Abstract
Cytomegalovirus (CMV) infection is the most common opportunistic infection of the central nervous system in patients with human immunodeficiency virus or AIDS or on immunosuppressive drug therapy. Despite medical management, infection may be refractory to treatment and continues to cause significant morbidity and mortality. We investigated adoptive transfer as an approach to treat and prevent neurotropic CMV infection in an adult immunodeficient mouse model. SCID mice were challenged with intracranial murine CMV (MCMV) and reconstituted with MCMV- or vesicular stomatitis virus (VSV)-sensitized splenocytes, T cells, or T-cell subsets. T cells labeled with vital dye or that constitutively generated green fluorescent protein (GFP) were identified in the brain as early as 3 days following peripheral transfer. Regardless of specificity, activated T cells localized to regions of the brain containing CMV, however, only those specific for CMV were effective at clearing virus. Reconstitution with unsorted MCMV-immune splenocytes, enriched T-cell fractions, or CD4+ cells significantly reduced virus levels in the brain within 7 days and also prevented clinical disease, in significant contrast with mice given VSV-immune unsorted splenocytes, MCMV-immune CD8+ T cells, and SCID control mice. Results suggest CMV-immune T cells (particularly CD4+) rapidly cross the blood-brain barrier, congregate at sites of specific CMV infection, and functionally eliminate acute CMV within the brain. In addition, when CMV-immune splenocytes were administered prior to a peripheral CMV challenge, CMV entry into the immunocompromised brain was prevented. Systemic adoptive transfer may be a rapid and effective approach to preventing CMV entrance into the brain and for reducing neurotropic infection.
Cytomegalovirus (CMV) infection is a major cause of morbidity and mortality among neonates and adults immunosuppressed due to human immunodeficiency virus (HIV) or AIDS or immunosuppressive therapy. CMV infection, which is normally asymptomatic in immunocompetent individuals, is highly prevalent throughout the world, with a seropositivity rate of 50 to 90% (1, 37, 43). Infection is usually acquired early in life, with up to 80% of children aged 12 to 18 months actively shedding virus (1, 21). Virtually all organ systems can be affected, leading to mononucleosis, severe respiratory infection, liver and kidney damage, intestinal disease, and central nervous system (CNS) damage. In a healthy population CMV dissemination to the CNS is uncommon; however, as the population of immunosuppressed adults has continued to rise, so has the incidence of CMV infection of the brain (21, 67).
CMV is the most common opportunistic viral pathogen in AIDS patients, infecting more than 90% and contributing to disease and death in 50 to 70% of infected patients, despite medical management. CMV frequently disseminates to the CNS in late stages of HIV infection, when the CD4+ T-cell count is low (16). CMV infection of mature CNS may result in retinitis, encephalitis, myeloradiculitis, subcortical dementia, obtundation, and other significant deficits (1, 3, 8, 21, 39, 40, 58, 64, 67). Following the introduction of highly active antiretroviral therapy, there has been a reduction of peripheral CMV infection; however, treatment has limitations that warrants alternative therapies (21, 65). Highly active antiretroviral therapy may be associated with potential serious side effects (further immune suppression, liver damage, and gastrointestinal maladies), failure to eliminate latency, development of drug resistance, inability to achieve therapeutic levels of drug in target organs like the brain, patient compliance, and cost issues may limit effectiveness (2). The understanding of immune responses to CMV infection in the CNS remains unclear, and new approaches to treatment of CNS disease are needed.
Adoptive immune reconstitution is a promising treatment alternative for persistent CMV infection. Murine models provide evidence for reconstituted immune T-cell protection against CMV disease in the lung, spleen, and eye (9, 25, 51, 60). In human trials, lymphocytes primed against CMV have been harvested from healthy individuals and transfused into immunosuppressed patients to reduce peripheral illness (47, 56, 66). These studies support adoptive immune therapy as a means of preventing or alleviating existing infection in peripheral organs; however, diversity in organ clearance mechanisms remains a factor (24, 44, 48). In terms of immunity against viral infection, the brain is considered a unique organ, with different and sometimes absent expression of the major histocompatibility complex (MHC) cell surface molecules that normally bind and present antigenic peptides on the surfaces of cells for recognition (binding) by the antigen-specific T-cell receptors of lymphocytes.
Entry of some cells of the systemic immune system into the CNS is impeded by a protective blood-brain barrier. Resident cells, including microglia and astrocytes, may act locally at a site of infection and may play a primary role in regulation of acute inflammation. These factors may significantly alter molecular signaling responses to viral infection in the brain compared to peripheral organs and distort the ability of a systemic immune response to adequately control disease. In an immunosuppressed individual, it is not known whether systemic transfer of active lymphocytes can effectively enter the brain in response to a CMV infection or whether transferred lymphocytes can alleviate acute neurotropic CMV disease.
Studies of human CMV in vivo are limited because of species specificity, but animal models using murine CMV (MCMV) are parallel to many aspects of human CNS infection. MCMV behaves similar to human CMV by entering the brain only in immunodeficient individuals, infecting multiple cell types, causing pathology, and the absence of functional immunity, progressing to lethality (54). We employed MCMV as a paradigm in studies aimed at developing treatment against the virus. Using an immunodeficient mouse model of neurotropic CMV infection, we tested whether transferred MCMV-immune lymphocytes were able to penetrate, target, and eliminate CMV infection in the CNS and whether adoptive transfer of immune cells might also prevent CMV invasion of the brain if administered prior to CMV inoculation.
MATERIALS AND METHODS
Mice.
Six-week-old female immunodeficient C.B-17/Icr-Prkdc-scid/Crl-BR (SCID) and congenic immunocompetent BALB/cAnNCrl-BR (donor) mice were obtained from Charles River Laboratories (Wilmington, MA) and acclimated 3 to 5 days before challenge. The SCID mice carry the immunoglobulin heavy-chain allele (Igh-1b) from a C57BL/Ka strain on a BALB/c background, and both donor and SCID mice have similar H-2 haplotypes (H-2d), allowing for acceptance of cell transfer from donors without rejection. Lymphocyte tracking studies used transgenic Swiss Webster mice carrying the enhanced green fluorescent protein (EGFP) gene TgN(beta-act-EGFP), expressing green fluorescent protein (GFP) (gift from M Okabe, Osaka University, Osaka, Japan) (41). These mice were crossed 15 times onto a Swiss Webster background.
All mice were free of murine viruses (including MCMV), pathogenic bacteria, and endo- and ectoparasites. Control and experimental groups were housed separately in static microisolator cages on corncob bedding, and all animal manipulations were performed in a class IIA biological safety cabinet using standard microisolation techniques. Animals were housed at a temperature of 22 to 24°C and humidity of 40 to 60% and a 12-h:12-h light-dark cycle. All procedures were approved by the Yale University Institutional Animal Care and Use Committee.
Virus and immune priming.
Recombinant MCMV-GFP (strain K181 MC.55, ie2− GFP+) strongly and rapidly expresses green fluorescent protein under control of the human elongation factor 1a promoter inserted at the immediate-early gene 2 (IE2) site (63). Expression of GFP enables the direct detection of MCMV in living and fixed, unstained tissue. MCMV-GFP and K181 wild-type virus stocks were minimally passaged in 3T3 cells (American Type Culture Collection, Manassas, VA). Cells were harvested, semipurified, titered using plaque assays, and stored in aliquots at −70°C. Recombinant vesicular stomatitis virus (VSV) was grown and titered as previously described for recombinant VSV-HA (55, 57). Plaque-purified recombinant VSV was grown and titered on baby hamster kidney (BHK-21, American Type Culture Collection) cells. Viruses were thawed and diluted with Dulbecco's modified Eagle's medium to appropriate titers immediately prior to inoculation.
To generate CMV-activated immune cells, donor mice were inoculated with MCMV-GFP (106 PFU), wild-type MCMV (4.83 × 105 PFU), or VSV (3.25 × 105 PFU) intraperitoneally and assessed daily for clinical illness. Eight days following priming, spleens were excised into cold, sterile calcium- and magnesium-free PBS (CMF-PBS). Spleens were transferred aseptically into glass tissue grinders containing ice-cold CMF-PBS and disrupted, and suspensions were collected. Cells were washed and resuspended, and an aliquot was taken for counting and trypan blue exclusion (>98% viability). For control purposes, aliquots of isolated splenocytes were cultured for 5 days with either 3T3 or BHK cells to verify lack of replicating virus. CMV-immune and VSV-immune cell suspensions were distributed for fluorescence-activated cell sorting (FACS), cytokine expression analysis, panning for T-cell enrichment, PKH labeling, and adoptive reconstitution.
Cytokine analysis.
Unsorted, purified CD4+ and CD8+ T-cell donor splenocytes were cultured in RPMI medium containung 10% fetal bovine serum, penicillin, streptomycin, and l-glutamine and with either 10 μg/ml phorbol myristate acetate/ionomycin (400 ng/ml) or 0.2 to 0.5 μg/μl UV-inactivated viral antigen (MCMV or VSV). UV inactivation of virus was accomplished with two exposures to 5,000 s 100 μJ, rotating 90° between treatments. Inactivation was confirmed in cell culture by lack of cytopathic effects. Cell culture supernatant was assayed after 24, 48, and 72 h in triplicate for cytokines tumor necrosis factor alpha (TNF-α), interleukin-4 (IL-4), and gamma interferon (IFN-γ) using OptEIA enzyme-linked immunosorbent assay (ELISA) kits as directed (BD Biosciences, San Diego, CA). T-cell-depleted, irradiated syngeneic feeder cells were used to provide antigen presentation to purified T-cell subsets.
