substantially affected by anti-Ad5 immunity, with mean tetramer-positive CD8+ T lymphocyte responses of 4.4% to 4.6% on day 14. In fact, rAd35-Gag and rAd11-Gag proved significantly more immunogenic than rAd5-Gag in the presence of preexisting anti-Ad5 immunity (P < 0.001 comparing mean tetramer responses among groups of mice on day 14 using analyses of variance with Bonferroni adjustments to account for multiple comparisons). Thus, rAd11-Gag effectively evaded anti-Ad5 immunity, comparable with our previous observations for rAd35-Gag (3).
To compare more rigorously the relative immunogenicity of rAd5-Gag, rAd35-Gag, and rAd11-Gag, we evaluated Gag-specific CD8+ T-lymphocyte responses in groups of mice (n = 4/group) that received lower vaccine doses of 108 vp or 107 vp of each vaccine vector. As shown in Fig. 2, rAd35-Gag and rAd11-Gag appeared comparably immunogenic, but rAd5-Gag proved substantially more immunogenic than both rAd35-Gag and rAd11-Gag when inoculated at these lower doses. These data suggest that rAd5 vectors were intrinsically more immunogenic than rAd35 and rAd11 vectors in the absence of anti-Ad5 immunity.
FIG. 2.
Immunogenicity of low doses of rAd5, rAd35, and rAd11. Naïve mice were immunized with (A) 108 vp or (B) 107 vp rAd5-Gag, rAd35-Gag, rAd11-Gag, or a sham vector control. Gag-specific CD8+ T-lymphocyte responses were assessed by Db/AL11 tetramer binding assays. The rAd5-Gag responses are depicted in the open circles.
Immunogenicity of heterologous rAd prime-boost regimens.
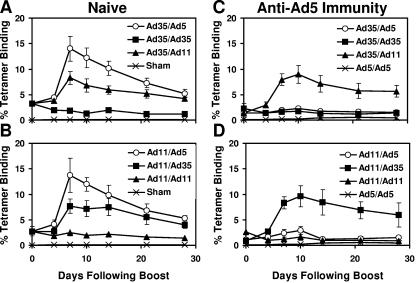
We next sought to determine the immunogenicity of homologous and heterologous rAd prime-boost regimens in mice both with and without anti-Ad5 immunity. Groups of naïve C57/BL6 mice (n = 4/group) were primed at week 0 with 109 vp rAd35-Gag or rAd11-Gag and boosted at week 4 with 109 vp rAd5-Gag, rAd35-Gag, or rAd11-Gag. Gag-specific cellular immune responses were assessed by Db/AL11 tetramer binding assays following the boost immunization. As shown in Fig. 3A, naïve mice primed with rAd35-Gag could not be boosted by a second immunization of rAd35-Gag, presumably as a result of vaccine-elicited anti-vector immunity. In contrast, mice primed with rAd35-Gag were effectively boosted by rAd11-Gag and in particular by rAd5-Gag. Similarly, as shown in Fig. 3B, mice primed with rAd11-Gag could not be boosted by a second administration of rAd11-Gag but were efficiently boosted by rAd35-Gag and in particular by rAd5-Gag. These data demonstrate that heterologous rAd prime-boost regimens were substantially more immunogenic than homologous rAd regimens. These results were confirmed by pooled peptide IFN-γ ELISPOT assays (data not shown).
FIG. 3.
Heterologous rAd prime-boost regimens. (A and B) Naïve mice and (C and D) mice with anti-Ad5 immunity were primed at week 0 with (A and C) 109 vp rAd35-Gag or (B and D) 109 vp rAd11-Gag and then boosted at week 4 with 109 vp rAd5-Gag, rAd35-Gag, rAd11-Gag, or a sham vector control. In mice with anti-Ad5 immunity, the rAd5-Gag prime, rAd5-Gag boost regimen was also included as a negative control. Gag-specific CD8+ T lymphocyte responses were assessed by Db/AL11 tetramer binding assays following the boost immunization. The rAd5-Gag boost responses are depicted in the open circles.
