Abstract
The outcome of a viral infection or of immunization with a vaccine can be influenced by the local cytokine environment. In studies of experimental vaccines against respiratory syncytial virus (RSV), an increased stimulation of Th2 (T helper 2) lymphocytes was associated with increased immunopathology upon subsequent RSV infection. For this study, we investigated the effect of increased local expression of the Th2 cytokine interleukin-4 (IL-4) from the genome of a recombinant RSV following primary infection and after a challenge with wild-type (wt) RSV. Mice infected with RSV/IL-4 exhibited an accelerated pulmonary inflammatory response compared to those infected with wt RSV, although the wt RSV group caught up by day 8. In the first few days postinfection, RSV/IL-4 was associated with a small but significant acceleration in the expansion of pulmonary T lymphocytes specific for an RSV CD8+ cytotoxic T-lymphocyte (CTL) epitope presented as a major histocompatibility complex class I tetramer. However, by day 7 the response of tetramer-positive T lymphocytes in the wt RSV group caught up and exceeded that of the RSV/IL-4 group. At all times, the CTL response of the RSV/IL-4 group was deficient in the production of gamma interferon and was nonfunctional for in vitro cell killing. The accelerated inflammatory response coincided with an accelerated accumulation and activation of pulmonary dendritic cells early in infection, but thereafter the dendritic cells were deficient in the expression of B7-1, which governs the acquisition of cytolytic activity by CTL. Following a challenge with wt RSV, there was an increase in Th2 cytokines in the animals that had previously been infected with RSV/IL-4 compared to those previously infected with wt RSV, but the CD8+ CTL response and the amount of pulmonary inflammation were not significantly different. Thus, a strong Th2 environment during primary pulmonary immunization with live RSV resulted in early inflammation and a largely nonfunctional primary CTL response but had a minimal effect on the secondary response.
Respiratory syncytial virus (RSV) is the most important viral agent of serious pediatric respiratory tract disease worldwide. It can also cause significant respiratory tract disease in adolescents and adults and is particularly important in the elderly and in bone marrow transplant recipients (reviewed in reference 11). A licensed vaccine is not yet available, although several promising live attenuated intranasal RSV vaccine candidates have been described and are under evaluation (27).
T helper (Th) lymphocyte subsets 1 and 2 are defined by the cytokines they produce. The Th1 subset is defined by an increased secretion of gamma interferon (IFN-γ) and also can have an increased secretion of interleukin-2 (IL-2). IL-12 and IL-18 are also considered Th1 cytokines because, even though they are produced by other cell types, they induce the secretion of IFN-γ by T cells. In contrast, the Th2 subset is defined by an increased secretion of IL-4 and also can have an increased secretion of IL-5, IL-6, IL-10, and IL-13 (24). This type 1 and type 2 distinction also extends to major histocompatibility complex (MHC) class I-restricted CD8+ T lymphocytes (CTL) (34) and possibly to natural killer (NK) cells (25). In several models of infectious disease, including RSV, Th1-augmented responses have been associated with disease resolution and Th2-augmented responses have been associated with disease progression (1, 13, 30). Th1 and Th2 responses can be self-stimulatory and cross-inhibitory, but they are complex and are not necessarily mutually antagonistic.
The cytokine environment has been associated with differences in the response to experimental RSV vaccines in animal models. For example, the immunization of mice or cotton rats with formalin-inactivated RSV, with purified RSV F and G glycoproteins, or with a vaccinia virus expressing the RSV G glycoprotein resulted in an elevated stimulation of Th2 cells (11, 17). A challenge of these immunized animals with wild-type (wt) RSV was followed by enhanced pulmonary histopathology and weight loss (as disease markers) (13, 28, 31, 32). Depletion of the Th2 cytokines IL-4 and IL-10 following immunization with purified protein and prior to the RSV challenge prevented disease enhancement (13, 40). Conversely, immunization by infection with live RSV is associated with an increased stimulation of type 1 T helper (Th1) lymphocytes and is protective rather than disease-enhancing. Also, augmentation of the Th1 response or suppression of the Th2 response during immunization with inactivated RSV resulted in an increased protective efficacy (40, 41). The factors that influence the cytokine response to RSV vaccines are complex and include whether the viral antigen is synthesized in situ versus supplied as a purified protein, the antigen involved (e.g., F glycoprotein versus G glycoprotein), the membrane-bound versus secreted status of an antigen expressed in situ, the site of immunization and nature of the adjuvant, and the genetic background of the host (5, 11, 28).
For the present study, we constructed a recombinant RSV that expresses murine IL-4 from an added gene inserted between the RSV G and F genes. Thus, the ectopic expression of this cytokine would be tied temporally and spatially to the replication of the infecting virus and the expression of the viral antigens. We evaluated the effect of ectopically expressed IL-4 on the outcome of the primary infection and a subsequent challenge with wt RSV with regard to the level of viral replication, the elaboration of pulmonary cytokines, the development of a virus-specific CTL response, and the proliferation and activation of pulmonary dendritic cells.
MATERIALS AND METHODS
Construction and in vitro growth of recombinant RSV/IL-4.
A cDNA containing the open reading frame of murine IL-4 was amplified by PCR to add RSV-specific gene start and gene end transcription signals and flanking XmaI sites. The forward and reverse primers were TATACCCGGGATGGGGCAAATATGGGTCTCAACCCCCAGCT and ATTACCCGGGAATTTTTAATAACTCTACGAGTAATCCATTTGCA, respectively (XmaI restriction endonuclease sites are shown in bold, and gene start and end sequences are underlined [Fig. 1, top panel]). The PCR product was digested with XmaI and inserted into an XmaI site that had been engineered into the G-F intergenic sequence of a cloned cDNA of the RSV antigenome (8). The recombinant viruses were recovered by cotransfection of the antigenomic plasmid and plasmids expressing the N, P, L, and M2-1 support proteins into HEp-2 cells along with the recombinant MVA vaccinia virus expressing T7 polymerase (12). The viruses were propagated in HEp-2 cells supplemented with 2% fetal bovine serum (Invitrogen, San Diego, CA) and were quantitated by a plaque assay (28). In initial experiments, RSV/IL-4 and the control wt RSV were purified from the infected cell supernatants by sucrose gradient centrifugation as previously described (7), with no difference compared to experiments using unpurified virus. IL-4 was quantified by a Quantikine colorimetric sandwich enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN) according to the manufacturer's recommendations. To examine the stability of the IL-4 insert in the viral genome, total RNA was isolated from HEp-2 cells infected with RSV/IL-4 following six passages and then analyzed by reverse transcription (RT) coupled with PCR as described previously (8).
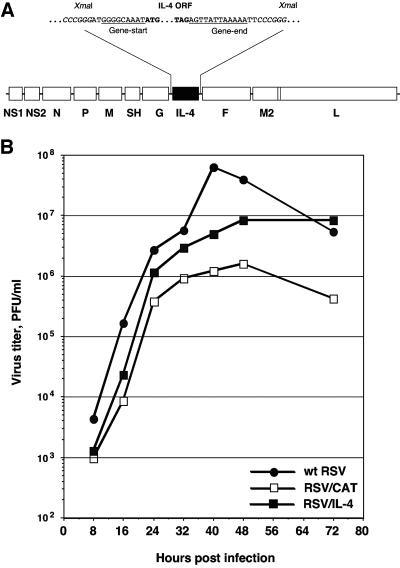
FIG. 1.
(A) Insertion of a transcription cassette encoding murine IL-4 into the RSV genome. A cDNA of the IL-4 open reading frame was engineered to be flanked by RSV-specific gene start and gene end transcription signals. It was inserted into a cloned cDNA of the RSV antigenome at an XmaI site that had been engineered into the intergenic region between the viral G and F genes (8). (B) Comparison of growth kinetics of wt RSV, RSV/IL-4, and RSV/CAT in HEp-2 cells (multiplicity of infection, 2 PFU).
Virus replication in mice.
Seven- to 12-week-old BALB/c mice (Charles River Laboratories, Wilmington, MA) were infected intranasally under methoxyflurane anesthesia with 106 PFU of the indicated virus in a 100-μl inoculum. On the indicated days after infection, animals were sacrificed by carbon dioxide intoxication, the nasal turbinates and lung tissues were harvested and homogenized, and viruses were titrated by plaque staining (28).