Stimulated lymphocytes were collected and analyzed by FACS. Lymphocytes (5 × 105) were incubated with staining buffer (PBS containing 3% fetal bovine serum and 0.2% NaN3) and monoclonal antibody for specific cell types for 30 min at 4°C in V-bottomed 96-well plates. Cells were washed with staining buffer and fetal bovine serum, and then fluorescein isothiocyanate-labeled rat anti-mouse immunoglobulin G F(ab)′2 was diluted 1:50 and added for 30 min at 4°C. Monoclonal antibodies to detect the following mouse cell subtypes were purchased from PharMingen (BD PharMingen): T cells: Thy-1.2 for all T cells, CD4+CD8+ (17A2), CD4 (RM4 to 5), CD8a (5H10-1); B cells: CD45R (RA3-6B2) or CD19 (ID3). Cells were washed, fixed with 2% paraformaldehyde, and stored at 4°C until analyzed. Fluorescence was observed with a Becton-Dickinson Analyzer I.
Cell sorting and isolation.
T-cell enrichment of splenocytes was conducted by negative selection using a modified panning technique (68). One day before selection, 100-mm petri dishes (BD Falcon) were coated with 10 μg/ml goat anti-mouse immunoglobulin G (heavy and light chain) (Jackson Immuno Research, West Grove, PA) in CMF-PBS. Plates were swirled and incubated overnight at 4°C; 2 × 109 MCMV-immunized splenocytes were resuspended in 5% fetal bovine serum-RPMI, incubated in 50-ml aliquots in a T75 tissue culture flask (BD Falcon) for 1 h at 37°C to remove accessory cells by plastic adherence. Nonadherent cells were collected, washed, resuspended to 8 × 106/ml, and added to immunoglobulin G-coated dishes for positive removal of B lymphocytes. The cells were incubated 70 min at 4°C and swirled after 30 min. Nonadherent cells were collected, washed, counted, and adjusted to 1.5 × 108 cells/ml. Confirmation of sorted cells was obtained by flow cytometry.
CD4+ and CD8+ T cells were isolated by negative selection with R&D Systems Magcellect specific T-cell isolation kits (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. Isolated splenocytes depleted of erythrocytes were incubated with biotinylated antibodies from the selected kit for 15 min on ice followed by incubation with streptavidin-coated microbeads. Volume was adjusted with buffer, and the reaction tube was placed in the MagCellect magnet for 8 to 10 min at room temperature. Supernatant was separated from magnetically retained cells. The purity of cell fractions pre- and postselection was analyzed by flow cytometry using a FACScalibur (BD Immunocytometry Systems, San Jose, CA). Fluorescein-conjugated antibodies were purchased from BD PharMingen, San Diego, CA (CD45R/B220-fluorescein isothiocyanate and CD45-phycoerythrin) and CALTAG Laboratories, Burlingame, CA (rat anti-mouse CD4-allophycocyanin and CD8a-peridinin chlorophyll protein).
Tracking of reconstituted lymphocytes.
Using the PKH26 red fluorescent cell linker kit (Sigma, St. Louis, MO) 108 VSV- or MCMV-immune splenocytes were resuspended in diluent (107 cells/ml). Cell suspensions were rapidly added to a 2x concentration of PKH (4 × 10−6 M) and incubated for 3 min at room temperature. Labeling was stopped with 10 ml fetal bovine serum (HyClone, Logan, Utah) per 10 ml cell-PKH suspension. Cells were washed and labeling was confirmed by fluorescent microscopy with a Zeiss Axioskop (Germany).
To confirm results with the cell dye, an additional experiment used splenocytes harvested from donor Swiss Webster mice that constitutively express GFP. GFP expression in splenocytes was examined using several methods, including cell culture, fluorescent histological examination, and FACS analysis. Reconstituted cells in SCID mice were identified in cryosectioned tissues (spleen, liver, and brain) using fluorescent microscopy.
Adoptive reconstitution.
All immune cell reconstitutions were given intravenously through the tail vein. Control mice were given medium only. SCID mice were given 108 unsorted CMV-immune splenocytes, 108 unsorted VSV-immune splenocytes, 2 × 107 CMV-immune enriched T cells, 5 × 106 to 1 × 107 CMV-immune CD8+ T cells, or 107 CMV-immune CD4+ T cells. Additional mice were given 108 PHK26-labeled or Swiss Webster GFP-labeled splenocytes (either VSV-immune or CMV-immune). Adoptive transfer was performed from 3 days prior to 1 day following viral challenge. Reconstitution was confirmed at necropsy using flow cytometry of splenic homogenate.
MCMV challenge.
For acute neurotropic challenge, mice were anesthetized with subcutaneous ketamine (100 mg/kg) and xylazine (10 mg/kg) and inoculated intracranially with 1.0 μl MCMV-GFP (4.4 × 105 PFU) or wild-type MCMV (3.22 × 103 PFU) injected in the left cerebrum 1 to 2 mm lateral to the midline, 3 to 4 mm caudal to the orbit through a 25-gauge burr hole using a 1-μl Hamilton syringe. Mice were assessed daily for clinical illness, and any moribund mouse was humanely euthanized. Mice were euthanized with carbon dioxide gas between 3 and 7 days following challenge; target tissues were snap frozen in liquid nitrogen for subsequent analysis or processed for flow cytometry. Some mice were immediately perfused transcardially with heparin-saline followed by 4% paraformaldehyde in phosphate buffer for cryosectioning.
An additional experiment was designed to measure the ability of adoptive reconstitution to prevent dissemination of peripheral MCMV to the CNS and to test whether transfer of CMV-immune cells was requisite for protection. Three days following reconstitution with either unsorted naïve or MCMV-immune splenocytes, SCID mice were challenged with 4.4 × 105PFU MCMV by injection into the lateral tail vein. Mice were assessed daily for adverse clinical signs and virus quantification was performed after 28 days. Serum MCMV antibody levels were assessed using indirect fluorescent antibody staining using previously described polyclonal anti-MCMV sera (54).
Quantification of virus.
MCMV in the brain was titered using either plaque assay or real-time PCR. The relationship and agreement between the two assays was determined using correlation analysis. The group means were compared using a two-sample Student's t test. Tissues were collected and snap frozen in liquid nitrogen. A 10% wt/vol homogenate was made in Dulbecco's modified Eagle's medium and diluted aliquots were incubated on 3T3 cells for 1 h at 37°C on a rocker. The suspension was removed and cells were overlaid with 0.95% SeaKem agar in 2x growth medium (45% Dulbecco's modified Eagle's medium, 45% L15, fetal bovine serum, 1% l-glutamine, 1% penicillin-streptomycin; Life Technologies, GibcoBRL, Grand Island, NY). Plaque assays were incubated for 6 days at 37°C with 5% CO2, before monolayers were fixed with paraformaldehyde, stained with crystal violet, and visualized for cytopathic effects.
Real-time PCR was run on DNA samples extracted from 25 mg of the 10% homogenate using a DNeasy tissue kit (Qiagen, Valencia, CA). DNA was extracted into 100 ul of buffer, and the concentration was quantified using a spectrophotometer; 500 ng of sample DNA was loaded with previously described PCR primers for MCMV gB (4) and QuantiTect SYBR Green real-time PCR kit (QIAGEN, Valencia, CA). Real-time PCR and melting curve analysis were performed in triplicate with the DNA Engine Opticon 2 PCR system (MJ Research, Inc.). DNA standardization was verified using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (housekeeping gene) with a VIC probe in a separate analysis (TaqMan Rodent GAPDH Control Reagents; Applied Biosystems, Foster City, CA). The sensitivity of gB primers and correlation to total viral PFU were optimized according to the manufacturer's protocols. DNA results are given as relative levels compared to other groups in the experimental cohort. Statistical analysis of group means was performed using one-way analysis of variance and Student's t test.
Fluorescent analysis.
Perfused brain and spleen were immersed overnight in 4% paraformaldehyde, and cryoprotected in 10% then 30% sucrose-PBS solution in 4% paraformaldehyde for 48 h before immersion in o-chlorotoluene (Sakura Finetek, Torrance, CA). Cryosectioning was done with a Leica CM 3000 cryostat (Leica Instruments, Nussloch, Germany). Tissues were coronally cut in either 5-μm or 30-μm serial sections. Representative sections from spleen were analyzed. To identify wild-type MCMV, sections were incubated with a 1:200 dilution of polyclonal mouse anti-MCMV antiserum (54) followed by detection with a 1:500 dilution of goat anti-mouse immunoglobulin -Cy3 (Chemicon, Int., Temecula, CA). Mounted sections were preserved with cryoprotectant (1% polyvinyl pyrrolidone, 30% sucrose, 30% ethylene glycol) and stored at 4°C in the dark. GFP fluorescence was recorded using filters with a wavelength of 395 nm for GFP excitation and 530 to 590 nm for PHK26 and Cy3 excitation on a Zeiss Axioscope, captured with a Zeiss AxioCam digital camera. Contrast and intensity of images were adjusted using Zeiss AxioVision digital imaging software and Adobe Photoshop. Lymphocyte and virus measurements in fluorescent tissue sections were performed using ImageJ 1.33u software (National Institutes of Health).
RESULTS
Lymphocyte activity against CMV.