We next assessed the immunogenicity of heterologous rAd prime-boost regimens in mice with anti-Ad5 immunity. Mice were preimmunized twice with rAd5-Empty as described above. As shown in Fig. 3C and D, rAd5-Gag was no longer an effective boosting vector in the presence of anti-Ad5 immunity. Immune responses primed by rAd35-Gag or rAd11-Gag could not be boosted by rAd5-Gag. Moreover, the homologous rAd5-rAd5 regimen elicited negligible responses in these mice. These data demonstrate that anti-Ad5 immunity essentially abrogated the ability of rAd5 to serve both as a priming vector and as a boosting vector. Importantly, heterologous rAd35-rAd11 and rAd11-rAd35 regimens elicited high-frequency cellular immune responses that proved more immunogenic than all regimens involving rAd5 in the presence of anti-Ad5 immunity (P < 0.01 comparing mean tetramer responses among groups of mice on day 7 following the boost immunization). These findings suggest that the optimal rAd vaccine regimen should involve two heterologous rAd serotype vectors that are both distinct from Ad5.
Immunogenicity of heterologous DNA-rAd prime-boost regimens.
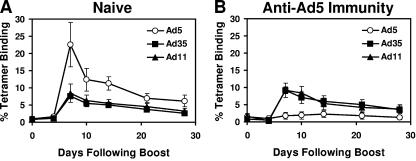
Priming with plasmid DNA vaccines and boosting with rAd5 vectors has been shown to elicit particularly potent immune responses in a variety of preclinical models (15, 24, 30). We therefore evaluated the immunogenicity of heterologous DNA-rAd prime-boost regimens in mice both with and without anti-Ad5 immunity. Groups of naïve C57/BL6 mice (n = 4/group) were primed at week 0 with 50 μg plasmid DNA vaccine expressing SIV Gag-Pol-Nef and boosted at week 4 with 109 vp rAd5-Gag, rAd35-Gag, or rAd11-Gag. Gag-specific cellular immune responses were assessed by Db/AL11 tetramer binding assays following the boost immunization. As shown in Fig. 4A, naïve mice primed with the DNA vaccine were boosted efficiently by all three vectors but in particular by rAd5-Gag. Mean tetramer-positive CD8+ T-lymphocyte responses were 22.6% on day 7 following the rAd5-Gag boost immunization, 7.5% following the rAd35-Gag boost immunization, and 8.5% following the rAd11 boost immunization. These data confirm the potent intrinsic immunogenicity of rAd5 vectors in the absence of anti-Ad5 immunity (Fig. 2).
FIG. 4.
Heterologous DNA-rAd prime-boost regimens. (A) Naïve mice and (B) mice with anti-Ad5 immunity were primed at week 0 with 50 μg plasmid DNA vaccine expressing Gag-Pol-Nef and then boosted at week 4 with 109 vp rAd5-Gag, rAd35-Gag, or rAd11-Gag. Gag-specific CD8+ T-lymphocyte responses were assessed by Db/AL11 tetramer binding assays following the boost immunization. The rAd5-Gag boost responses are depicted in the open circles.
In the presence of anti-Ad5 immunity, however, rAd5-Gag proved only marginally effective as a boosting vector, whereas rAd35-Gag and rAd11-Gag were not substantially affected. As shown in Fig. 4B, mean tetramer-positive CD8+ T-lymphocyte responses in these mice were only 1.8% on day 7 following the rAd5-Gag boost immunization, but they were 8.2% following the rAd35-Gag boost immunization and 8.1% following the rAd11 boost immunization. Thus, in mice with anti-Ad5 immunity, DNA-rAd35 and DNA-rAd11 regimens were significantly more immunogenic than the DNA-rAd5 regimen (P < 0.01 comparing mean tetramer responses among groups of mice on day 7 following the boost immunization). These data demonstrate that anti-Ad5 immunity substantially suppressed the immunogenicity of all vaccine regimens containing rAd5 vectors. In contrast, vaccine regimens containing rAd35 and rAd11 vectors proved immunogenic both in the presence and in the absence of anti-Ad5 immunity.
Cross-reactive Ad35/Ad11-specific immunity.