Histopathological studies.
Mice were infected and then sacrificed by carbon dioxide intoxication, and the lungs were removed, inflated intratracheally with 10% neutral buffered formalin, and fixed for 48 h. Following fixation, a single lung block was cut in the midcoronal plane such that all five lobes were on one slide and air passages of all sizes were present. The slides were stained with hematoxylin and eosin, and pathology was scored under a blinded code on a semiquantitative scale as described in Table 2.
TABLE 2.
Comparison of histopathological changes induced by infection with wt RSV or RSV/IL-4a
| Day | Virusb | Mean score ± SE (P)c,d
|
|||
|---|---|---|---|---|---|
| Peribronchiolitis | Perivasculitis | Interstitial pneumonitis | Alveolitis | ||
| 4 | wt RSV | 2.5 ± 0.8 | 4.5 ± 0.5 | 0.5 ± 0.5 | 0 |
| RSV/IL-4 | 17.0 ± 3.3 (<0.001) | 24.0 ± 6.4 (<0.01) | 13.0 ± 3.3 (<0.01) | 4.5.0 ± 0.5 (<0.001) | |
| Mock | 1.2 ± 1.2 | 1.2 ± 1.2 | 0 | 0 | |
| 8 | wt RSV | 17.0 ± 3.3 | 19.0 ± 3.1 | 9.0 ± 3.6 | 2.5 ± 0.8 |
| RSV/IL-4 | 23.0 ± 2.0 | 15.0 ± 3.3 | 3.5 ± 2.5 | 2.0 ± 0.8 | |
| Mock | 0 | 1.2 ± 1.25 | 0 | 0 | |
| 32 (4) | wt RSV | 30.6 ± 5.6 | 77.8 ± 2.8 | 20.6 ± 10.6 | 8.3 ± 3.2 |
| RSV/IL-4 | 28.0 ± 5.6 | 62.5 ± 8.5 | 14.5 ± 3.5 | 13.5 ± 7.1 | |
| Mock | 3.7 ± 1.2 | 10.0 ± 5.0 | 0 | 0 | |
| 36 (8) | wt RSV | 28.0 ± 5.6 | 60.0 ± 7.6 | 2.5 ± 0.8 | 1.5 ± 0.8 |
| RSV/IL-4 | 23.0 ± 2.0 | 40.0 ± 7.6 | 0 (<0.001) | 1.00 ± 0.67 | |
| Mock | 20.0 ± 5.0 | 5.0 ± 0 | 15.0 ± 5.6 | 5.0 ± 0 | |
Groups of mice were infected on day 0 with wt RSV or RSV/IL-4 (9 or 10 animals per group per day) or were mock infected (4 animals per group per day). Animals were sacrificed on days 4 and 8 postinfection, and the lungs were removed for histopathologic analysis. The remaining animals (including the mock controls) were challenged on day 28 with wt RSV and then sacrificed on days 32 and 36 (4 or 8 days postchallenge, respectively), and the lungs were removed for histopathologic analysis.
All groups are designated according to the inoculum on day 0.
Scoring was done on a semiquantitative scale from 0 (no inflammation) to 100 (maximum). Peribronchiolitis, accumulation of inflammatory cells, primarily lymphocytes, surrounding a bronchiole; perivasculitis, inflammatory cells surrounding a blood vessel; interstitial pneumonitis, increased thickness of alveolar walls associated with inflammatory cells, primarily neutrophils; alveolitis, inflammatory cells, primarily neutrophils, within alveolar spaces.
Statistical significances of differences between the RSV/IL-4 and the wt RSV groups are shown in bold for P values of <0.05.
Analysis of pulmonary cytokine mRNAs.
As previously described (7), total RNAs were isolated from the lungs of mice by use of the Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's recommendations. Cytokine mRNAs were assayed with the RiboQuant Multi-Probe RNase protection assay system (BD Biosciences, San Diego, CA) with the multiprobe template sets mCK-1 and mCK-2B according to the instruction manual. Radioactive bands corresponding to mRNAs of individual cytokines were quantitated using a PhosphorImager 445 SI (Molecular Dynamics, Sunnyvale, CA).
Analysis of RSV-specific MHC class I-restricted T cells.
Total pulmonary mononuclear cells (PMC) were isolated from mice (7) on the indicated days, washed twice in phosphate-buffered saline containing 2% fetal bovine serum (FBS), and stained with an optimized amount of phycoerythrin-conjugated MHC class I H-2Kd tetramer complexes loaded with the peptide SYIGSINNI, representing the immunodominant epitope of the RSV M2-1 protein (21, 22), or of similar complexes loaded with the peptide TYQRTRALV, representing amino acids 147 to 155 of the influenza virus nucleoprotein, as a negative control (both tetramers were supplied by the NIAID Tetramer Facility, Yerkes Regional Primate Research Center, Atlanta, GA). The cells were also stained with an optimized amount of the fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse CD8α monoclonal antibody clone 53-6.7 (BD Biosciences). For some experiments, the cells were additionally stained with one of the following allophycocyanin (APC)-conjugated antibodies: anti-mouse CD3 (T-cell marker) clone 145-2C11, anti-mouse CD19 (B-cell marker) clone 1D3, and anti-mouse CD49 (NK cell marker) clone DX5 (all from BD Biosciences).
For the quantitation of cells that secrete IFN-γ in response to RSV-specific stimulation, PMC were resuspended in RPMI medium 1640 (Invitrogen, Carlsbad, CA) containing 10% FBS, 100 U of penicillin/ml, and 100 μg of streptomycin sulfate/ml. The cells were counted and incubated overnight with 1 μM of the M2-1 peptide in the presence of GolgiStop (Invitrogen, Carlsbad, CA). Following stimulation, cells were washed twice with phosphate-buffered saline containing 2% FBS, treated with Fc Block (BD Biosciences) to block Fc receptors, stained as described above with the FITC-conjugated anti-mouse CD8α monoclonal antibody, washed twice, fixed, and permeabilized with Cytofix/Cytoperm solution (BD Biosciences). They were next stained with the APC-conjugated rat anti-mouse IFN-γ antibody clone XMG1.2 or the APC-conjugated rat anti-mouse IL-4 antibody clone BVD4-1D11 (both from BD Biosciences). Flow cytometry analysis was performed using a FACSCalibur flow cytometer (BD Biosciences). A total of 30,000 cells were analyzed per sample.
An analysis of the RSV-specific cytolytic activity of PMC was performed by a 51Cr release assay using target P815 mouse mastocytoma cells loaded with 1 μM of the M2-1 peptide as described previously (4, 7).
Analysis of pulmonary dendritic cells.
Briefly, PMC were stained with predetermined optimal amounts of FITC-labeled anti-mouse CD11b antibodies (clone M1/70; this and all of the subsequent antibodies were purchased from BD Biosciences) and phycoerythrin-labeled anti-mouse CD11c antibodies (clone HL3), as described previously (7, 42). The labeled cell suspensions were divided into four parts, incubated with an anti-mouse biotin-labeled antibody (specific for H-2Kd [clone SF1-1.1], I-Ad/I-Ed [clone 2G9], B7-1 [CD80] [clone 16-10A1], or B7-2 [CD86] [clone GL1]), washed, and stained with APC-labeled streptavidin (BD Biosciences). Flow cytometry was performed with 30,000 cells per sample.
Data analysis.
Data are shown as means ± standard errors of the means. Differences were evaluated by the Student t test and were considered statistically significant when the P value was <0.05.
RESULTS
Recovery of RSV/IL-4 and replication in mice.
A cDNA of the open reading frame encoding murine IL-4 (23, 29) was engineered by PCR to be flanked by RSV-specific gene start and gene end transcription signals and was inserted between the RSV G and F genes in a cloned cDNA of the RSV antigenome (8) (Fig. 1, top panel). The recombinant virus, designated RSV/IL-4, was recovered from this antigenomic cDNA by previously described methods (12). The stability of the IL-4 gene insert after six passages of the virus in HEp-2 cells was confirmed by RT-PCR, which resulted in a single band of the expected size (data not shown). The growth of RSV/IL-4 in HEp-2 cells was somewhat reduced compared to that of wt RSV (Fig. 1, bottom panel) but was greater than that of a control RSV (RSV/CAT) expressing the chloramphenicol acetyltransferase (CAT) gene, an insert that is somewhat larger than the IL-4 insert (468 bp versus 761 bp). This is consistent with our experience that the level of attenuation of RSV replication in cell culture is inversely related to the length of the insert. The accumulation of IL-4 in the medium of HEp-2 cells infected with RSV/IL-4 reached 250 ng/ml at 24 h and 1 μg/ml at 48 h postinfection; in cells infected with wt RSV, the level of the cytokine did not exceed 100 pg/ml (not shown).