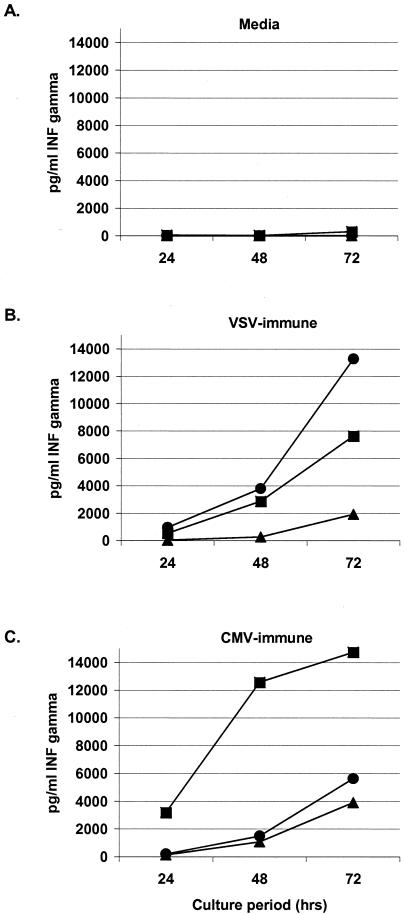
Before assessing the functionality of lymphocyte reconstitution against neurotropic CMV disease in immunodeficient mice, we tested the specificity and cytokine expression pattern of primed lymphocytes, as cytokine expression would demonstrate a cellular response to the virus. We measured IFN-γ, TNF-α, and IL-4 cytokine release following exposure to UV-inactivated virus. Splenocytes harvested from mice following exposure to live MCMV showed a specific (Fig. 1) and antigen dose-responsive (not shown) increase in IFN-γ production when incubated with inactivated homologous CMV antigen (7 × 104 pg/ml). Although robust IFN-γ secretion was seen at all periods, production of both IL-4 and TNF-α was negligible, suggesting a primarily Th1 and cytotoxic T-lymphocyte response. VSV-immune cells cocultured with inactivated VSV antigen produced 13,000 pg/ml IFN-γ, 200 pg/ml IL-4, and 80 pg/ml TNF-α. The responses of VSV-immune splenocytes to inactivated MCMV and those of MCMV-immune splenocytes to inactivated VSV were negligible, confirming specificity of function.
FIG. 1.
Antigen specificity of MCMV-immune lymphocyte cellular response. IFN-γ secretion from activated splenocytes following stimulation with killed virus. (A) Unprimed splenocytes from naive mice; (B) VSV-primed splenocytes; (C) MCMV-primed splenocytes cocultured with: ▴ = medium; • = inactivated VSV, ▪ = inactivated MCMV. IL-4 and TNF-α release was not detectable following stimulation with killed MCMV.
Intracellular cytokine staining following incubation of lymphocytes with UV light-inactivated MCMV confirmed the functionality of both CD4+ and CD8+ T cells. Primed CD8+ T cells expressing IFN-γ in response to MCMV antigen were 1.71%, compared with 0.89% with VSV antigen or 0.82% with medium. Primed CD4+ T cells expressing IFN-γ in response to MCMV antigen were 3.37%, compared with 1.54% with VSV antigen or 1.75% with medium. Phorbol myristate acetate plus ionomycin mitogen stimulation resulted in 6.94% CD8+ and 8.44% CD4+ T cells expressing IFN-γ.
Lymphocyte infiltration and targeting in the brain.
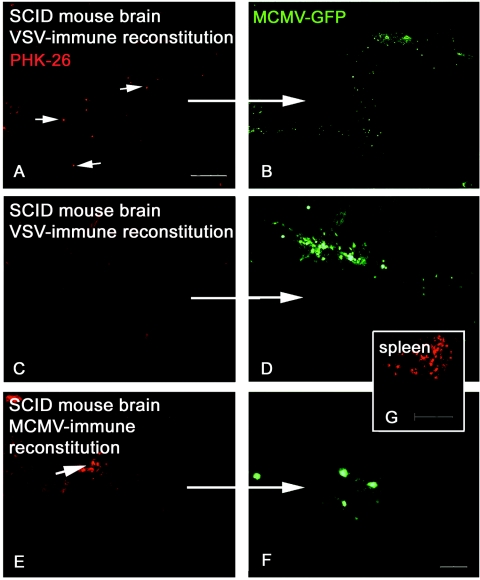
To test whether activated lymphocytes were physiologically capable of translocating across the blood-brain barrier, we tracked the migration of reconstituted lymphocytes labeled by a vital dye (PKH-26). SCID mice were reconstituted with 108 unsorted CMV-immune cells or VSV-immune cells (three mice per group) and challenged with intracranial CMV 1 day later. Mice were perfused 7 days following CMV challenge. Brains were serially sectioned at 5 or 30 μm from the middle rostrally to the olfactory bulbs, incorporating the site of MCMV injection. Virus replication was observed in cryosections using the GFP reporter protein. Brain was serially sectioned from 3 mm rostral to 3 mm caudal to the inoculation site. All sections were visualized for fluorescence, and representative sections were analyzed for lymphocyte and virus quantification. As fixation killed the virus, quantification of total viral levels in the brain was done in additional mice described in the experiments below. The specificity of cells for MCMV was tested by incorporation of an additional group of SCID mice that received cells sensitized to an unrelated virus (VSV).
PHK-26-labeled lymphocytes rapidly repopulated lymphoid organs (Fig. 2G) and entered and migrated through the brain following intracranial MCMV inoculation. Regardless of antigenic specificity, activated lymphocytes were observed within the brain, and higher numbers of cells were seen in proximity to infection (Fig. 2). Approximately 90% of all activated cells were seen within 500 μm of virus antigen. Specifically, an average of 17.1 MCMV-immune and 10.7 VSV-immune lymphocytes were seen near antigen per 0.6-mm2 area section. The number of corresponding MCMV-infected cells expressing GFP in these sections was 17 cells (MCMV-immune brain) and 36.6 cells (VSV-immune brain). Activation of lymphocytes against a different virus, VSV, although sufficient for responding to signaling stimuli, did not impart functionality to clear virus from MCMV-infected CNS cells.
FIG. 2.
Infiltration and targeting of reconstituted MCMV-immune lymphocytes in the brain. PHK-26-labeled lymphocytes (red) were visualized following systemic reconstitution in SCID mice. Both VSV- and MCMV-immune lymphocytes penetrated the blood-brain barrier and surveyed brain parenchyma, but MCMV antigen specificity was required for recognition and elimination of MCMV infection (green). A to D) Brain of representative SCID mouse following reconstitution with VSV-immune cells; note scattered red lymphocytes throughout the parenchyma (arrows). E and F) Brain of representative SCID mouse following reconstitution with MCMV-immune cells. G) Repopulated spleen of SCID mouse, showing transferred red lymphocytes. A, C, E, and G) Red PHK-26-labeled lymphocytes; B, D, and F) green MCMV-GFP. PKH-26 and MCMV-GFP images are of the same field. Bar: 500 μm in A; 100 μm in G; 50 μm in F.
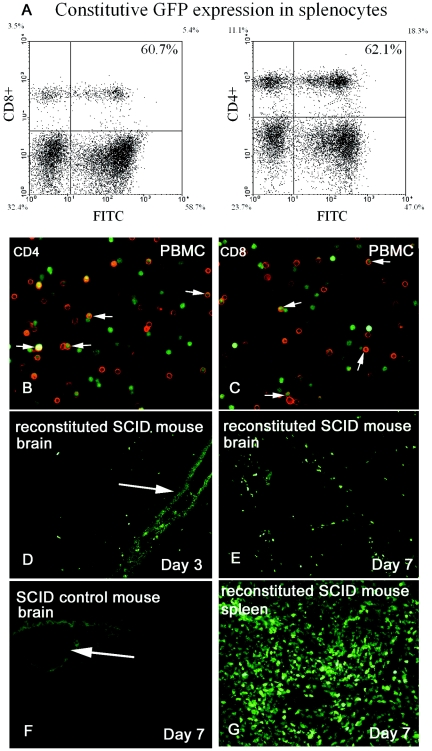
Because of a potential for artifacts associated with release of the vital dye to neighboring cells and loss of dye labeling with cellular replication, we confirmed the initial results by an additional experiment using lymphocytes that constitutively express green fluorescent protein harvested from transgenic mice. GFP fluorescence was measured within the spleens of several transgenic GFP donor mice using flow cytometry; 60.7% of CD4+ and 62.1% of CD8+ T cells demonstrated positive GFP fluorescence (Fig. 3A). GFP expression in peripheral blood mononuclear cells was also identified from these mice using immunohistochemistry staining (Fig. 3B and C), confirming GFP expression in a majority of both CD4+ and CD8+ T cells.
FIG. 3.
In vivo tracking of repopulated lymphocytes. Lymphocytes constitutively expressing GFP were used to reconstitute SCID mice infected with wild-type MCMV. (A) FACS analysis of constitutive GFP expression in splenocytes from transgenic Swiss Webster mice. Peripheral blood CD4+ (B) and CD8+ (C) T lymphocytes (red) constitutively expressing GFP; arrows, double-stained cells. (D to F) Cryosections of SCID mouse brain following intracranial infection of MCMV; arrows highlight the injection track. Activate lymphocytes (green) translocate across the blood-brain barrier within 3 days of systemic reconstitution and are seen in proximity to CMV infection. (E and F) SCID mouse brain 3 days (E) and 7 days (F) following reconstitution with CMV-immune splenocytes. F) SCID mouse reconstituted with medium; arrows highlight the injection tract. (G) Lymphocyte (green)-repopulated SCID mouse spleen 7 days following reconsititution.