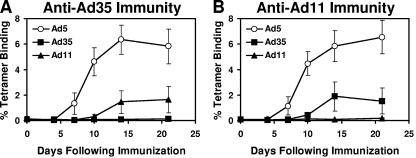
In the absence of anti-Ad5 immunity, heterologous rAd35-rAd5 and rAd11-rAd5 vaccine regimens proved substantially more immunogenic than rAd35-rAd11 and rAd11-rAd35 regimens (Fig. 3A and B). These findings presumably reflected in part the potent intrinsic immunogenicity of rAd5 vectors (Fig. 2). However, we also reasoned that the high degree of genetic homology between Ad35 and Ad11 (11) could potentially result in cross-reactive Ad35/Ad11-specific immune responses that may also limit the immunogenicity of these heterologous prime-boost regimens. To assess for possible cross-reactive anti-vector immune responses, groups of mice (n = 4/group) were preimmunized with 1010 vp rAd35-Empty or rAd11-Empty prior to immunization with 109 vp rAd5-Gag, rAd35-Gag, or rAd11-Gag. All three of these vectors elicited comparable cellular immune responses when inoculated at this dose in naïve mice (Fig. 1A). As shown in Fig. 5A, anti-Ad35 immunity abrogated the immunogenicity of rAd35-Gag as expected. Interestingly, anti-Ad35 immunity also substantially suppressed the immunogenicity of rAd11-Gag, indicating the presence of functionally significant cross-reactive Ad35/Ad11-specific immune responses. Conversely, as depicted in Fig. 5B, anti-Ad11 immunity abrogated the immunogenicity of rAd11-Gag but also suppressed cellular immune responses elicited by rAd35-Gag.
FIG. 5.
Cross-reactive Ad35/Ad11-specific immunity. Mice with (A) anti-Ad35 immunity or (B) anti-Ad11 immunity were immunized with 109 vp rAd5-Gag, rAd35-Gag, or rAd11-Gag. Gag-specific CD8+ T lymphocyte responses were assessed by Db/AL11 tetramer binding assays. The rAd5-Gag responses are depicted in the open circles.
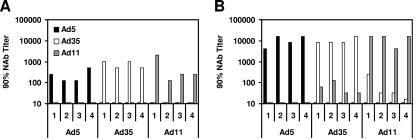
To quantitate titers of cross-reactive vector-specific NAbs, mice were immunized with one or two doses of 1010 vp rAd5-Empty, rAd35-Empty, or rAd11-Empty. Ad5-, Ad35-, and Ad11-specific NAbs were measured at week 4 following each immunization using luciferase-based virus neutralization assays (28). As shown in Fig. 6A, no detectable cross-reactive NAbs were detected following the first immunization, indicating titers of <16. Following the second immunization, as depicted in Fig. 6B, rAd35-immunized mice generated low but clearly detectable Ad11-specific NAb titers, and rAd11-immunized mice exhibited low Ad35-specific NAb titers. The cross-reactive NAb titers were approximately 100-fold lower than the NAb titers against the homologous vectors. Interestingly, no cross-reactive Ad5/Ad35- or Ad5/Ad11-specific NAbs were detected in these animals.
FIG. 6.
Cross-reactive Ad35/Ad11-specific NAbs. Mice were immunized either (A) once or (B) twice with 1010 vp rAd5-Empty, rAd35-Empty, or rAd11-Empty. Serum was assessed for Ad5-, Ad35-, and Ad11-specific NAbs 4 weeks following immunization.
Adoptive transfer studies.