We compared the level of replication of RSV/IL-4 in the respiratory tracts of mice with that of wt RSV and RSV/CAT on days 3, 4, 5, 7, and 9 following intranasal administration of the virus (Table 1). The levels of replication of the three viruses in the nasal turbinates and lungs on days 3, 4, and 5 were essentially indistinguishable. On days 7 and 9, RSV/IL-4 and wt RSV were detected only in trace amounts, indicating that the expression of IL-4 did not result in delayed clearance of the virus. Additional groups of mice were each infected with one of the three viruses and challenged 56 days later with wt RSV. Challenge virus replication in the nasal turbinates and lungs could not be detected when the animals were sacrificed 4 days later (not shown).
TABLE 1.
Replication of RSV/IL-4 in BALB/c micea
| Virus | Tissueb | Mean virus titer (log10 PFU/g ± SE) on indicated day postinfection
|
||||
|---|---|---|---|---|---|---|
| 3 | 4 | 5 | 7 | 9 | ||
| wt RSV | NT | 3.8 ± 0.1 | 3.7 ± 0.1 | 3.8 ± 0.1 | 2.2 ± 0.1 | <2.0 |
| L | 4.1 ± 0.1 | 4.6 ± 0.1 | 4.5 ± 0.0 | 2.3 ± 0.1 | 1.8 ± 0.1 | |
| RSV/CAT | NT | 4.0 ± 0.1 | 4.0 ± 0.1 | 5.0 ± 0.0 | ND | ND |
| L | 3.7 ± 0.1 | 4.4 ± 0.1 | 4.7 ± 0.1 | ND | ND | |
| RSV/IL-4 | NT | 4.0 ± 0.1 | 3.7 ± 0.1 | 3.7 ± 0.1 | 2.2 ± 0.2 | <2.0 |
| L | 4.0 ± 0.1 | 4.5 ± 0.1 | 4.7 ± 0.1 | <1.7 | <1.7 | |
Mice were infected with 106 PFU of the indicated viruses. On days 3, 4, 5, 7, and 9, five mice per group were killed, nasal turbinates and lungs were removed, and virus titers were quantitated by a plaque assay. The detection limits were as follows: for nasal turbinates; 2.0 log10 PFU/g; for lungs, 1.7 log10 PFU/g. ND, not determined.
NT, nasal turbinates; L, lungs.
Lung histopathology during primary infection and following challenge with wt RSV.
Mice infected with wt RSV or RSV/IL-4 were examined for pulmonary histopathology on days 4 and 8 following primary infection or were challenged with wt RSV on day 28 and examined 4 or 8 days later. The histopathologic indicators and scores used are shown in Table 2 (see footnote c for a description of the indicators and the scoring system), and examples of lung sections are shown in Fig. 2. During primary infection with either virus, perivasculitis was the most predominant histopathologic effect, followed in order by peribroncholitis, interstitial pneumonitis, and alveolitis. On day 4 of the primary infection, mice infected with RSV/IL-4 had significantly higher levels of all four indicators than those for the wt RSV-infected animals (Table 2; Fig. 2). By day 8, however, the levels of all four parameters had increased in wt RSV-infected animals to become approximately equivalent to those of the RSV/IL-4 group.
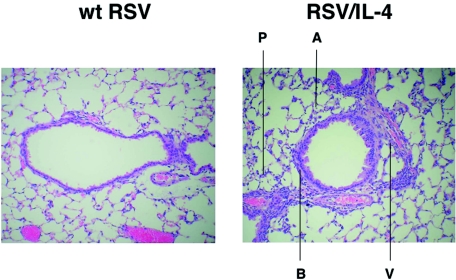
FIG. 2.
Histopathology in the lungs of mice after primary infection with wt RSV or RSV/IL-4. Mice were infected with RSV/IL-4 or wt RSV and sacrificed on day 4. The lungs were removed, fixed, cut along the midcoronal plane, and stained with hematoxylin and eosin. In animals infected with wt RSV (left), only a minimal amount of lymphocytic infiltration around the airways and blood vessels was observed, and there was no inflammation in the alveoli and alveolar walls. In contrast, in animals infected with RSV/IL-4 (right), a cellular infiltrate consisting primarily of lymphocytes was readily apparent around the airways (peribronchiolitis, indicated by B) and blood vessels (perivasculitis, V). A cellular infiltrate consisting primarily of lymphocytes and macrophages was also observed in the alveoli (alveolitis, A) and alveolar walls (interstitial pneumonitis, P). Magnification, ×100.
When animals that had been infected with wt RSV or RSV/IL-4 were challenged with wt RSV, elevated levels of all four histopathologic indicators, especially perivasculitis and peribronchiolitis, were observed for both groups (Table 2). However, there was no significant difference in the overall inflammatory response following challenge between animals that had received wt RSV and those that received RSV/IL-4 as the primary infection, with the exception of intestinal pneumonitis, which was not detected in the RSV/IL-4 group on day 8 (Table 2). We also monitored the weight of the mice following the primary and secondary infections; no significant difference between the groups was found (data not shown), indicating a lack of vaccine-enhanced disease, as measured by this widely used parameter.
Pulmonary Th1 and Th2 cytokine mRNAs.
We compared the profiles of Th1 and Th2 cytokine mRNAs in the lungs of mice on days 1 and 4 following the primary infection with wt RSV or RSV/IL-4 and on days 1 and 4 following challenge on day 28 with wt RSV (Table 3; the values for the RSV/IL-4 group that differ significantly [P < 0.05] from those for the wt RSV group are shown in bold). Following the primary infection, mice infected with RSV/IL-4 had reductions in the levels of mRNA for the Th1 cytokines IL-12 p40 (the inducible subunit of IL-12), IL-18, and IFN-γ (although the difference in the levels of IFN-γ was not statistically significant due to the high variability within the groups) compared to the levels induced by wt RSV. Mice infected with RSV/IL-4 had an abundant band of IL-4 mRNA that was not present with wt RSV infection and presumably was transcribed from the RSV/IL-4 genome. Thus, infection with RSV/IL-4 was associated with a modest down-regulation of Th1 cytokines compared to wt RSV infection.
TABLE 3.