Additional mice were used for splenic cultures, and cells were visualized for constitutive GFP expression. We observed a majority of cells (lymphocytes and fibroblasts alike) expressing GFP (>70%), with a gradient of fluorescence from weak to very strong. Wild-type MCMV, which does not produce GFP, was used for priming of these GFP-expressing mice and for virus challenge of reconstituted SCID mice to allow rapid identification of lymphocytes. One group of five SCID mice was reconstituted with 108 CMV-immune cells followed by CMV challenge a day later. A second group of five mice was challenged with CMV followed by immune reconstitution a day later, along with a third group of six control SCID mice. Mice were perfused day 4 (two mice) and day 7 (three mice) following CMV challenge from group 1 and day 3 (two mice) and day 7 (three mice) following CMV challenge from group 2. Control SCID mice were sacrificed at day 3, 4, or 7 following challenge.
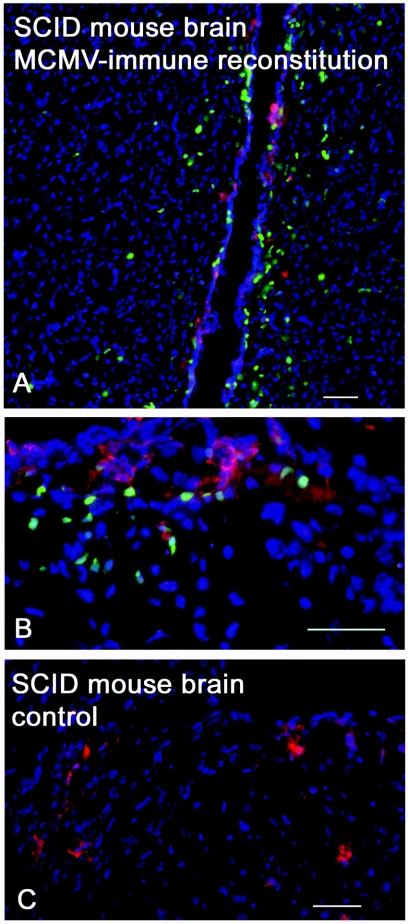
Successful reconstitution of cells in SCID mice was confirmed by observing transferred GFP-expressing cells within the spleen (Fig. 3G). Transferred GFP-expressing lymphocytes rapidly entered and migrated through the brain within 3 days of MCMV challenge. Continual cell recruitment to the CNS was observed, and the number of GFP-expressing cells in the brain was much greater at day 7 than day 3 (Fig. 3D and E). Using immunohistochemistry, wild-type MCMV was observed in the cortex, striatum, ventricular system, choroid plexus, and cells of the meninges. An average of 121 MCMV-immune lymphocytes were observed within 500 μm of virus antigen per 0.6-mm2 area section (Fig. 4). A few cells were occasionally identified at sites distant to virus antigen. Together, these experiments demonstrate rapid entry into the brain of identified lymphocytes and specific adaptive responses against virus in the brain.
FIG. 4.
Activated lymphocytes invade brain and target antigen. Immunohistochemistry of stained cryosections of SCID mouse brain 8 days following MCMV challenge and 7 days following adoptive transfer of unsorted MCMV-immune splenocytes expressing GFP. (A) Periventricular migrating lymphocytes. (B) Foci of lymphocytes targeting MCMV infection in the cortex. (C) Control SCID mouse with active MCMV infection. Green, activated lymphocytes; red, MCMV antigen; blue, 4′,6′-diamidino-2-phenylindole stain of cellular nuclei. Bar, 50 μm.
Immune reconstitution reduces acute neurotropic CMV infection.
The efficacy of primed, reconstituted cells against neurotropic MCMV infection was tested using several parameters to identify major effector cells in the brain. The composition of donor cells was verified by flow cytometry (Fig. 5). Unsorted MCMV-immune splenocytes consisted of approximately 70 to 85% lymphocytes (30 to 40% B cells, 15 to 23% CD4+ and 10% CD8α+ T cells). T-cell-enriched fractions had 56.1% CD3+ staining (40% CD4+ and 15% CD8+ T cells) with a significantly reduced B-lymphocyte population (3.6%). CD4+ and CD8+ T-cell subset isolation using MagCellect negative selection resulted in a population >95% pure for each cell population. Reconstitution of SCID mice was confirmed at necropsy using flow cytometry of splenocytes or histological analysis of labeled lymphocytes in spleen (Fig. 2G and 3G).
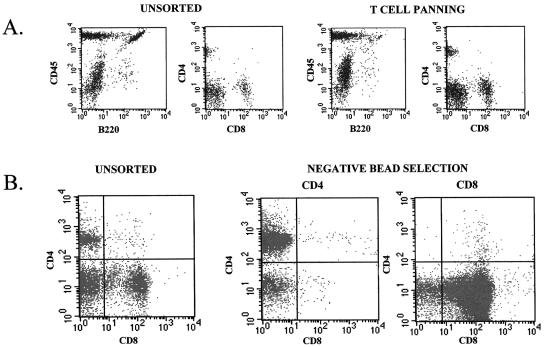
FIG. 5.
Flow cytometric verification of sorting efficiency. Scatter plots selected by cellular phenotype confirm cell-sorting effectiveness for adoptive reconstitution of SCID mice. (A) Unsorted splenocytes versus T-cell-enriched splenocytes following panning, <5% remaining; B lymphocytes (B220+ cells); (B) >95% pure isolation by negative selection of CD4 or CD8+ T cells using magnetic bead separation.
An initial experiment was designed to define the potential adaptive response in mice with neurotropic MCMV infection. Ten BALB/c mice were inoculated with systemic MCMV (donor immunization protocol) to prime splenocytes against viral antigen. Splenocytes from five of these mice were harvested 8 days later, and 108 viable cells were administered to SCID mice. The remaining group of five CMV-immune BALB/c mice were then challenged with intracranial virus, while groups of six reconstituted and four control SCID mice were challenged with intracranial CMV 3 days following reconstitution with either CMV-immune splenocytes or medium only. Brains were harvested from BALB/c, reconstituted, and control SCID mice 7 days after viral challenge. Residual CMV DNA was detectable in the brain (Fig. 6A), but using virus isolation in culture, no live virus was recovered from any of the treated mice. All control SCID mice had significantly elevated virus DNA levels and replicating live virus in the brain 7 days following intracranial challenge.
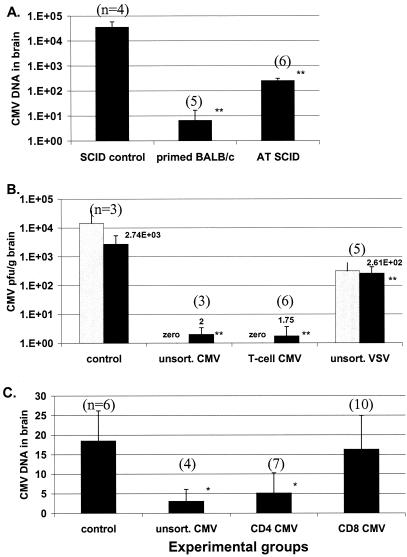
FIG. 6.
Transfer of MCMV-immune T cells successfully clears acute neurotropic disease. (A) Maximal adaptive immune response against neurotropic disease. MCMV-immune BALB/c mice that had previously cleared an initial MVMV infection were then challenged intracranially with virus. All mice in this group rapidly and effectively cleared acute neurotropic CMV infection within 7 days of challenge. SCID mice reconstituted with immune splenocytes (AT SCID) from BALB/c mice that had cleared initial infection also significantly reduced viral DNA load as tested by real-time quantitative PCR, but to a lesser extent than fully immunocompetent mice. (B) Quantification of MCMV in the brain was determined using viral gB DNA (real-time PCR, black bars) and recovery of live virus (plaque assay, gray bars). No live virus remained in groups receiving either unsorted splenocytes or T-cell-enriched fractions. (C) CD4+ Th1 cells are a major contributor in the clearance of active MCMV in the brain, while reconstituted CD8+ T cells showed no difference in virus load compared to SCID controls. Results for DNA quantification using real-time PCR (black bars in all graphs) are expressed as relative fold increase in the amount of viral DNA compared to other experimental groups. *, P ≤ 0.1; **, P ≤ 0.05; n, number of mice per group.
To test whether virus clearance from the brain was a result of specific T-lymphocyte recognition or a nonspecific outcome mediated by lymphocyte activation, we administered 108 MCMV-immune or VSV-immune activated splenocytes or 2 × 107 MCMV immune T-cell-enriched splenocytes to SCID mice (five mice per group). These mice along with a group of three control SCID mice receiving medium only were challenged intracranially with virus the following day. All mice from this experiment were sacrificed 7 days later.
Control SCID mice not receiving cells showed significant MCMV replication and overt clinical disease, including dehydration, scruffy appearance, and photophobia. In preliminary experiments, it was determined that control SCID mice invariably died between 7 and 14 days after intracranial MCMV challenge (data not shown). SCID mice treated with unsorted MCMV-immune cells experienced a significantly greater viral reduction compared to mice receiving VSV-immune splenocytes (P value = 0.004). Mice receiving unsorted or T-cell-enriched MCMV-immune cells equally cleared virus from brain, suggesting that T cells are a predominant contributor to virus reduction in the brain. Virus DNA levels in the brain were reduced 100-fold following adoptive transfer of either unsorted or T-cell-enriched MCMV-immune splenocytes, and based on plaque assays, no active MCMV was recovered from the brain (Fig. 6B) compared with virus DNA levels and proliferating MCMV in mice given VSV-immune splenocytes.