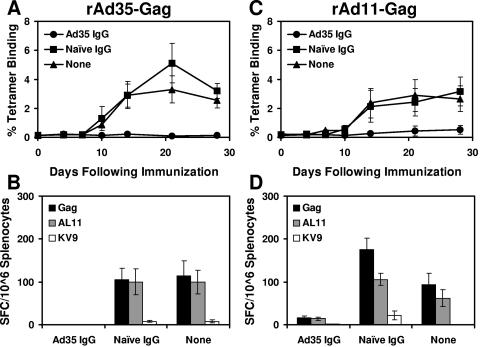
We next performed adoptive transfer studies to investigate the relative functional significance of cross-reactive Ad35/Ad11-specific NAbs and CD8+ T lymphocytes. Donor mice were immunized twice with either 1010 vp rAd35-Empty or saline. IgG was purified from serum, and 500 μl was adoptively transferred to naïve recipient mice (n = 8/group) prior to immunization with 2 × 108 vp rAd35-Gag or rAd11-Gag. As shown in Fig. 7A and B, Ad35-specific IgG but not control IgG abrogated cellular immune responses elicited by rAd35-Gag as measured by tetramer binding assays and IFN-γ ELISPOT assays using pooled Gag peptides and individual epitope peptides. Importantly, as shown in Fig. 7C and D, Ad35-specific IgG also markedly suppressed the immunogenicity of rAd11-Gag, demonstrating the functional significance of cross-reactive Ad35/Ad11-specific NAbs.
FIG. 7.
Adoptive transfer of Ad35-specific IgG. Donor mice were immunized twice with 1010 vp rAd35-Empty or saline control. IgG was purified from serum, and 500 μl was adoptively transferred to naïve recipient mice prior to vaccination with (A and B) 2 × 108 vp rAd35-Gag or (C and D) 2 × 108 vp rAd11-Gag. Mice that received no adoptive transfer were used as an additional control (None). Gag-specific cellular immune responses were assessed by (A and C) Db/AL11 tetramer binding assays and (B and D) IFN-γ ELISPOT assays using pooled Gag peptides and individual AL11 and KV9 epitope peptides.
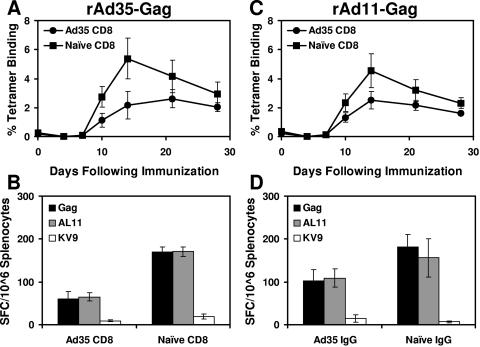
We next performed a similar adoptive transfer study using CD8+ T lymphocytes purified from splenocytes of mice immunized with rAd35-Empty or saline as described above. CD8+ T lymphocytes (5 × 107) were adoptively transferred to naïve recipient mice prior to immunization with 2 × 108 vp rAd35-Gag or rAd11-Gag (n = 8/group). As shown in Fig. 8A and B, Ad35-specific CD8+ T lymphocytes partially suppressed cellular immune responses elicited by rAd35-Gag. Moreover, as shown in Fig. 8C and D, Ad35-specific CD8+ T lymphocytes also partially suppressed the immunogenicity of rAd11-Gag. These data suggest that both NAbs and CD8+ T lymphocytes contributed to cross-reactive Ad35/Ad11-specific immunity, although NAbs likely served the critical and primary role.
FIG. 8.
Adoptive transfer of Ad35-specific CD8+ T lymphocytes. Donor mice were immunized twice with 1010 vp rAd35-Empty or saline control. CD8+ T lymphocytes were purified from splenocytes, and 5 × 107 cells were adoptively transferred to naïve recipient mice prior to vaccination with (A and B) 2 × 108 vp rAd35-Gag or (C and D) 2 × 108 vp rAd11-Gag. Gag-specific cellular immune responses were assessed by (A and C) Db/AL11 tetramer binding assays and (B and D) IFN-γ ELISPOT assays using pooled Gag peptides and individual AL11 and KV9 epitope peptides.