Comparison of the levels of selected pulmonary cytokine mRNAs in mice infected with wt RSV or RSV/IL-4a
| Cytokine | Virusb | % Expression (mean ± SE)c
|
|||
|---|---|---|---|---|---|
| Day 1 | Day 4 | Day 29 (1) | Day 32 (4) | ||
| IFN-γ | wt RSV | 2.63 ± 0.81 | 0 | 4.95 ± 0.28 | 2.99 ± 0.22 |
| RSV/IL-4 | 1.29 ± 0.05 | 0 | 6.49 ± 0.76 | 4.45 ± 0.49 | |
| Mock | 0.92 ± 0.24 | 0 | 0 | 0 | |
| IL-12 p35 | wt RSV | 0.82 ± 0.12 | 1.12 ± 0.12 | 0.45 ± 0.03 | 0.47 ± 0.01 |
| RSV/IL-4 | 0.56 ± 0.04 | 1.16 ± 0.03 | 0.42 ± 0.03 | 0.58 ± 0.08 | |
| Mock | 1.11 ± 0.30 | 1.37 ± 0.05 | 0.55 ± 0.07 | 0.73 ± 0.14 | |
| wt RSV | 0.74 ± 0.12 | 1.12 ± 0.08 | 0.57 ± 0.03 | 0.18 ± 0.08 | |
| IL-12 p40 | RSV/IL-4 | 0.38 ± 0.03 | 1.02 ± 0.06 | 0.41 ± 0.03 | 0.17 ± 0.08 |
| Mock | 0.91 ± 0.29 | 0.91 ± 0.09 | 0.83 ± 0.07 | 0.14 ± 0.06 | |
| IL-18 | wt RSV | 5.93 ± 0.99 | 5.14 ± 0.11 | 5.21 ± 0.18 | 2.75 ± 0.12 |
| RSV/IL-4 | 2.73 ± 0.10 | 3.87 ± 0.27 | 7.40 ± 0.61 | 3.75 ± 0.16 | |
| Mock | 3.80 ± 0.20 | 5.42 ± 0.23 | 6.76 ± 0.13 | 4.65 ± 0.22 | |
| IL-4 | wt RSV | 0 | 0 | 0 | 0 |
| RSV/IL-4 | 8.20 ± 0.51 | 8.58 ± 0.72 | 1.46 ± 0.16 | 0.34 ± 0.03 | |
| Mock | 0 | 0 | 0 | 0 | |
| IL-5 | wt RSV | 0 | 0 | 0 | 0 |
| RSV/IL-4 | 0 | 0 | 1.27 ± 0.13 | 0.34 ± 0.04 | |
| Mock | 0 | 0 | 0 | 0 | |
| IL-13 | wt RSV | 0 | 0 | 0 | 0 |
| RSV/IL-4 | 0 | 0 | 1.54 ± 0.20 | 0.50 ± 0.04 | |
| Mock | 0 | 0 | 0 | 0 | |
Groups of mice (five animals per group per day) were infected on day 0 with wt RSV or RSV/IL-4 or were mock infected. Animals were sacrificed on days 1 and 4 postinfection, and the lungs were removed and processed to quantify cytokine mRNAs by a ribonuclease protection assay. The remaining animals (including the mock controls) were challenged on day 28 with wt RSV and sacrificed on days 29 and 32 (1 and 4 days postchallenge, respectively), and the lungs were processed for RNA analysis. The abundance of each mRNA is expressed as the percentage of the L32 housekeeping gene mRNA in the same sample, with the standard error indicated. Values for the RSV/IL-4 group that have a statistically significant (P < 0.05) difference from those for the wt RSV group are shown in bold. Initial experiments on mRNA quantitation used gradient-purified virus preparations, with no difference seen compared to unpurified preparations.
All groups are designated according to the inoculum on day 0.
Zero values mean that the corresponding mRNA was not detected.
In mice that had previously been infected with RSV/IL-4, the wt RSV challenge resulted in the expression of detectable levels of the Th2 cytokines IL-4, IL-5, and IL-13, whereas these cytokines were below the level of detection in mice that had previously been infected with wt RSV. Thus, the initial infection with RSV/IL-4 resulted in an increase in Th2 cytokines upon wt RSV challenge. The effect on Th1 cytokines during the challenge was mixed: for the RSV/IL-4 group, there were modest increases in mRNAs for IFN-γ and IL-18 and a decrease in mRNA for IL-12 p40 compared to the levels in the wt RSV group.
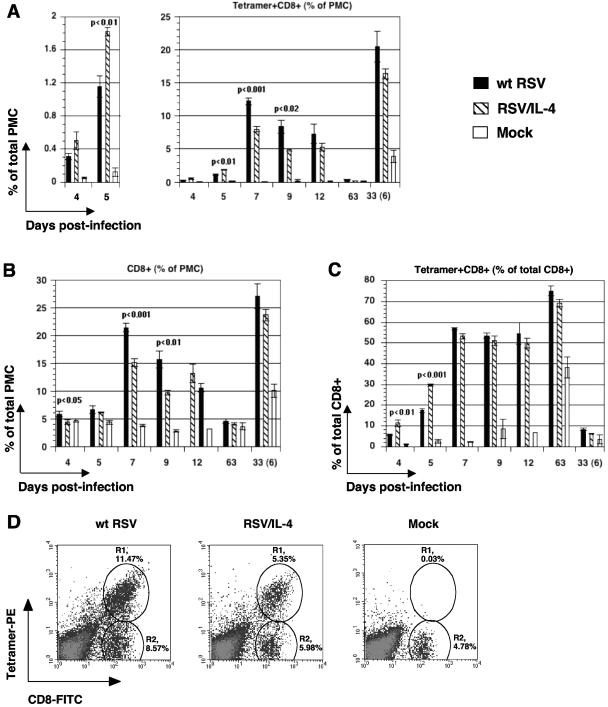
MHC class I-restricted T cells specific for an immunodominant RSV epitope, assessed by staining with a peptide-MHC tetramer.
PMC were isolated from BALB/c mice on days 4, 5, 7, 9, 12, and 63 following a primary infection with wt RSV or RSV/IL-4 or a mock infection. Other animals were infected or mock infected in the same way, challenged on day 27 with wt RSV, sacrificed 6 days later, and processed for PMC isolation. Following the primary infection, the yield of PMC from mice infected with either virus exceeded that from mock-infected animals by approximately sevenfold; there was no consistent difference between the two viruses or between the first and second infections for either virus (data not shown). The PMC were then analyzed for binding to MHC class I H-2Kd tetramer complexes loaded with the peptide SYIGSINNI, which is the RSV immunodominant CD8+ CTL epitope in BALB/c mice and represents amino acids 82 to 90 of the viral M2-1 protein (9, 21, 22) (Fig. 3). The specificity of the assay was confirmed by the lack of significant binding of the tetramer to PMC isolated from mock-infected mice (Fig. 3A and D) and by the lack of significant binding of an irrelevant (influenza virus-specific) H-2Kd tetramer (see Materials and Methods) to PMC isolated from RSV-infected mice (data not shown). The population of tetramer-positive CD8+ cells was further characterized as CD3+ CD19− CD49−, which is the phenotype of T cells, and was viable based on a lack of staining with propidium iodide (data not shown).
FIG. 3.
Characterization of RSV-specific pulmonary MHC class I-restricted T cells following infection with wt RSV or RSV/IL-4 or mock infection, based on staining with antibodies to CD8 and an MHC class I tetramer loaded with an RSV peptide epitope. (A) Tetramer-positive CD8+ cells as a percentage of total PMC (in addition to the main panel, the data for days 4 and 5 are shown in a separate panel with a larger scale); (B) CD8+ cells as a percentage of total PMC; (C) tetramer-positive CD8+ cells as a percentage of total CD8+ cells. Each cell population is expressed as the mean of the percentage of total pulmonary mononuclear cells (PMC), with the standard error, based on four to six mice per group per day for wt RSV or RSV/IL-4 and one to three mice for the mock infection group. The samples were analyzed on days 4, 5, 7, 9, 12, and 63 after the primary infection or 6 days (day 33) following the challenge with wt RSV performed on day 27 (the mock control was included in the challenge). When the difference between the wt RSV and RSV/IL-4 groups is statistically significant (P < 0.05), the P value is indicated above the bars. The experiment was performed two times that resulted in similar data, and the results of a single representative experiment are shown. (D) Examples of primary flow cytometry data from individual mice on day 9 following primary infection with wt RSV or RSV/IL-4 or mock infection. Each cell population is expressed as the mean of the percentage of the total PMC. R1, tetramer-positive CD8+ cells; R2, tetramer-negative CD8+ cells.
We first measured the response to wt RSV infection and challenge. On days 4 and 5, a time when RSV-specific CTL activity typically is not evident (2; see below), we detected a population of tetramer-positive CD8+ cells that represented 0.3% (day 4) to 1.1% (day 5) of the total PMC population (Fig. 3A). This increased dramatically on day 7 postinfection, to a peak of 12% of the total PMC; remained elevated, although somewhat reduced in magnitude, on days 9 and 12; and was reduced to a value of <0.5% by day 63 (unchallenged animals). In the group that was challenged on day 27 and analyzed 6 days later, the percentage of tetramer-positive cells increased to a level that was much higher than the primary response of the original mock-infected group that was challenged in parallel primary infections (Fig. 3A). This demonstrated a rapid secondary MHC class I-restricted T-cell response, even under conditions where there was no detectable challenge virus replication. The kinetics of accumulation of the total population of CD8+ cells (Fig. 3B) generally paralleled the kinetics of the tetramer-positive cells. Thus, there was a large expansion of CD8+ cells in response to wt RSV infection and challenge, most of which were specific for this single epitope. Representative direct flow cytometry data for individual animals sacrificed on day 9 are shown in Fig. 3D.
