Abstract
Although it is well documented that CD8 T cells play a critical role in controlling chronic viral infections, the mechanisms underlying the regulation of CD8 T-cell responses are not well understood. Using the mouse model of an acute and chronic lymphocytic choriomeningitis virus (LCMV) infection, we have examined the relative importance of peripheral T cells and thymic emigrants in the elicitation and maintenance of CD8 T-cell responses. Virus-specific CD8 T-cell responses were compared between mice that were either sham thymectomized or thymectomized (Thx) at ∼6 weeks of age. In an acute LCMV infection, thymic deficiency did not affect either the primary expansion of CD8 T cells or the proliferative renewal and maintenance of virus-specific lymphoid and nonlymphoid memory CD8 T cells. Following a chronic LCMV infection, in Thx mice, although the initial expansion of CD8 T cells was normal, the contraction phase of the CD8 T-cell response was exaggerated, which led to a transient but striking CD8 T-cell deficit on day 30 postinfection. However, the virus-specific CD8 T-cell response in Thx mice rebounded quickly and was maintained at normal levels thereafter, which indicated that the peripheral T-cell repertoire is quite robust and capable of sustaining an effective CD8 T-cell response in the absence of thymic output during a chronic LCMV infection. Taken together, these findings should further our understanding of the regulation of CD8 T-cell homeostasis in acute and chronic viral infections and might have implications in the development of immunotherapy.
It is well recognized that CD8 T cells play a critical role in control of chronic viral infections with human immunodeficiency virus (HIV), Epstein-Barr virus, cytomegalovirus, hepatitis B virus, and hepatitis C virus (HCV) (15, 21, 28, 34, 40, 43). The elicitation and maintenance of a potent CD8 T-cell response is crucial for long-term virus control and prevention of viral recrudescence. The inability of patients to control HIV, hepatitis B virus, and HCV is strongly correlated with a loss of virus-specific CD8 T-cell responses (7, 22, 23, 28, 43, 44). However, the homeostatic mechanisms underlying the regulation of CD8 T-cell responses during chronic viral infections are not well understood. Virus-specific CD8 T-cell numbers can be sustained during chronic viral infections by two distinct mechanisms that are not mutually exclusive: (i) one which is dependent upon the proliferation and/or maintenance of antigen-specific T cells that are present in the peripheral pool and (ii) one which is dependent upon recruitment and activation of recent thymic emigrants (38). Although T-cell maturation can occur in extrathymic sites, the thymus is considered the primary source of naive T cells for the maintenance of a diverse repertoire of T cells in the periphery (24). It is well documented that thymic output decreases with age as a result of a reduction in the thymic epithelial space, which is the site of active thymopoiesis (17, 24). Consequent to lower thymic output, there is an age-dependent reduction in the ratio of naive T cells to memory T cells in the peripheral lymphoid tissues and restriction of the peripheral T-cell repertoire (24, 41). However, the consequence of deficient thymic output in the maintenance of antiviral CD8 T-cell responses during a chronic viral infection is not known. Additionally, the ability of peripheral T cells to sustain an effective CD8 T-cell response during a chronic viral infection has not been tested in thymus-deficient animals. Furthermore, the impact of virus-induced thymic suppression (17) on the elicitation of antiviral CD8 T-cell responses in the periphery is not well understood. These issues need to be addressed because of their implication in the development of strategies to enhance T-cell responses in subjects with persistent viral infections.