Relative virus levels in tissue determined using the plaque assay or with real-time PCR using gB primers were very well correlated (r2 = 0.99). After validation, the plaque assay was used primarily for verifying complete elimination of replicating virus in samples with corresponding low DNA levels as measured by real-time PCR. DNA levels from PCR were computed based on the level of gB DNA in the sample. In preliminary trials, we found the sensitivity of gB primers to detect CMV DNA to be greater than for either E1 and IE1 (data not shown). Although CMV detection methodology has yet to be universally standardized, gB is routinely used to measure viral levels, which are significantly reduced in latent infection (7, 32). Thus, the levels of gB DNA obtained using real-time PCR are representative of measurement of the total viral genome.
Previous work on peripheral organ systems has suggested that CD8+ T cells might play a pivotal role in combating CMV infection (19, 51). We therefore tested the hypothesis that CD8+ T cells were the major effector cells against virus within the CNS. Isolation of T-cell subsets was done using negative selection to avoid inadvertent activation of cells. SCID mice were given either medium only (6 mice), 5 × 106 to 107 CD8+ T cells (10 mice), 5 × 106 CD4+ T cells (seven mice), or a combination of 108 CD4 and CD8+ T and accessory cells in unsorted splenocytes (four mice). All mice were challenged with intracranial CMV the same day as reconstitution and sacrificed 6 days later. Reconstitution of infected SCID mice using MCMV-immune T-cell fractions revealed a significant CD4+ T-cell dependency for virus clearance in the brain (Fig. 6C), as a significant difference was not observed between groups receiving either CD4+ T cells or unsorted splenocytes. MCMV-primed CD8+ T cells alone were ineffective against live virus. Because of the unexpected death of a mouse in each of the SCID control and CD8+ T-cell treated groups before the conclusion of the 7-day period, remaining mice were euthanized at day 6.
Given the successful short-term outcome of primed T-cell transfer to significantly reduce neurotropic virus levels, we tested the longevity of protection offered by reconstituted cells. Following the donor protocol in 30 BALB/c mice, splenocytes were harvested and given at a concentration of 108 cells to 18 SCID mice. A group of three control mice received medium only. The same day, all mice in this experiment were challenged with intracranial CMV. Cohorts of mice were sacrificed 3, 7, 14, 21, and 28 days later. Following reconstitution and challenge, a progressive decline in the level of MCMV DNA in the brain was observed in CMV-immune, reconstituted SCID mice, plateauing 3 to 4 weeks following adoptive transfer (Fig. 7A). The onset of viral DNA reduction in the brain corresponded in time with increased translocation of immune cells into the brain (day 3) observed by using fluorescently labeled lymphocytes.
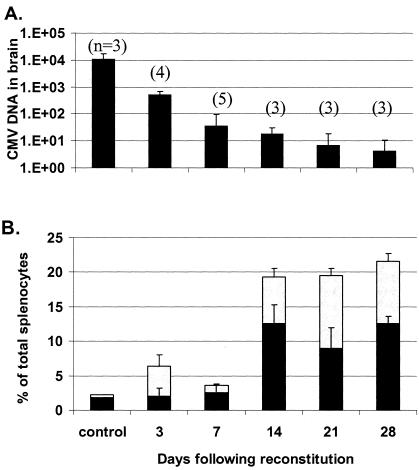
FIG. 7.
Duration of protection offered through transfer of CMV-immune T cells. (A) Following adoptive transfer of MCMV-immune splenocytes and intracranial challenge with MCMV, viral DNA load in the brain assessed by real-time PCR progressively declined throughout the study period and corresponded to clearance of active infection 7 days following reconstitution and remaining latent through the 28-day study endpoint. (B) Splenic repopulation and T-cell proliferation following reconstitution. The percentage of total splenocyte population identified as CD4+ T cells (black bars) and CD8+ cells (gray bars). n, number of mice per group.
Reconstituted cells inhibited MCMV replication throughout the study, and we could not recover live virus in any organ beginning at 7 days following reconstitution. Nonreconstituted control SCID mice that had been infected intracranially with CMV displayed progressive clinical signs associated with MCMV disease and were humanely euthanized after 2 weeks. Following administration of systemic immune cells, significant CD4+ and CD8+ T-cell proliferation and expansion occurred in the spleen, particularly strong 1 to 2 weeks following reconstitution (Fig. 7B).
Blocking peripheral MCMV from invading the brain by adoptive immune reconstitution.
Peripheral inoculation with MCMV results in invasion of the brain within 3 weeks in SCID mice, whereas immunocompetent controls show no CMV in the brain (54). Extending results from experiments directed at testing the efficacy of adoptive reconstitution for treatment of acute neurotropic disease, we postulated that adoptive transfer could also prevent dissemination of peripheral MCMV infection to brains of immunodeficient mice. SCID mice were given medium only, 108 CMV-immune splenocytes or 108 naïve splenocytes (n = 5 mice/group). One day later, all mice were challenged with peripheral CMV. Mice were sacrificed 28 days following viral challenge. Reconstitution of SCID mice with either MCMV-immune cells or naïve splenocytes provided for a strong protective response within the brain against CMV following systemic virus challenge. No adverse clinical signs of disease were seen throughout the entire study in reconstituted mice of both groups, unlike the nonreconstituted SCID control mice that did show significant viral proliferation and after 21 days begin to develop clinical signs of hunched posturing, scruffy hair coat, and lethargy (54).
No live virus was recovered from brain homogenate of either reconstituted group, while an average of 318 PFU/g was cultured from the brains of nonreconstituted SCID mice. Additionally, reconstituted mice developed specific humoral MCMV antibody beginning at 10 days (titer > 1:1,000 by immunofluorescent assay); in contrast, no antibody was detected in nonreconstituted SCID animals (titer < 1:5). Adoptive transfer of either naïve cells or MCMV-immune splenocytes was sufficient to prevent neurotropic CMV disease.
Although no replicating virus was detected in either reconstituted group, the genome of CMV can remain latent in infected cells. As a test for the presence of MCMV DNA, real-time PCR was used. MCMV DNA levels were significantly reduced in all organs and brain of reconstituted mice compared to control SCID mice (Fig. 8). Viral DNA was detected in the brain homogenate from five of five mice receiving naïve cells and one of five receiving CMV-immune cells and in significantly higher amounts in all nonreconstituted control SCID mice.
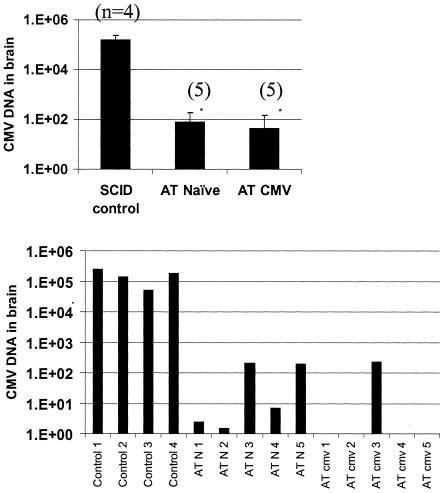
FIG. 8.
Protection against viral invasion of the CNS. Adoptive reconstitution with primed or naïve lymphocytes was tested as a means of preventing dissemination of CMV to the CNS in immunosuppressed mice. Mice were given either naïve (AT naïve) or CMV-immune (AT CMV) splenocytes on day zero and inoculated with 4.4 × 105 PFU CMV intravenously the following day. Brains were harvested at 28 days following reconstitution, and viral DNA was quantified using real-time PCR. Both naïve and CMV-primed splenocytes were effective at limiting the spread of virus to the brain. No virus was recovered from either reconstituted group, compared to 3.2 × 102 PFU/g in the SCID control group (not graphed). A) Relative fold increase in MCMV levels in the brain, group means. B) Individual MCMV DNA levels in mice.*, P ≤ 0.001; n, number of mice per group.
DISCUSSION
Treatment of neurotropic CMV infection remains challenging because of difficulty in achieving therapeutic levels of antiviral drugs in the CNS, systemic toxicity, and bystander damage to vital cells. Preemptive adoptive transfer to immunocompromised mice has been effective for controlling MCMV infection in several peripheral tissues outside the brain (25, 52, 60). Using multiple experimental paradigms with over 250 mice, we demonstrate successful reduction and prevention of acute CMV infection within the CNS using systemic adoptive transfer. Clearance of acute neurotropic CMV infection required specific, CMV-immune T cells.
Lymphocyte activation using other viral antigens (e.g., VSV) enabled entry and migration through the CNS, but cells failed to eliminate CMV infection. Mice receiving VSV-immune cells served as controls for two issues. First, transfer of cells into these mice controlled for homeostatic proliferation, which normally occurs after transfer into a lymphopenic recipient, and second, to control for nonspecific effects of activated splenocytes against virus. Since there was no significant cytokine production from VSV-immune cells incubated with MCMV antigen in culture, we attribute the partial reduction of MCMV DNA levels of mice receiving unsorted VSV-immune splenocytes to the nonspecific presence of innate immune (accessory) cells such as monocytes, NK cells, and granulocytes present in the reconstitute. These accessory cells were present in unsorted cells and likely played a minor antivirus role in mice receiving unsorted MCMV-immune splenocytes, but not in groups receiving enriched T cells or T-cell fractions. Intrinsic cytokine production from stimulated microglia and astrocytes may also have contributed to some viral reduction, but resultant changes in themselves were negligible, as unreconstituted SCID mice experienced significant proliferative CMV titers.