DISCUSSION
Accumulating evidence has confirmed the importance of virus-specific CD8+ T-lymphocyte responses in the control of HIV-1 replication (18, 23). Vaccine strategies such as rAd5 vector-based vaccines are therefore being developed to elicit high-frequency HIV-1-specific cellular immune responses (15, 24, 25). However, rAd5 vaccines will likely be substantially suppressed by the high prevalence of anti-Ad5 immunity in human populations, particularly in sub-Saharan Africa (13), and thus alternative serotype rAd vectors are being developed. In this study, we demonstrate the immunogenicity of novel rAd11 vectors as well as heterologous rAd prime-boost strategies involving rAd5 (8), rAd35 (32), and rAd11 (11) vectors in mice both with and without anti-Ad5 immunity. Importantly, all regimens that contained rAd5 vectors were markedly suppressed by anti-Ad5 immunity. In contrast, heterologous rAd prime-boost regimens involving two rare serotype rAd vectors elicited the highest magnitude immune responses in mice with anti-Ad5 immunity. Such strategies should therefore be explored further as candidate vaccines for both HIV-1 and other pathogens.
Cross-reactive anti-vector immune responses, however, may limit the utility of this approach, presumably by reducing the efficiency of the boost immunization. We detected low but functionally significant cross-reactive Ad35/Ad11-specific NAbs and CD8+ T lymphocytes that suppressed vaccine immunogenicity in both preimmunization studies (Fig. 5) and adoptive transfer studies (Fig. 7 and 8). These data demonstrate that closely related serotype rAd vectors are not necessarily immunologically distinct in this model. It is clear from the adoptive transfer studies that both NAbs and CD8+ T lymphocytes contribute to cross-reactive Ad35/Ad11-specific immunity, although NAbs likely play the critical and primary role. These observations are consistent with our prior observations regarding the relative importance of NAbs and CD8+ T lymphocytes to Ad5-specific immunity (29).
Ad35 and Ad11 are highly related viruses from Ad subfamily B that share >98% genetic homology (11). It is therefore not surprising that cross-reactive Ad35/Ad11-specific immunity exists. Cross-reactive CD4+ and CD8+ T-lymphocyte responses have also been described in humans (9, 19, 27), although cross-reactive NAbs and the functional significance of these responses have not previously been investigated. Interestingly, we did not detect cross-reactive Ad35/Ad11-specific NAbs in our previous studies in which we assessed human serum samples for the presence of Ad35- and Ad11-specific NAbs (11). Presumably the relatively low titers of Ad35- and Ad11-specific NAbs typically found in humans compared with those generated by immunization of mice obscured detection of cross-reactive NAbs in these seroprevalence studies.
Studies from our laboratory and others have previously reported that heterologous rAd prime-boost regimens can elicit high-frequency immune responses (3, 20, 25). The present study extends these prior observations by evaluating the immunogenicity of rAd prime-boost regimens in the presence of preexisting anti-Ad5 immunity and by assessing the extent of cross-reactive anti-vector immunity. Our results indicate that in the presence of anti-Ad5 immunity, the optimal rAd vaccine regimen requires two rare serotype rAd vectors that are both distinct from rAd5. Both rAd35 and rAd11 are attractive candidate vaccine vectors that are not substantially suppressed by anti-Ad5 immunity but may be limited by cross-reactive anti-vector immunity. Moreover, rAd35 and rAd11 vectors appear less immunogenic than rAd5 vectors in the absence of anti-Ad5 immunity (3, 25), although it is possible that our studies may have underestimated the immunogenicity of rAd35 and rAd11 vectors since mice lack the optimal CD46 receptor (7).
Although we observed clear cross-reactive Ad35/Ad11-specific NAbs, we failed to detect cross-reactive Ad5/Ad35- or Ad5/Ad11-specific NAbs (Fig. 6). These data suggest that certain rAd vector combinations may not be inhibited by cross-reactive anti-vector immunity. Whereas Ad35 and Ad11 are from Ad subfamily B, Ad5 is from Ad subfamily C and thus shares less genetic homology with these other vectors. Interestingly, rAd35-rAd5 and rAd11-rAd5 vaccine regimens proved substantially more immunogenic than rAd35-rAd11 and rAd11-rAd35 regimens in the absence of anti-Ad5 immunity (Fig. 3). These findings likely reflect both the potent intrinsic immunogenicity of rAd5 vectors as well as the absence of substantial cross-reactive anti-vector immunity. These data suggest that the optimal heterologous rAd prime-boost regimen should involve two vectors that are both rare in humans, distinct from Ad5, and derived from different Ad subfamilies. We predict that such a regimen will prove substantially more immunogenic than regimens involving vectors derived from the same Ad subfamily as a result of reduced cross-reactive anti-vector immunity. Additional rare serotype rAd vectors that are immunologically distinct from rAd35 and rAd11 should therefore be constructed. Future studies could investigate the immunogenicity and protective efficacy of such rAd vector-based vaccine regimens for both HIV-1 and other pathogens.