For mice infected with RSV/IL-4, a higher percentage of PMC were positive for the tetramer and CD8 on day 4 and day 5 (1.8% versus 1.2% for wt RSV; P < 0.01) than for mice infected with wt RSV. This is also evident in Fig. 3C, which shows the level of tetramer-positive CD8+ cells as a percentage of the total CD8+ cells: on days 4 and 5, the population was increased by 108% (P < 0.01) and 69% (P < 0.001), respectively, in the RSV/IL-4 group compared to the wt RSV-infected group. However, by day 7 and thereafter, the response of tetramer-positive cells in RSV/IL-4-infected animals was less than that in wt RSV-infected animals (for example, 8% versus 12% on day 7; P < 0.001) (Fig. 3A; see Fig. 3D for direct data from individual representative animals). Thus, the increase in tetramer-positive CD8+ cells occurred more rapidly than that in the wt RSV group but reached a significantly lower peak. There was no increase in total CD8+ cells on days 4 and 5 in the RSV/IL-4 group compared to the wt RSV group (Fig. 3B). On the subsequent days, the total number of CD8+ cells increased in the RSV/IL-4 group but remained significantly lower than that for the wt RSV group. This is also evident by the direct flow cytometric data for individual mice on day 9 postinfection or post-mock infection (Fig. 3D), which showed that in mice infected with RSV/IL-4 and analyzed on day 9, the numbers of tetramer-positive CD8+ cells (population R1) and tetramer-negative CD8+ cells (R2) were reduced compared to those in wt RSV-infected animals. For the group that was challenged on day 27 and analyzed 6 days later, there was a very substantial secondary response of tetramer-positive CD8+ T cells, although it was slightly less than that in animals originally infected with wt RSV (Fig. 3A).
Pulmonary CD8+ cells secrete IFN-γ in response to RSV-specific stimulation.
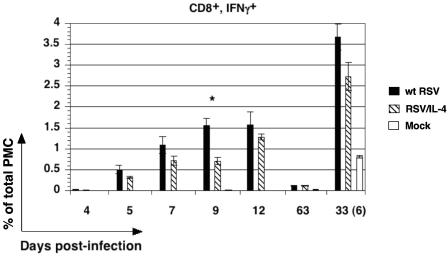
PMC were isolated from mice on days 4, 5, 7, 9, 12, and 63 following infection with wt RSV or RSV/IL-4 or mock infection and were stimulated overnight in vitro with the RSV peptide. We then quantified the number of CD8+ cells that secreted IFN-γ, expressed as a percentage of the total PMC (Fig. 4). For mice infected with wt RSV, CD8+ IFN-γ+ cells were detectable beginning on day 5, reached a maximum level on days 9 and 12, and by day 63 had diminished to only a very small fraction of the total PMC (about 0.1%). For animals that were challenged with wt RSV on day 27 and analyzed 6 days later, there was a large increase in the number of CD8+ IFN-γ+ cells that significantly exceeded that for the mock group that was challenged in parallel and represented a primary infection with wt RSV. For mice infected with RSV/IL-4, the number of CD8+ IFN-γ+ cells was reduced on all days compared to that for animals infected with wt RSV, although the difference was highly significant only on day 9 (more than twofold reduction; P < 0.01). Following the secondary infection with wt RSV, the number of CD8+ IFN-γ+ cells was also reduced in the RSV/IL-4-primed group, but the difference was not statistically significant. Following the peptide-specific in vitro stimulation described above, we also performed double staining for CD8 and IL-4 to analyze the possibility of the induction of CD8+ cells producing type 2 cytokines (Tc2 cells) following infection with RSV/IL-4. However, we did not detect IL-4+ cells in a total CD8+ population of pulmonary PMC on any day for either virus (data not shown).
FIG. 4.
Abundance of pulmonary CD8+ IFN-γ+ T cells on the indicated days following primary infection with wt RSV, RSV/IL-4, or placebo and on day 6 (day 33) following a challenge with wt RSV performed on day 27 (the mock control group was included in the challenge). The expression of CD8+ IFN-γ+ cells was made following specific stimulation in vitro using the RSV peptide epitope. Each cell population is expressed as the mean of the percentage of total PMC, with the standard error, based on four mice per group for wt RSV and RSV/IL-4 and one or two mice for mock infection. The total PMC were isolated, stimulated with the RSV-specific peptide as described in Materials and Methods, stained for CD8, permeabilized, stained for IFN-γ, and analyzed by flow cytometry. The data for day 9, when the difference between the wt RSV and RSV/IL-4 groups was statistically significant (P < 0.05), are indicated by a star. On days 4, 5, 7, and 12, the fraction of IFN-γ+ cells in the mock-infected group was very low and cannot be seen with this scale.
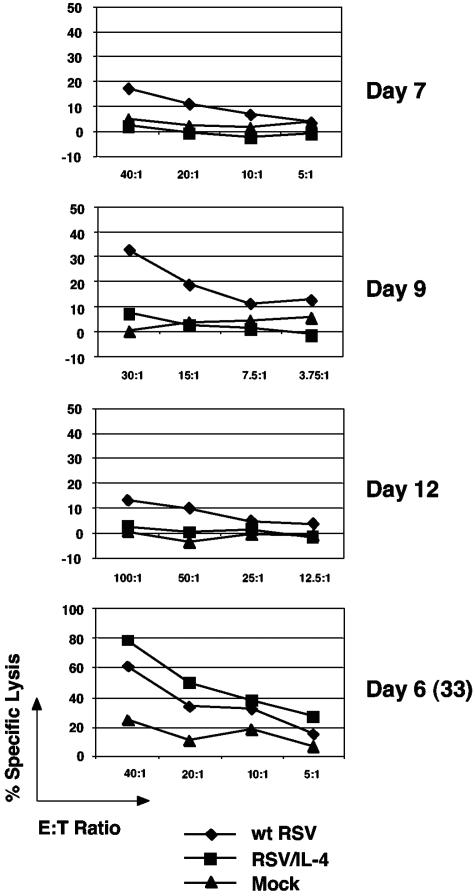
Cytolytic activity of pulmonary MHC class I-restricted CTL in vitro.
Mice were infected with wt RSV or RSV/IL-4 or mock infected and then were sacrificed on days 4, 5, 7, 9, 12, and 56 following the primary infection. On each day of sacrifice, freshly isolated total PMC (without in vitro stimulation) were tested for cytolytic activity against mouse P815 cells pulse labeled with the above-mentioned RSV-specific M2-1 peptide in a standard 51Cr release assay (Fig. 5). For cells from mice infected with wt RSV, cytolytic activity was not detected on days 4 and 5 (not shown), was detected on day 7, reached a maximum on day 9, was reduced by day 12 (Fig. 5), and was undetectable on day 56. Additional animals that had been infected or mock infected as described above were challenged with wt RSV on day 27, and cells were harvested and analyzed 6 days later (day 33). Cells from the wt RSV-infected group that had been challenged had a strong secondary cytolytic response that was severalfold higher than the primary response of the mock group that was challenged in parallel (Fig. 5). In contrast, little or no cytolytic response was detected in cells from RSV/IL-4-infected animals on any day following the primary infection. However, when additional animals were challenged with wt RSV on day 27 and cells were analyzed 6 days later, a high-level secondary cytolytic response was observed. Thus, RSV/IL-4 was associated with a greatly reduced, essentially negligible primary pulmonary CTL response, but the secondary response to a wt RSV challenge was largely intact.
FIG. 5.
In vitro RSV-specific, MHC class I-restricted cytolytic activity of primary PMC isolated on days 7, 9, and 12 after administration of wt RSV, RSV/IL-4, or placebo and 6 days (day 33) following the challenge on day 27 with wt RSV (the mock control was included in the challenge). 51Cr-labeled P815 cells that had been incubated with 1 μM of the RSV M2-1 epitope peptide were used as the target. PMC from four mice per group for wt RSV and RSV/IL-4 or from one or two mice for mock infection were pooled, and the cytolytic activity was determined by incubation with target cells followed by the quantitation of released 51Cr. The 51Cr release from target cells without peptide was subtracted from that with the peptide. E:T ratio, effector/target ratio.