Reconstituted lymphocytes enter the brain.
Activation of lymphocytes enables rapid transmigration through the blood-brain barrier, infiltration into the CNS within days of adoptive transfer, and requisite immunologic surveillance of the CNS (18). Irrespective of the site of initial antigenic encounter, activated lymphocytes are able to migrate to many nonlymphoid organs (including the brain) (35). We tracked the migration of reconstituted lymphocytes in peripheral organs and brain using a vital dye, PKH-26. As there was the potential for artifactual but inadvertent leakage of dye from labeled cells to host cells and a further problem of serial dilution of the dye as cells divided, we verified results using lymphocytes that constitutively expressed GFP.
As studied with both tracking methods, entry and localization of T cells to sites of infection within the brain occurred within days of transfer. Activated cells (CMV specific or VSV nonspecific) both were observed in high numbers near viral antigen within the brain. Although some lymphocytes were observed in the brain at distant sites from the virus, the majority of cells were seen in close proximity to the virus. It is likely that activation allows lymphocytes to follow chemokine gradients within the brain, but recognition of specific antigen is essential for functionality. Rapid and specific immune responses help limit the degree of viral dissemination and thereby minimize secondary damage to healthy cells (10) and may reduce the magnitude of subsequent viral latency (50). This can be supported by our observation of a drastically reduced incidence of residual DNA levels in the brain from SCID mice given MCMV-immune versus naïve lymphocytes following systemic MCMV challenge. The delay experienced in generating specific adaptive responses to MCMV in mice receiving naïve cells resulted in an 80% increase in CMV DNA present in the brain homogenate.
The CNS is immunologically unique, in part, because of low expression of class I and II MHC molecules from resident cells of a quiescent CNS and restriction of cellular entry by the blood-brain barrier, resulting in very low frequencies of naïve T cells in the brain (34). Inflammatory responses in acute CMV CNS disease have been described (5, 6, 53), but clearance mechanisms in the mature brain are still unresolved. Kosugi and colleagues (29) found immune evasion by MCMV-infected neurons to macrophages and NK cells, suggesting that alternative mechanisms likely exist for clearing infection, such as adaptive immunity. In our study, we also observed a partial reduction of virus in the mature brain (mice receiving VSV-immune cells), likely caused by cells of the innate immune system, although this effect was not sufficient to prevent disease.
In previous work, we demonstrated that CMV levels in the brain 1 week following intracranial inoculation of immunocompetent mice are equivalent to levels in immunocompromised SCID mice (62), supporting the idea that innate immunity alone is insufficient for viral clearance. Within 2 to 3 weeks, immunocompetent mice subsequently mounted effective adaptive immune responses and cleared infection, whereas immune-deficient mice did not clear infection. As we demonstrate, viral clearance in BALB/c mice is expedited through prior exposure to virus and activation of lymphocytes. Neurons are also permissive to MCMV infection in the mature brain (54), and as our data show, adoptive therapy completely cleared active infection from all cells of the CNS, suggesting an important role for T cells in control of CMV infection in the brain. MHC expression in neurons is variable (31, 42, 49), T-cell-induced viral clearance mechanisms from these cells may differ from those in other organs.
CMV clearance from brain.
Mechanisms of CMV clearance are organ dependent, and our findings suggest the effector cell responsible for CMV elimination in the brain differs from that in other organs, relying to a large degree on functional CD4+ Th1 and not CD8+ T cells. Intracellular cytokine staining and IFN-γ secretion in culture independently confirmed the functionality of both CD4+ and CD8+ T cells against CMV. CD8+ T cells are reported to be the primary antiviral effectors in BALB/c mice, effective against MCMV in spleen, lung, liver, and adrenals (19, 50). It is likely that multiple cell types have the capacity to exert antiviral effects, and it is unknown whether another functional cell phenotype may exist in the unsorted splenocyte population that may also contribute to CMV clearance.
CD4+ T cells are necessary for viral clearance primarily in the salivary gland (23), while transfer of immune CD4+ T cells is not protective against systemic disease (51, 52). However, animal models completely depleted of CD8+ T cells can eliminate CMV in acute or chronic infection because of redundant mechanisms contributed by CD4+ T cells and NK cells (22, 23). One possible mechanism for viral clearance in the brain is through interaction of activated T cells with adjacent microglia or astrocytes functioning as antigen-presenting cells. Both cell types are capable of presenting epitopes though class II MHC presentation to CD4+ T cells (10, 12, 17, 45). Additionally, NK cells secrete IFN-γ following CNS infection, which may divert the immune response to a Th1-type CD4+ T-dominated response. Infiltrating T cells may then upregulate antigen-presenting cell MHC expression, further stimulating virus elimination (10).
Lymphocyte activation in our study resulted in demonstrable expression of IFN-γ but not TNF-α or IL-4. It is likely that noncytolytic immune responses played a crucial role in viral clearance in the CNS, as CD4+ T cells are thought to also mediate antiviral effects in infected salivary glands by releasing IFN-γ (30). Noncytolytic mechanisms of clearance are reported with several other virus infections of the CNS, such as dengue virus (59), lymphocytic choriomeningitis virus (61) measles virus (45), VSV, poliovirus type 1, and herpes simplex virus type 1 (28). However, it seems unlikely that noncytolytic mechanisms alone can result in viral clearance from the brain. Naive SCID mice remain competent for NK cell and microglia cell activity without adoptive transfer of immune cells, and we show they remain very susceptible to CMV pathology. Additionally, recombinant IFN-γ could not replace the function of Th1 cells in vivo against CMV and had limited direct antiviral activity in vitro (33), unlike that seem with other viruses (15).
Reconstituted CMV-immune CD8+ T cells were surprisingly ineffective at clearing active CMV infection from brain. Potential explanations for this may be viral immune evasion and inefficient lymphocyte priming. CMV can evade MHC class I host responses by expressing several proteins (HCMV: US2, US3, US6, US8, US10, US11, and UL18; and MCMV:gp34, gp40, and gp48) (46). Although these proteins interfere with MHC class I binding, when they are removed, as from mutant viruses, CD8+ T-cell responses are not significantly altered (14), suggesting that alternative mechanisms for CD8+ T-cell inhibition likely exist. Priming of nonfunctional CD8+ T cells is also reported, thereby misleading the host defense response (20). The priming method used in our studies may have limited the number of epitopes for lymphocyte response and skewed recognition to IE1 proteins and m164 gene product (13, 50), as these are the major immunodominant responses against live virus infection in healthy hosts. T-cell responses to these epitopes, however, are very effective at clearing active infection. Additionally, CD8+ T cells primed against other, masked antigens can effectively protect against diseases (19, 38), suggesting that selective priming against T-cell epitopes may enhance the antiviral responses. Irrespective of which cell type is ultimately responsible for virus clearance in the brain, CMV latency remains.
CMV DNA was detectable in the brain over a prolonged period, even after clearance of active infection by reconstituted immune cells. We were unable to eliminate viral DNA, even with full immune reconstitution (using unsorted CMV-immune splenocytes) or priming of the immune system in immunocompetent mice, suggesting viral latency. Sites of latency within the CNS are still unclear, but multiple cells are susceptible to infection (54) and endothelial cells and vascular walls have been reported to harbor latent CMV (26, 36). Although adoptive transfer of immune cells clears active CMV from other peripheral tissues, latency invariably develops and data indicate that functional T cells cannot prevent latency (60). For maximal protection against disease, it may be necessary to provide several components during immunotherapy, as CMV-immune CD8+ T cells successfully reconstituted cellular immunity against CMV, but CD4+ Th cells were needed for maintenance of CD8+ cell levels (66). This has important implications regarding the duration of protection offered by immunotherapy, as reactivation of latent virus may be possible.
Lymphocyte transfer blocks CMV presence in the brain.
Prevention of disseminated CMV disease in immunocompromised mice was accomplished using adoptive reconstitution. Both sensitized and naïve lymphocytes successfully cleared live virus and limited systemic dissemination. Although no significant differences were seen between group averages of remaining CMV DNA levels within the brain, the overall incidence of detectable viral DNA in the CNS from SCID mice receiving cells primed against CMV was small, whereas every mouse receiving naïve cells was positive for viral DNA in the brain. We found no evidence of active infection within the brain parenchyma in the naïve cell group, but endothelial cells are known to be a major site for MCMV latency (11, 27) and are susceptible to infection in this model (54). This suggests that, although naïve cells responded to virus, the longer a viremic state exists before control by the adaptive immune system, the greater the possible extent of viral latency. Although we did not test the overall length of protection offered through adoptive transfer of cells, successful immune reconstitution may permit life-long protection against disease. Rapid viral clearance achieved through the use of CMV-immune lymphocytes may additionally offer the significant benefit of reduced viral latency.
In summary, we show rapid and complete clearance of CMV from the brain following adoptive reconstitution using CMV-immune T cells. CD4+ T cells may play a major role in viral clearance from the brain, possibility in concert with IFN-γ expression. Activated cells enter the CNS and specifically respond to virus within 3 days of transfer, clearing infection in about a week and, together with other cells of the adaptive immune system, offer prolonged protection from reactivation. Adoptive immune reconstitution is also effective at preventing entrance from the periphery to the brain, and both naïve and activated lymphocytes block CMV from getting into the brain.
Acknowledgments
Support was provided in part by NIH grant 1RO1 AI/NS48854 and NCRR grant 1K01 RR17017.
We thank Frank Paturzo, Elizabeth Johnson, and Akiko Iwasaki for help and advice.