Acknowledgments
We thank Gary Nabel, Norman Letvin, Raphael Dolin, Michael Kishko, Jerome Custers, and Sandra Verhaagh for generous advice, assistance, and reagents. The SIV Gag peptides were obtained from the NIH AIDS Research and Reference Reagent Program.
We acknowledge support from NIH grants AI-60368 (D.H.B.) and AI-51223 (D.H.B.). D.H.B. is also a recipient of a Doris Duke Clinical Scientist Development Award.
REFERENCES
- 1.Altman, J. D., P. A. H. Moss, P. J. R. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94-96. [DOI] [PubMed] [Google Scholar]
- 2.Barouch, D. H., P. F. McKay, S. M. Sumida, S. Santra, S. S. Jackson, D. A. Gorgone, M. A. Lifton, B. K. Chakrabarti, L. Xu, G. J. Nabel, and N. L. Letvin. 2003. Plasmid chemokines and colony-stimulating factors enhance the immunogenicity of DNA priming-viral vector boosting HIV-1 vaccines. J. Virol. 77:8729-8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barouch, D. H., M. G. Pau, J. H. Custers, W. Koudstaal, S. Kostense, M. J. Havenga, D. M. Truitt, S. M. Sumida, M. G. Kishko, J. C. Arthur, B. Korioth-Schmitz, M. H. Newberg, D. A. Gorgone, M. A. Lifton, D. L. Panicali, G. J. Nabel, N. L. Letvin, and J. Goudsmit. 2004. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of preexisting anti-Ad5 immunity. J. Immunol. 172:6290-6297. [DOI] [PubMed] [Google Scholar]
- 4.Casimiro, D. R., L. Chen, T. M. Fu, R. K. Evans, M. J. Caulfield, M. E. Davies, A. Tang, M. Chen, L. Huang, V. Harris, D. C. Freed, K. A. Wilson, S. Dubey, D. M. Zhu, D. Nawrocki, H. Mach, R. Troutman, L. Isopi, D. Williams, W. Hurni, Z. Xu, J. G. Smith, S. Wang, X. Liu, L. Guan, R. Long, W. Trigona, G. J. Heidecker, H. C. Perry, N. Persaud, T. J. Toner, Q. Su, X. Liang, R. Youil, M. Chastain, A. J. Bett, D. B. Volkin, E. A. Emini, and J. W. Shiver. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 77:6305-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farina, S. F., G. P. Gao, Z. Q. Xiang, J. J. Rux, R. M. Burnett, M. R. Alvira, J. Marsh, H. C. Ertl, and J. M. Wilson. 2001. Replication-defective vector based on a chimpanzee adenovirus. J. Virol. 75:11603-11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzgerald, J. C., G. P. Gao, A. Reyes-Sandoval, G. N. Pavlakis, Z. Q. Xiang, A. P. Wlazlo, W. Giles-Davis, J. M. Wilson, and H. C. Ertl. 2003. A simian replication-defective adenoviral recombinant vaccine to HIV-1 gag. J. Immunol. 170:1416-1422. [DOI] [PubMed] [Google Scholar]
- 7.Gaggar, A., D. M. Shayakhmetov, and A. Lieber. 2003. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 9:1408-1412. [DOI] [PubMed] [Google Scholar]
- 8.Havenga, M. J., A. A. Lemckert, J. M. Grimbergen, R. Vogels, L. G. Huisman, D. Valerio, A. Bout, and P. H. Quax. 2001. Improved adenovirus vectors for infection of cardiovascular tissues. J. Virol. 75:3335-3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heemskerk, B., L. A. Veltrop-Duits, T. van Vreeswijk, M. M. ten Dam, S. Heidt, R. E. Toes, M. J. van Tol, and M. W. Schilham. 2003. Extensive cross-reactivity of CD4+ adenovirus-specific T cells: implications for immunotherapy and gene therapy. J. Virol. 77:6562-6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofmann, C., P. Loser, G. Cichon, W. Arnold, G. W. Both, and M. Strauss. 