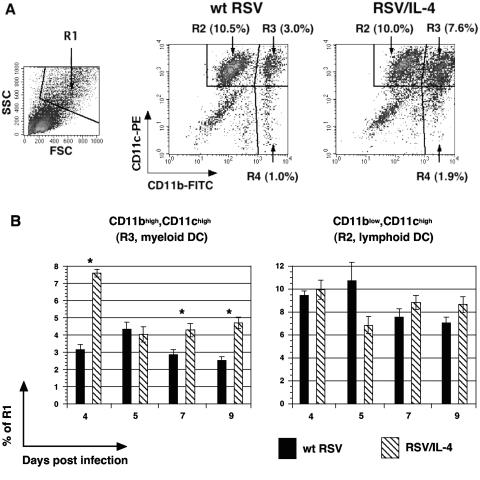
Abundance and activation of pulmonary dendritic cells.
IL-4 has been shown to influence the cytolytic activity and proliferation of CTL through its effects on dendritic cells (20). Therefore, it was of interest to investigate the accumulation and activation of pulmonary dendritic cells in mice infected with RSV/IL-4 compared to those in mice infected with wt RSV. Total PMC were isolated on various days postinfection and then stained for CD11b (Mac1) and CD11c, markers specific for dendritic cells. Cells were gated on the properties of high forward and side scatter (Fig. 6A, population R1) and were analyzed for the expression of CD11b and CD11c (Fig. 6A), as described previously (42). The exogenous expression of IL-4 resulted in a significant increase in the proportion of CD11bhigh CD11chigh cells (Fig. 6A, population R3), representing myeloid dendritic cells (42). The mean percentage of this cell population was elevated on most of the days analyzed, namely, days 4, 7, and 9, with the greatest increase (143%) observed on day 4 (Fig. 6B). In contrast, no difference was found in the mean percentages of CD11blow CD11chigh (R2) cells, representing mainly lymphoid dendritic cells (Fig. 6B), and CD11bhigh CD11clow cells, representing mainly macrophages (not shown) (42).
FIG. 6.
Flow cytometry of pulmonary myeloid and lymphoid dendritic cells in mice infected with RSV/IL-4 or wt RSV. (A) Example of primary data from individual mice on day 4 following infection with the indicated virus. The population with high forward scatter (FSC) and side scatter (SSC) was gated (designated R1, left panel) and analyzed for CD11b versus CD11c expression. The CD11blow CD11chigh (designated R2) and CD11bhigh CD11chigh (designated R3) cell populations represent pulmonary lymphoid and myeloid dendritic cells, respectively (42), and the CD11bhigh CD11clow population (R4) represents mostly macrophages (42). The percentage of the total PMC is indicated for each cell population. (B) Amounts of myeloid and lymphoid dendritic cells on days 4, 5, 7, and 9 following primary infection with wt RSV or RSV/IL-4, expressed as the mean percentages of the total PMC, with standard errors based on five or six mice per group. The days when the difference was statistically significant (P < 0.05) are indicated by stars.
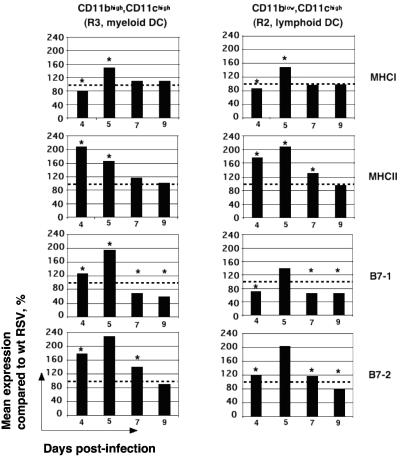
We evaluated the expression of MHC I, MHC II, B7-1, and B7-2, the main markers of dendritic cell activation, on lymphoid and myeloid dendritic cells. The results for mice infected with RSV/IL-4, normalized to the results for the wt RSV group (100%), are shown in Fig. 7. In general, the mean expression levels of the markers in the RSV/IL-4 group were increased early in the infection (days 4 and 5) and returned to or below the levels associated with wt RSV by days 7 and 9. In particular, there was a strong reduction in the level of expression of B7-1, which governs the acquisition of cytolytic activity by CTL (20), in both myeloid and lymphoid dendritic cells on days 7 and 9.
FIG. 7.
Expression of MHC I, MHC II, B7-1, and B7-2 by CD11blow CD11chigh (R2, lymphoid dendritic) cells and CD11bhigh CD11chigh (R3, myeloid dendritic) cells following infection with wt RSV or RSV/IL-4. Expression following infection with RSV/IL-4 is shown as a percentage of that for wt RSV (100%, indicated by dashed lines). When the difference between wt RSV and RSV/IL-4 is statistically significant (P < 0.05), the bars are indicated by a star.
DISCUSSION
In this study, primary pulmonary infections of mice with RSV/IL-4 were associated with an accelerated pulmonary inflammatory response compared to that in wt RSV-infected mice and with an MHC class I-restricted pulmonary T-cell response that remained largely nonfunctional throughout the infection. Interestingly, when these animals were challenged with wt RSV, the secondary CTL response was largely intact and functional, and the level of protective immunity and inflammation was the same as that for animals that had originally been infected with wt RSV. Thus, expression of IL-4 from recombinant RSV greatly perturbed the primary but not the secondary CTL and inflammatory responses.
IL-4 is produced predominantly by stimulated Th2 lymphocytes and has wide-ranging immunomodulatory effects. It was originally described as a B-cell growth factor (16). It up-regulates Th2 responses, down-regulates Th1 responses (39), and affects monocytes, macrophages, NK cells, CTL, and other cell types (reviewed in reference 26). The effect of IL-4 on the CTL response has been of particular interest with regard to viral infection and viral vaccines because CTL are important antiviral effectors, can contribute to regulating the immune response, and may be important for long-term immunity (17, 38). Several studies have shown that an increased expression of IL-4 during a viral infection suppresses the virus-specific CTL response. For example, mice infected with a recombinant vaccinia virus that had been engineered to express IL-4 exhibited a reduction in the number of virus-specific CTL (37). The expression of murine IL-4 by a recombinant ectromelia (mousepox) virus suppressed the CTL response in infected mice and dramatically increased the pathogenicity of the virus (18). The expression of murine IL-4 by a recombinant vaccinia virus that had been engineered to express the M2 protein of RSV was associated with a reduced CTL number and cytolytic activity against the M2 protein (3). Systemic overproduction of IL-4 by transgenic mice was associated with a delayed clearance of RSV infection and suppression of the pulmonary CTL response (14). Conversely, however, the expression of murine IL-4 by a vaccinia virus coexpressing the RSV F protein did not affect the cytolytic activity of memory T cells in infected mice (6), and the increased expression of Th2 cytokines in response to a recombinant vaccinia virus expressing a secreted form of the RSV F protein also did not interfere with the development of a CTL response (5). Moreover, IL-4 has been shown to be a growth factor for CTL (19, 35, 36) and does not inhibit the induction of CTL activity in immune spleen cells in vitro (I. M. Belyakov and J. A. Berzofsky, unpublished data). Thus, observations on the effect of IL-4 on the CTL response to viral infections have been inconsistent and difficult to relate to pulmonary RSV infections, perhaps reflecting experimental variables such as the use of vectored antigens rather than live RSV and the use of in vitro conditions and nonpulmonary sites of immunization.
In the present study, we observed a combination of effects of IL-4 on pulmonary CTL during primary infection. First, infection with RSV/IL-4 was associated with a modest but significant and reproducible acceleration in the increase in tetramer-positive T cells on days 4 and 5. However, on subsequent days (7, 9, and 12), the abundance of tetramer-positive cells dropped to below that of the wt RSV group. At all times postinfection, CTL from animals infected with RSV/IL-4 lacked in vitro cytolytic activity. Thus, proliferation appeared to be enhanced during the first few days after infection but not thereafter, and the activation of cytolytic activity was strongly suppressed throughout.
Dendritic cells play a role in mediating the effects of IL-4 on CTL (22). For example, the treatment of antigen-loaded dendritic cells with IL-4 augmented the proliferation of CTL but suppressed the development of cytolytic activity (22). These effects were associated with an increased expression of B7-2 and a decreased expression of B7-1 on dendritic cells (20). In the present study, we observed a modest enhancement of CTL proliferation in RSV/IL-4-infected animals that occurred on days 4 and 5 and was associated with a transient increase in the expression of B7-2 on dendritic cells. It is also possible that this modest increase in tetramer-positive T cells was mediated directly by IL-4, since IL-4 has been shown to be a growth factor for CTL (19, 35, 36). In any event, any apparent proliferative effects associated with the ectopic expression of IL-4 were modest and limited to the first few days. The more striking effect was the complete suppression of CTL cytolytic activity that, consistent with the results of a previous study (20), was associated with a decrease in the expression of B7-1 by dendritic cells on most, but not all, of the days examined.