REFERENCES
- 1.Alford, C. A., and W. J. Britt. 1996. Cytomegalovirus, p. 2493-2534. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology. Lippincott-Raven, New York, NY.
- 2.Anduze-Faris, B. M., A. M. Fillet, J. Gozlan, R. Lancar, N. Boukli, J. Gasnault, E. Caumes, J. Livartowsky, S. Matheron, C. Leport, D. Salmon, D. Costagliola, and C. Katlama. 2000. Induction and maintenance therapy of cytomegalovirus central nervous system infection in HIV-infected patients. AIDS 14:517-524. [DOI] [PubMed] [Google Scholar]
- 3.Arribas, J. R., G. A. Storch, D. B. Clifford, and A. C. Tselis. 1996. Cytomegalovirus encephalitis. Ann. Intern. Med. 125:577-587. [DOI] [PubMed] [Google Scholar]
- 4.Bevan, I. S., C. C. Sammons, and C. Sweet. 1996. Investigation of murine cytomegalovirus latency and reactivation in mice using viral mutants and the polymerase chain reaction. J. Med. Virol. 48:308-320. [DOI] [PubMed] [Google Scholar]
- 5.Bigger, J. E., C. A. Thomas 3rd, and S. S. Atherton. 1998. NK cell modulation of murine cytomegalovirus retinitis. J. Immunol. 160:5826-5831. [PubMed] [Google Scholar]
- 6.Booss, J., P. R. Dann, B. P. Griffith, and J. H. Kim. 1989. Host defense response to cytomegalovirus in the central nervous system. Predominance of the monocyte. Am. J. Pathol. 134:71-78. [PMC free article] [PubMed] [Google Scholar]
- 7.Bradford, R. D., G. Cloud, A. D. Lakeman, S. Boppana, D. W. Kimberlin, R. Jacobs, G. Demmler, P. Sanchez, W. Britt, S. J. Soong, and R. J. Whitley. 2005. Detection of cytomegalovirus (CMV) DNA by polymerase chain reaction is associated with hearing loss in newborns with symptomatic congenital CMV infection involving the central nervous system. J. Infect. Dis. 191:227-233. [DOI] [PubMed] [Google Scholar]
- 8.Chimelli, L., S. Rosemberg, M. D. Hahn, M. B. Lopes, and M. B. Netto. 1992. Pathology of the central nervous system in patients infected with the human immunodeficiency virus (HIV): a report of 252 autopsy cases from Brazil. Neuropathol. Appl. Neurobiol. 18:478-488. [DOI] [PubMed] [Google Scholar]
- 9.Del Val, M., H. J. Schlicht, H. Volkmer, M. Messerle, M. J. Reddehase, and U. H. Koszinowski. 1991. Protection against lethal cytomegalovirus infection by a recombinant vaccine containing a single nonameric T-cell epitope. J. Virol. 65:3641-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorries, R. 2001. The role of T-cell-mediated mechanisms in virus infections of the nervous system. Curr. Top. Microbiol. Immunol. 253:219-245. [DOI] [PubMed] [Google Scholar]
- 11.Fish, K. N., C. Soderberg-Naucler, L. K. Mills, S. Stenglein, and J. A. Nelson. 1998. Human cytomegalovirus persistently infects aortic endothelial cells. J. Virol. 72:5661-5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontana, A., P. Erb, H. Pircher, R. Zinkernagel, E. Weber, and W. Fierz. 1986. Astrocytes as antigen-presenting cells. Part II: Unlike H-2K-dependent cytotoxic T cells, H-2Ia-restricted T cells are only stimulated in the presence of interferon-gamma. J. Neuroimmunol. 12:15-28. [DOI] [PubMed] [Google Scholar]
- 13.Gibson, L., G. Piccinini, D. Lilleri, M. G. Revello, Z. Wang, S. Markel, D. J. Diamond, and K. Luzuriaga. 2004. Human cytomegalovirus proteins pp65 and immediate early protein 1 are common targets for CD8+ T-cell responses in children with congenital or postnatal human cytomegalovirus infection. J. Immunol. 172:2256-2264. [DOI] [PubMed] [Google Scholar]
- 14.Gold, M. C., M. W. Munks, M. Wagner, C. W. McMahon, A. Kelly, D. G. Kavanagh, M. K. Slifka, U. H. Koszinowski, D. H. Raulet, and A. B. Hill. 2004. Murine cytomegalovirus interference with antigen presentation has little effect on the size or the effector memory phenotype of the CD8 T-cell response. J. Immunol. 172:6944-6953. [DOI] [PubMed] [Google Scholar]
- 15.Guidotti, L. G., and F. V. Chisari. 2001. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu. Rev. Immunol. 19:65-91. [DOI] [PubMed] [Google Scholar]
- 16.Harrison, M. J. G., and J. C. McArthur. 1995. AIDS and neurology. Churchill Livingstone, New York, NY.
- 17.Hart, M. N., and Z. Fabry. 1995. CNS antigen presentation. Trends Neurosci. 18:475-481. [DOI] [PubMed] [Google Scholar]
- 18.Hickey, W. F. 1991. Migration of hematogenous cells through the blood-brain barrier and the initiation of CNS inflammation. Brain Pathol. 1:97-105. [DOI] [PubMed] [Google Scholar]
- 19.Holtappels, R., J. Podlech, N. K. Grzimek, D. Thomas, M. F. Pahl-Seibert, and M. J. Reddehase. 2001. Experimental preemptive immunotherapy of murine cytomegalovirus disease with CD8 T-cell lines specific for ppM83 and pM84, the two homologs of human cytomegalovirus tegument protein ppUL83 (pp65). J. Virol. 75:6584-6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holtappels, R., J. Podlech, M. F. Pahl-Seibert, M. Julch, D. Thomas, C. O. Simon, M. Wagner, and M. J. Reddehase. 2004. Cytomegalovirus misleads its host by priming of CD8 T cells specific for an epitope not presented in infected tissues. J. Exp. Med. 199:131-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson, R. T. 1998. Viral infections of the nervous system, 2nd ed. Lipincott-Raven, Philadelphia, Pa.
- 22.Jonjic, S., W. Mutter, F. Weiland, M. J. Reddehase, and U. H. Koszinowski. 1989. Site-restricted persistent cytomegalovirus infection after selective long-term depletion of CD4+ T lymphocytes. J. Exp. Med. 169:1199-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jonjic, S., I. Pavic, P. Lucin, D. Rukavina, and U. H. Koszinowski. 1990. Efficacious control of cytomegalovirus infection after long-term depletion of CD8+ T lymphocytes. J. Virol. 64:5457-5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadima-Nzuji, M., and J. E. Craighead. 1990. T-lymphocyte effects on murine cytomegalovirus pulmonary infection. Am. J. Pathol. 137:907-912. [PMC free article] [PubMed] [Google Scholar]
- 25.Kercher, L., and B. M. Mitchell. 2000. Immune transfer protects severely immunosuppressed mice from murine cytomegalovirus retinitis and reduces the viral load in ocular tissue. J. Infect. Dis. 182:652-661. [DOI] [PubMed] [Google Scholar]
- 26.Koffron, A. J., M. Hummel, B. K. Patterson, S. Yan, D. B. Kaufman, J. P. Fryer, F. P. Stuart, and M. I. Abecassis. 1998. Cellular localization of latent murine cytomegalovirus. J. Virol. 72:95-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koffron, A. J., K. H. Mueller, D. B. Kaufman, F. P. Stuart, B. Patterson, and M. I. Abecassis. 1995. Direct evidence using in situ polymerase chain reaction that the endothelial cell and T-lymphocyte harbor latent murine cytomegalovirus. Scand. J. Infect. Dis. Suppl. 99:61-62. [PubMed] [Google Scholar]
- 28.Komatsu, T., Z. Bi, and C. S. Reiss. 1996. Interferon-gamma induced type I nitric oxide synthase activity inhibits viral replication in neurons. J. Neuroimmunol. 68:101-108. [DOI] [PubMed] [Google Scholar]
- 29.Kosugi, I., H. Kawasaki, Y. Arai, and Y. Tsutsui. 2002. Innate immune responses to cytomegalovirus infection in the developing mouse brain and their evasion by virus-infected neurons. Am. J. Pathol. 161:919-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krmpotic, A., I. Bubic, B. Polic, P. Lucin, and S. Jonjic. 2003. Pathogenesis of murine cytomegalovirus infection. Microbes Infect. 5:1263-1277. [DOI] [PubMed] [Google Scholar]
- 31.Lee, E. M., J. Y. Kim, B. R. Cho, W. K. Chung, B. W. Yoon, S. U. Kim, B. C. Lee, W. S. Hwang, S. Y. Moon, J. S. Lee, and C. Ahn. 2005. Down-regulation of MHC class I expression in human neuronal stem cells using viral stealth mechanism. Biochem. Biophys. Res. Commun. 326:825-835. [DOI] [PubMed] [Google Scholar]
- 32.Lenzo, J. C., D. Fairweather, V. Cull, G. R. Shellam, and C. M. J. Lawson. 2002. Characterisation of murine cytomegalovirus myocarditis: cellular infiltration of the heart and virus persistence. J. Mol. Cell Cardiol. 34:629-640. [DOI] [PubMed] [Google Scholar]
- 33.Lucin, P., I. Pavic, B. Polic, S. Jonjic, and U. H. Koszinowski. 1992. Gamma interferon-dependent clearance of cytomegalovirus infection in salivary glands. J. Virol. 66:1977-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marten, N. W., S. A. Stohlman, J. Zhou, and C. C. Bergmann. 2003. Kinetics of virus-specific CD8+-T-cell expansion and trafficking following central nervous system infection. J. Virol. 77:2775-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masopust, D., V. Vezys, E. J. Usherwood, L. S. Cauley, S. Olson, A. L. Marzo, R. L. Ward, D. L. Woodland, and L. Lefrancois. 2004. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J. Immunol. 172:4875-4882. [DOI] [PubMed] [Google Scholar]