1999. Ovine adenovirus vectors overcome preexisting humoral immunity against human adenoviruses in vivo. J. Virol. 73:6930-6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holterman, L., R. Vogels, R. van der Vlugt, M. Sieuwerts, J. Grimbergen, J. Kaspers, E. Geelen, E. van der Helm, A. Lemckert, G. Gillissen, S. Verhaagh, J. Custers, D. Zuijdgeest, B. Berkhout, M. Bakker, P. Quax, J. Goudsmit, and M. Havenga. 2004. Novel replication-incompetent vector derived from adenovirus 11 for vaccination and gene therapy: low seroprevalence and non-cross-reactivity with Ad5. J. Virol. 78:13207-13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kass-Eisler, A., L. Leinwand, J. Gall, B. Bloom, and E. Falck-Pedersen. 1996. Circumventing the immune response to adenovirus-mediated gene therapy. Gene Ther. 3:154-162. [PubMed] [Google Scholar]
- 13.Kostense, S., W. Koudstaal, M. Sprangers, G. J. Weverling, G. Penders, N. Helmus, R. Vogels, M. Bakker, B. Berkhout, M. Havenga, and J. Goudsmit. 2004. Adenovirus types 5 and 35 seroprevalence in AIDS risk groups supports type 35 as a vaccine vector. AIDS 18:1213-1216. [DOI] [PubMed] [Google Scholar]
- 14.Letvin, N. L., D. H. Barouch, and D. C. Montefiori. 2002. Prospects for vaccine protection against HIV-1 infection and AIDS. Annu. Rev. Immunol. 20:73-99. [DOI] [PubMed] [Google Scholar]
- 15.Letvin, N. L., Y. Huang, B. K. Chakrabarti, L. Xu, M. S. Seaman, K. Beaudry, B. Korioth-Schmitz, F. Yu, D. Rohne, K. L. Martin, A. Miura, W. P. Kong, Z. Y. Yang, R. S. Gelman, O. G. Golubeva, D. C. Montefiori, J. R. Mascola, and G. J. Nabel. 2004. Heterologous envelope immunogens contribute to AIDS vaccine protection in rhesus monkeys. J. Virol. 78:7490-7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mack, C. A., W. R. Song, H. Carpenter, T. J. Wickham, I. Kovesdi, B. G. Harvey, C. J. Magovern, O. W. Isom, T. Rosengart, E. Falck-Pedersen, N. R. Hackett, R. G. Crystal, and A. Mastrangeli. 1997. Circumvention of anti-adenovirus neutralizing immunity by administration of an adenoviral vector of an alternate serotype. Hum. Gene Ther. 8:99-109. [DOI] [PubMed] [Google Scholar]
- 17.Mastrangeli, A., B. G. Harvey, J. Yao, G. Wolff, I. Kovesdi, R. G. Crystal, and E. Falck-Pedersen. 1996. “Sero-switch” adenovirus-mediated in vivo gene transfer: circumvention of anti-adenovirus humoral immune defenses against repeat adenovirus vector administration by changing the adenovirus serotype. Hum. Gene Ther. 7:79-87. [DOI] [PubMed] [Google Scholar]
- 18.Musey, L., J. Hughes, T. Schacker, T. Shea, L. Corey, and M. J. McElrath. 1997. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N. Engl. J. Med. 337:1267-1274. [DOI] [PubMed] [Google Scholar]
- 19.Olive, M., L. Eisenlohr, N. Flomenberg, S. Hsu, and P. Flomenberg. 2002. The adenovirus capsid protein hexon contains a highly conserved human CD4+ T-cell epitope. Hum. Gene Ther. 13:1167-1178. [DOI] [PubMed] [Google Scholar]
- 20.Pinto, A. R., J. C. Fitzgerald, W. Giles-Davis, G. P. Gao, J. M. Wilson, and H. C. Ertl. 2003. Induction of CD8+ T cells to an HIV-1 antigen through a prime boost regimen with heterologous E1-deleted adenoviral vaccine carriers. J. Immunol. 171:6774-6779. [DOI] [PubMed] [Google Scholar]