Previously, the infection of mice with a recombinant ectromelia (mousepox) virus expressing IL-4 was associated with a strong suppression of the CTL response and a high mortality rate compared to infection with the wild-type virus, even in previously infected animals (18). In the present study, the infection of mice with RSV/IL-4 also strongly suppressed the CTL response but, remarkably, did not result in delayed clearance of the virus. The latter finding differs from the results for wt RSV infections of transgenic mice engineered to overexpress IL-4, for which delayed clearance was observed (14). That might reflect additional changes in the transgenic animals in response to the constitutive overexpression of IL-4, including the suppression of mechanisms other than CTL that control viral clearance, such as NK cells. CTL are known to play an important role in controlling and clearing RSV infections in BALB/c mice, as previously demonstrated by depletion studies (15). The finding in the present study that RSV was cleared efficiently in the apparent absence of functional CTL (at least as measured by in vitro cell killing) suggests that some other mechanism might be responsible for clearing the virus or, alternatively, there might have been a low level of CTL cytolytic activity that was not apparent in vitro but might have been sufficient to restrict the virus in vivo.
Animals infected with RSV/IL-4 had an accelerated pulmonary influx of inflammatory cells in the first few days postinfection compared to wt RSV-infected animals, but this difference disappeared by 8 days postinfection. At this later time point, there was no difference between groups with regard to the peak magnitudes, irrespective of the day, for the four histopathology indicators that were measured. Following the challenge with wt RSV, there was no significant difference in pulmonary histopathology between animals that had previously received RSV/IL-4 and those that had received wt RSV. A high level of histopathology had also been noted previously for cotton rats that had been infected and subsequently challenged with wt RSV (32). Thus, for both species of rodents, the virus challenge was associated with an extensive inflammatory response that was highly protective such that challenge virus replication could not be detected. Previously, interstitial pneumonitis and alveolitis (scored by the same laboratory and scoring system as that used for the present study) were found to be the primary indicators of disease enhancement associated with formalin-inactivated RSV in the cotton rat model (31). In the present study with mice, the indicators that were the most prominent during primary and secondary infections of mice were peribronchiolitis and perivascularitis. With the exceptions of day 4 following primary infection and intestinal pneumonitis on day 8 after secondary infection, there was no significant difference between the RSV/IL-4 and wt RSV groups. Since disease enhancement is not associated with wt RSV primary infection and reinfection, the observed histopathology appeared to reflect a protective rather than disease-enhancing response (31). The one caveat is that both the RSV/IL-4 and wt RSV groups exhibited a very high level of resistance to the wt RSV challenge, and a previous study showed that a strong protective effect can obscure an adverse reaction to an RSV vaccine (28). This would need to be studied under conditions where the challenge is delayed to allow immunity to diminish.
It is noteworthy that the perturbations associated with RSV/IL-4 were largely confined to the primary infection and, for the most part, were minimal following the challenge with wt RSV. For example, the secondary CTL response was at least as great in magnitude as that for the group that originally received wt RSV, the level of IFN-γ activation was nearly as great, and the in vitro cytolytic activity and lung histopathology were largely indistinguishable between the groups that had received RSV/IL-4 and wt RSV. This showed that a live intranasal RSV vaccine that is administered under the adverse conditions of a strong Th2 environment and an aberrant CTL response nonetheless provides a nearly normal secondary response. This might be due to the substantial expression of IFN-γ despite the increased expression of IL-4, perhaps due to the live, replicating nature of the vaccine. This is reassuring with regard to the potential safety and efficacy of a live intranasal vaccine for infants, given that the very young immune system is known to have a reduced cellular response (10) and has been described as being biased toward Th2 responses (33).
Acknowledgments
We thank Lijuan Yang and Elaine Lamirande for their technical assistance and David Stephany of the NIAID Flow Cytometry Section for his skilled assistance and advice. We are grateful to Brian Murphy for a critical reading of the manuscript.
REFERENCES
- 1.Alwan, W. H., W. J. Kozlowska, and P. J. Openshaw. 1994. Distinct types of lung disease caused by functional subsets of antiviral T cells. J. Exp. Med. 179:81-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, J. J., J. Norden, D. Saunders, G. L. Toms, and R. Scott. 1990. Analysis of the local and systemic immune responses induced in BALB/c mice by experimental respiratory syncytial virus infection. J. Gen. Virol. 71:1561-1570. [DOI] [PubMed] [Google Scholar]
- 3.Aung, S., Y. W. Tang, and B. S. Graham. 1999. Interleukin-4 diminishes CD8(+) respiratory syncytial virus-specific cytotoxic T-lymphocyte activity in vivo. J. Virol. 73:8944-8949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belyakov, I. M., J. Wang, R. Koka, J. D. Ahlers, J. T. Snyder, R. Tse, J. Cox, J. S. Gibbs, D. H. Margulies, and J. A. Berzofsky. 2001. Activating CTL precursors to reveal CTL function without skewing the repertoire by in vitro expansion. Eur. J. Immunol. 31:3557-3566. [DOI] [PubMed] [Google Scholar]
- 5.Bembridge, G. P., J. A. Lopez, R. Bustos, J. A. Melero, R. Cook, H. Mason, and G. Taylor. 1999. Priming with a secreted form of the fusion protein of respiratory syncytial virus (RSV) promotes interleukin-4 (IL-4) and IL-5 production but not pulmonary eosinophilia following RSV challenge. J. Virol. 73:10086-10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bembridge, G. P., J. A. Lopez, R. Cook, J. A. Melero, and G. Taylor. 1998. Recombinant vaccinia virus coexpressing the F protein of respiratory syncytial virus (RSV) and interleukin-4 (IL-4) does not inhibit the development of RSV-specific memory cytotoxic T lymphocytes, whereas priming is diminished in the presence of high levels of IL-2 or gamma interferon. J. Virol. 72:4080-4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bukreyev, A., I. M. Belyakov, J. A. Berzofsky, B. R. Murphy, and P. L. Collins. 2001. Granulocyte-macrophage colony-stimulating factor expressed by recombinant respiratory syncytial virus attenuates viral replication and increases the level of pulmonary antigen-presenting cells. J. Virol. 75:12128-12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bukreyev, A., E. Camargo, and P. L. Collins. 1996. Recovery of infectious respiratory syncytial virus expressing an additional, foreign gene. J. Virol. 70:6634-6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang, J., and T. J. Braciale. 2002. Respiratory syncytial virus infection suppresses lung CD8+ T-cell effector activity and peripheral CD8+ T-cell memory in the respiratory tract. Nat. Med. 8:54-60. [DOI] [PubMed] [Google Scholar]
- 10.Chiba, Y., Y. Higashidate, K. Suga, K. Honjo, H. Tsutsumi, and P. L. Ogra. 1989. Development of cell-mediated cytotoxic immunity to respiratory syncytial virus in human infants following naturally acquired infection. J. Med. Virol. 28:133-139. [DOI] [PubMed] [Google Scholar]
- 11.Collins, P. L., R. M. Chanock, and B. R. Murphy. 2001. Respiratory syncytial virus, p. 1443-1485. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 1. Lippincott-Raven Publishers, Philadelphia, Pa.