- 36.Melnick, J. L., C. Hu, J. Burek, E. Adam, and M. E. DeBakey. 1994. Cytomegalovirus DNA in arterial walls of patients with atherosclerosis. J. Med. Virol. 42:170-174. [DOI] [PubMed] [Google Scholar]
- 37.Mocarski, E. S., Jr., and G. W. Kemble. 1996. Recombinant cytomegaloviruses for study of replication and pathogenesis. Intervirology 39:320-330. [DOI] [PubMed] [Google Scholar]
- 38.Morello, C. S., L. D. Cranmer, and D. H. Spector. 2000. Suppression of murine cytomegalovirus (MCMV) replication with a DNA vaccine encoding MCMV M84 (a homolog of human cytomegalovirus pp65). J. Virol. 74:3696-3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Navia, B. A., E. S. Cho, C. K. Petito, and R. W. Price. 1986. The AIDS dementia complex: II. Neuropathology. Ann. Neurol. 19:525-535. [DOI] [PubMed] [Google Scholar]
- 40.Nelson, J. A., C. Reynolds-Kohler, M. B. Oldstone, and C. A. Wiley. 1988. HIV and HCMV coinfect brain cells in patients with AIDS. Virology 165:286-290. [DOI] [PubMed] [Google Scholar]
- 41.Okabe, M., M. Ikawa, K. Kominami, T. Nakanishi, and Y. Nishimune. 1997. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 407:313-319. [DOI] [PubMed] [Google Scholar]
- 42.Oliveira, A. L., S. Thams, O. Lidman, F. Piehl, T. Hokfelt, K. Karre, H. Linda, and S. Cullheim. 2004. A role for MHC class I molecules in synaptic plasticity and regeneration of neurons after axotomy. Proc. Natl. Acad. Sci. USA 101:17843-17848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osborn, J. E. 1982. Cytomegalovirus and other herpeseviruses, p. 267-292. In H. Foster, D. Small, and J. C. Fox (ed.), The mouse in biomedical research, vol. II. Academic Press, Inc, New York, NY. [Google Scholar]
- 44.Palmon, A., S. Blagerman, S. Tel-Or, M. Pecht, N. Trainin, Y. Burstein, and B. Rager-Zisman. 1996. Treatment of murine cytomegalovirus salivary-gland infection by combined therapy with ganciclovir and thymic humoral factor gamma 2. Antiviral Res. 33:55-64. [DOI] [PubMed] [Google Scholar]
- 45.Patterson, C. E., D. M. Lawrence, L. A. Echols, and G. F. Rall. 2002. Immune-mediated protection from measles virus-induced central nervous system disease is noncytolytic and gamma interferon dependent. J. Virol. 76:4497-4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petersen, J. L., C. R. Morris, and J. C. Solheim. 2003. Virus evasion of MHC class I molecule presentation. J. Immunol. 171:4473-4478. [DOI] [PubMed] [Google Scholar]
- 47.Podlech, J., R. Holtappels, N. Wirtz, H. P. Steffens, and M. J. Reddehase. 1998. Reconstitution of CD8 T cells is essential for the prevention of multiple-organ cytomegalovirus histopathology after bone marrow transplantation. J. Gen. Virol. 79:2099-2104. [DOI] [PubMed] [Google Scholar]
- 48.Polic, B., S. Jonjic, I. Pavic, I. Crnkovic, I. Zorica, H. Hengel, P. Lucin, and U. H. Koszinowski. 1996. Lack of MHC class I complex expression has no effect on spread and control of cytomegalovirus infection in vivo. J. Gen. Virol. 77:217-225. [DOI] [PubMed] [Google Scholar]
- 49.Rall, G. F. 1998. CNS neurons: the basis and benefits of low class I major histocompatibility complex expression. Curr. Top. Microbiol. Immunol. 232:115-134. [DOI] [PubMed] [Google Scholar]
- 50.Reddehase, M. J. 2002. Antigens and immunoevasins: opponents in cytomegalovirus immune surveillance. Nat. Rev. Immunol. 2:831-844. [DOI] [PubMed] [Google Scholar]
- 51.Reddehase, M. J., W. Mutter, K. Munch, H. J. Buhring, and U. H. Koszinowski. 1987. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J. Virol. 61:3102-3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reddehase, M. J., F. Weiland, K. Munch, S. Jonjic, A. Luske, and U. H. Koszinowski. 1985. Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J. Virol. 55:264-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reuter, J. D. 2005. Cytomegalovirus induces T-cell independent apoptosis in the brain during immunodeficiency. J. Clin. Virol. 32:218-223. [DOI] [PubMed] [Google Scholar]
- 54.Reuter, J. D., D. L. Gomez, J. H. Wilson, and A. N. van den Pol. 2004. Systemic immune deficiency necessary for cytomegalovirus invasion of the mature brain. J. Virol. 78:1473-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reuter, J. D., B. E. Vivas-Gonzalez, D. Gomez, J. H. Wilson, J. L. Brandsma, H. L. Greenstone, J. K. Rose, and A. Roberts. 2002. Intranasal vaccination with a recombinant vesicular stomatitis virus expressing cottontail rabbit papillomavirus L1 protein provides complete protection against papillomavirus-induced disease. J. Virol. 76:8900-8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roback, J. D., M. S. Hossain, L. Lezhava, J. W. Gorechlad, S. A. Alexander, D. L. Jaye, S. Mittelstaedt, S. Talib, J. E. Hearst, C. D. Hillyer, and E. K. Waller. 2003. Allogeneic T cells treated with amotosalen prevent lethal cytomegalovirus disease without producing graft-versus-host disease following bone marrow transplantation. J. Immunol. 171:6023-6031. [DOI] [PubMed] [Google Scholar]
- 57.Roberts, A., E. Kretzschmar, A. S. Perkins, J. Forman, R. Price, L. Buonocore, Y. Kawaoka, and J. K. Rose. 1998. Vaccination with a recombinant vesicular stomatitis virus expressing an influenza virus hemagglutinin provides complete protection from influenza virus challenge. J. Virol. 72:4704-4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Setinek, U., E. Wondrusch, K. Jellinger, A. Steuer, M. Drlicek, W. Grisold, and F. Lintner. 1995. Cytomegalovirus infection of the brain in AIDS: a clinicopathological study. Acta Neuropathol. 90:511-515. [DOI] [PubMed] [Google Scholar]
- 59.Shresta, S., J. L. Kyle, H. M. Snider, M. Basavapatna, P. R. Beatty, and E. Harris. 2004. Interferon-dependent immunity is essential for resistance to primary dengue virus infection in mice, whereas T- and B-cell-dependent immunity are less critical. J. Virol. 78:2701-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Steffens, H. P., S. Kurz, R. Holtappels, and M. J. Reddehase. 1998. Preemptive CD8 T-cell immunotherapy of acute cytomegalovirus infection prevents lethal disease, limits the burden of latent viral genomes, and reduces the risk of virus recurrence. J. Virol. 72:1797-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tishon, A., H. Lewicki, G. Rall, M. Von Herrath, and M. B. Oldstone. 1995. An essential role for type 1 interferon-gamma in terminating persistent viral infection. Virology 212:244-250. [DOI] [PubMed] [Google Scholar]
- 62.van den Pol, A. N., J. D. Reuter, and J. G. Santarelli. 2002. Enhanced cytomegalovirus infection of developing brain independent of the adaptive immune system. J. Virol. 76:8842-8854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van den Pol, A. N., J. Vieira, D. D. Spencer, and J. G. Santarelli. 2000. Mouse cytomegalovirus in developing brain tissue: analysis of 11 species with GFP-expressing recombinant virus. J. Comp. Neurol. 427:559-580. [DOI] [PubMed] [Google Scholar]
- 64.Vinters, H. V. 1989. AIDS, cytomegalovirus, and the brainstem. Ann. Neurol. 25:311-312. [DOI] [PubMed] [Google Scholar]
- 65.Vogel, J. U., M. Scholz, and J. Cinatl, Jr. 1997. Treatment of cytomegalovirus diseases. Intervirology 40:357-367. [DOI] [PubMed] [Google Scholar]
- 66.Walter, E. A., P. D. Greenberg, M. J. Gilbert, R. J. Finch, K. S. Watanabe, E. D. Thomas, and S. R. Riddell. 1995. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N. Engl. J. Med. 333:1038-1044. [DOI] [PubMed] [Google Scholar]
- 67.Wiley, C. A., R. D. Schrier, F. J. Denaro, J. A. Nelson, P. W. Lampert, and M. B. Oldstone. 1986. Localization of cytomegalovirus proteins and genome during fulminant central nervous system infection in an AIDS patient. J. Neuropathol. Exp. Neurol. 45:127-139. [DOI] [PubMed] [Google Scholar]
- 68.Wysocki, L. J., and V. L. Sato. 1978. “Panning” for lymphocytes: a method for cell selection. Proc. Natl. Acad. Sci. USA 75:2844-2848. [DOI] [PMC free article] [PubMed] [Google Scholar]