- 21.Reddy, P. S., N. Idamakanti, L. A. Babiuk, M. Mehtali, and S. K. Tikoo. 1999. Porcine adenovirus-3 as a helper-dependent expression vector. J. Gen. Virol. 80:2909-2916. [DOI] [PubMed] [Google Scholar]
- 22.Reddy, P. S., N. Idamakanti, Y. Chen, T. Whale, L. A. Babiuk, M. Mehtali, and S. K. Tikoo. 1999. Replication-defective bovine adenovirus type 3 as an expression vector. J. Virol. 73:9137-9144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 24.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 25.Shiver, J. W., and E. A. Emini. 2004. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu. Rev. Med. 55:355-372. [DOI] [PubMed] [Google Scholar]
- 26.Shiver, J. W. 2004. Development of an HIV-1 vaccine based on replication-defective adenovirus. HIV vaccine development: progress and prospects. Keystone Symposia, Whistler, British Columbia, Canada.
- 27.Smith, C. A., L. S. Woodruff, C. Rooney, and G. R. Kitchingman. 1998. Extensive cross-reactivity of adenovirus-specific cytotoxic T cells. Hum. Gene Ther. 9:1419-1427. [DOI] [PubMed] [Google Scholar]
- 28.Sprangers, M. C., W. Lakhai, W. Koudstaal, M. Verhoeven, B. F. Koel, R. Vogels, J. Goudsmit, M. J. Havenga, and S. Kostense. 2003. Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: addressing preexisting immunity to vaccine and gene therapy vectors. J. Clin. Microbiol. 41:5046-5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sumida, S. M., D. M. Truitt, M. G. Kishko, J. C. Arthur, S. S. Jackson, D. A. Gorgone, M. A. Lifton, W. Koudstaal, M. G. Pau, S. Kostense, M. J. Havenga, J. Goudsmit, N. L. Letvin, and D. H. Barouch. 2004. Neutralizing antibodies and CD8+ T lymphocytes both contribute to immunity to adenovirus serotype 5 vaccine vectors. J. Virol. 78:2666-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sullivan, N. J., A. Sanchez, P. E. Rollin, Z. Y. Yang, and G. J. Nabel. 2000. Development of a preventive vaccine for Ebola virus infection in primates. Nature 30:605-609. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan, N. J., T. W. Geisbert, J. B. Geisbert, L. Xu, Z. Y. Yang, M. Roederer, R. A. Koup, P. B. Jahrling, and G. J. Nabel. 2003. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature 424:681-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vogels, R., D. Zuijdgeest, R. van Rijnsoever, E. Hartkoorn, I. Damen, M. de Bethune, S. Kostense, G. Penders, N. Helmus, W. Koudstaal, M. Cecchini, A. Wetterwald, M. Sprangers, A. Lemckert, O. Ophorst, B. Koel, M. van Meerendonk, P. Quax, L. Panitti, J. Grimbergen, A. Bout, J. Goudsmit, and M. Havenga. 2003. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell interaction and bypass of preexisting adenovirus immunity. J. Virol. 77:8263-8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiang, Z., G. Gao, A. Reyes-Sandoval, C. J. Cohen, Y. Li, J. M. Bergelson, J. M. Wilson, and H. C. Ertl. 2002. Novel, chimpanzee serotype 68-based adenoviral vaccine carrier for induction of antibodies to a transgene product. J. Virol. 76:2667-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang, Z. Y., L. S. Wyatt, W. P. Kong, Z. Moodie, B. Moss, and G. J. Nabel. 2003. Overcoming immunity to a viral vaccine by DNA priming before vector boosting. J. Virol. 77:799-803. [DOI] [PMC free article] [PubMed] [Google Scholar]