- 12.Collins, P. L., M. G. Hill, E. Camargo, H. Grosfeld, R. M. Chanock, and B. R. Murphy. 1995. Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5′ proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. Proc. Natl. Acad. Sci. USA 92:11563-11567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connors, M., N. A. Giese, A. B. Kulkarni, C. Y. Firestone, H. C. Morse III, and B. R. Murphy. 1994. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J. Virol. 68:5321-5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer, J. E., J. E. Johnson, R. K. Kuli-Zade, T. R. Johnson, S. Aung, R. A. Parker, and B. S. Graham. 1997. Overexpression of interleukin-4 delays virus clearance in mice infected with respiratory syncytial virus. J. Virol. 71:8672-8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham, B. S., L. A. Bunton, P. F. Wright, and D. T. Karzon. 1991. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J. Clin. Investig. 88:1026-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howard, M., J. Farrar, M. Hilfiker, B. Johnson, K. Takatsu, T. Hamaoka, and W. E. Paul. 1982. Identification of a T cell-derived B cell growth factor distinct from interleukin 2. J. Exp. Med. 155:914-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hussell, T., C. J. Baldwin, A. O'Garra, and P. J. Openshaw. 1997. CD8+ T cells control Th2-driven pathology during pulmonary respiratory syncytial virus infection. Eur. J. Immunol. 27:3341-3349. [DOI] [PubMed] [Google Scholar]
- 18.Jackson, R. J., A. J. Ramsay, C. D. Christensen, S. Beaton, D. F. Hall, and I. A. Ramshaw. 2001. Expression of mouse interleukin-4 by a recombinant ectromelia virus suppresses cytolytic lymphocyte responses and overcomes genetic resistance to mousepox. J. Virol. 75:1205-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawakami, Y., S. A. Rosenberg, and M. T. Lotze. 1988. Interleukin 4 promotes the growth of tumor-infiltrating lymphocytes cytotoxic for human autologous melanoma. J. Exp. Med. 168:2183-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King, C., R. Mueller Hoenger, M. Malo Cleary, K. Murali-Krishna, R. Ahmed, E. King, and N. Sarvetnick. 2001. Interleukin-4 acts at the locus of the antigen-presenting dendritic cell to counter-regulate cytotoxic CD8+ T-cell responses. Nat. Med. 7:206-214. [DOI] [PubMed] [Google Scholar]
- 21.Kulkarni, A. B., P. L. Collins, I. Bacik, J. W. Yewdell, J. R. Bennink, J. E. Crowe, Jr., and B. R. Murphy. 1995. Cytotoxic T cells specific for a single peptide on the M2 protein of respiratory syncytial virus are the sole mediators of resistance induced by immunization with M2 encoded by a recombinant vaccinia virus. J. Virol. 69:1261-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulkarni, A. B., M. Connors, C. Y. Firestone, H. C. Morse III, and B. R. Murphy. 1993. The cytolytic activity of pulmonary CD8+ lymphocytes, induced by infection with a vaccinia virus recombinant expressing the M2 protein of respiratory syncytial virus (RSV), correlates with resistance to RSV infection in mice. J. Virol. 67:1044-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee, F., T. Yokota, T. Otsuka, P. Meyerson, D. Villaret, R. Coffman, T. Mosmann, D. Rennick, N. Roehm, C. Smith, et al. 1986. Isolation and characterization of a mouse interleukin cDNA clone that expresses B-cell stimulatory factor 1 activities and T-cell- and mast-cell-stimulating activities. Proc. Natl. Acad. Sci. USA 83:2061-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leonard, W. J. 2003. Type 1 cytokines and interferons and their receptors, p. 701-747. In W. E. Paul (ed.), Fundamental immunology, 5th ed., vol. 1. Lippincott-Raven Publishers, Philadelphia, Pa.
- 25.Loza, M. J., and B. Perussia. 2001. Final steps of natural killer cell maturation: a model for type 1-type 2 differentiation? Nat. Immunol. 2:917-924. [DOI] [PubMed] [Google Scholar]
- 26.Mire-Sluis, A. 1998. Interleukin-4, p. 53-68. In A. R. Mire-Sluis and R. Thorpe (ed.), Cytokines. Academic Press, San Diego, Calif.
- 27.Murphy, B. R., and P. L. Collins. 2002. Live-attenuated virus vaccines for respiratory syncytial and parainfluenza viruses: applications of reverse genetics. J. Clin. Investig. 110:21-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy, B. R., A. V. Sotnikov, L. A. Lawrence, S. M. Banks, and G. A. Prince. 1990. Enhanced pulmonary histopathology is observed in cotton rats immunized with formalin-inactivated respiratory syncytial virus (RSV) or purified F glycoprotein and challenged with RSV 3-6 months after immunization. Vaccine 8:497-502. [DOI] [PubMed] [Google Scholar]
- 29.Noma, Y., P. Sideras, T. Naito, S. Bergstedt-Lindquist, C. Azuma, E. Severinson, T. Tanabe, T. Kinashi, F. Matsuda, Y. Yaoita, et al. 1986. Cloning of cDNA encoding the murine IgG1 induction factor by a novel strategy using SP6 promoter. Nature 319:640-646. [DOI] [PubMed] [Google Scholar]
- 30.Polack, F. P., P. G. Auwaerter, S. H. Lee, H. C. Nousari, A. Valsamakis, K. M. Leiferman, A. Diwan, R. J. Adams, and D. E. Griffin. 1999. Production of atypical measles in rhesus macaques: evidence for disease mediated by immune complex formation and eosinophils in the presence of fusion-inhibiting antibody. Nat. Med. 5:629-634. [DOI] [PubMed] [Google Scholar]
- 31.Prince, G. A., S. J. Curtis, K. C. Yim, and D. D. Porter. 2001. Vaccine-enhanced respiratory syncytial virus disease in cotton rats following immunization with Lot 100 or a newly prepared reference vaccine. J. Gen. Virol. 82:2881-2888. [DOI] [PubMed] [Google Scholar]
- 32.Prince, G. A., J. P. Prieels, M. Slaoui, and D. D. Porter. 1999. Pulmonary lesions in primary respiratory syncytial virus infection, reinfection, and vaccine-enhanced disease in the cotton rat (Sigmodon hispidus). Lab. Investig. 79:1385-1392. [PubMed] [Google Scholar]
- 33.Roman, M., W. J. Calhoun, K. L. Hinton, L. F. Avendano, V. Simon, A. M. Escobar, A. Gaggero, and P. V. Diaz. 1997. Respiratory syncytial virus infection in infants is associated with predominant Th-2-like response. Am. J. Respir. Crit. Care Med. 156:190-195. [DOI] [PubMed] [Google Scholar]
- 34.Sad, S., R. Marcotte, and T. R. Mosmann. 1995. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity 2:271-279. [DOI] [PubMed] [Google Scholar]
- 35.Schuler, T., T. Kammertoens, S. Preiss, P. Debs, N. Noben-Trauth, and T. Blankenstein. 2001. Generation of tumor-associated cytotoxic T lymphocytes requires interleukin 4 from CD8(+) T cells. J. Exp. Med. 194:1767-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuler, T., Z. Qin, S. Ibe, N. Noben-Trauth, and T. Blankenstein. 1999. T helper cell type 1-associated and cytotoxic T lymphocyte-mediated tumor immunity is impaired in interleukin 4-deficient mice. J. Exp. Med. 189:803-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma, D. P., A. J. Ramsay, D. J. Maguire, M. S. Rolph, and I. A. Ramshaw. 1996. Interleukin-4 mediates down regulation of antiviral cytokine expression and cytotoxic T-lymphocyte responses and exacerbates vaccinia virus infection in vivo. J. Virol. 70:7103-7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srikiatkhachorn, A., and T. J. Braciale. 1997. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J. Exp. Med. 186:421-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swain, S. L., A. D. Weinberg, M. English, and G. Huston. 1990. IL-4 directs the development of Th2-like helper effectors. J. Immunol. 145:3796-3806. [PubMed] [Google Scholar]
- 40.Tang, Y. W., and B. S. Graham. 1994. Anti-IL-4 treatment at immunization modulates cytokine expression, reduces illness, and increases cytotoxic T lymphocyte activity in mice challenged with respiratory syncytial virus. J. Clin. Investig. 94:1953-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang, Y. W., and B. S. Graham. 1995. Interleukin-12 treatment during immunization elicits a T helper cell type 1-like immune response in mice challenged with respiratory syncytial virus and improves vaccine immunogenicity. J. Infect. Dis. 172:734-738. [DOI] [PubMed] [Google Scholar]
- 42.Wang, J., D. P. Snider, B. R. Hewlett, N. W. Lukacs, J. Gauldie, H. Liang, and Z. Xing. 2000. Transgenic expression of granulocyte-macrophage colony-stimulating factor induces the differentiation and activation of a novel dendritic cell population in the lung. Blood 95:2337-2345. [PubMed] [Google Scholar]