The mouse model of lymphocytic choriomeningitis virus (LCMV) infection has provided seminal insights into the mechanisms that regulate CD8 T-cell responses in vivo. Depending upon the strain of virus used, LCMV infection can either be acute or chronic. Infection with the Armstrong strain of LCMV (LCMV-Arm) elicits a potent CD8 T-cell response that clears the virus in 8 to 10 days (45). In striking contrast, infection of mice with the clone 13 strain of LCMV (LCMV-clone 13) results in a protracted infection lasting up to 6 months (45). Studies on the CD8 T-cell response to LCMV-clone 13 have shown that establishment of a chronic infection is associated with a deletion of CD8 T cells specific to the Db-restricted NP396-404 and Kb-restricted GP34-41 epitopes (45, 47). Additionally, in concert with high levels of circulating LCMV-clone 13, CD8 T cells of other specificities exhibit various degrees of functional impairment in vitro in response to antigenic stimulation (45, 47). The existence of such functionally impaired CD8 T cells has also been reported in simian immunodeficiency virus, HCV, and HIV infections (14, 16, 20, 35, 42, 46). The role of thymic output in the regulation of virus-specific CD8 T-cell responses during a chronic LCMV infection has not been studied. In this study, we have investigated the impact of thymic deficiency on antigen-specific CD8 T-cell homeostasis and viral clearance during an acute and chronic LCMV infection.
MATERIALS AND METHODS
Mice.
C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). Thymectomized (Thx) and sham-thymectomized (SThx) C57BL/6 mice were purchased from Charles River Laboratory (Wilmington, MA). Surgeries were performed when the mice were approximately 6 weeks old and used 2 to 3 weeks after surgery. Mice were kept under specific-pathogen-free conditions, and all experiments were performed per institutional animal care guidelines.
Virus infection.
Mice were infected with either 2 × 105 PFU of LCMV-Arm intraperitoneally to initiate an acute infection or 2 × 106 PFU of LCMV-clone 13 intravenously to initiate a chronic infection or for rechallenge (1). Infectious LCMV in the tissues was quantitated by a plaque assay using Vero cells (1).
Quantitation of LCMV-specific CD8 T cells using MHC-I tetramers.
The major histocompatibility complex class I (MHC-I) tetramers specific to the Db-restricted LCMV cytotoxic T-lymphocyte (CTL) epitopes NP396-404 (NP396), GP33-41 (GP33), and GP276-286 (GP276) were prepared as described previously (32). Single-cell suspensions of splenocytes were stained with allophycocyanin-labeled MHC-I tetramers, phycoerythrin-labeled anti-CD8, and fluorescein isothiocyanate-labeled anti-CD44 antibodies at 4°C for 1 h. Following staining, cells were fixed in 2% paraformaldehyde, and samples were acquired with a FACSCalibur flow cytometer (BD Biosciences, La Jolla, CA). Flow cytometry data were analyzed using CellQuest software (BD Biosciences). All antibodies were purchased from BD PharMingen (La Jolla, CA).
In vivo cytotoxicity assay.
The MHC-I-restricted cytotoxic activity of CD8 T cells was assessed in vivo as described previously (13). Briefly, splenocytes from naive uninfected C57BL/6 mice were labeled with either 0.1 μM or 2 μM of CFSE (Molecular Probes, Eugene, OR) at room temperature for 5 min. Fetal calf serum was added to the CFSE labeling reaction to a final concentration of 20% (vol/vol) to quench the dye. Splenocytes labeled with 2 μM CFSE were pulsed with the CTL epitope peptide GP33-41 for 1 h at 37°C, while splenocytes labeled with 0.1 μM CFSE were not pulsed with any peptide. Peptide-pulsed and unpulsed CFSE-labeled splenocytes (target cells) were mixed in equal proportions and transferred into uninfected, LCMV-Arm-infected, and clone 13-infected SThx and Thx mice by tail vein injection; each mouse received 107 peptide-pulsed and unpulsed target cells. Recipient mice were sacrificed 5 to 6 h after target cell transfer, and single-cell suspensions of splenocytes were prepared by standard procedures. The recovery of peptide-pulsed and unpulsed target cells in each mouse was quantitated by flow cytometry. The percent killing was calculated as follows: 100 − {[(% peptide pulsed in infected/% unpulsed in infected)/(% peptide pulsed in uninfected/% unpulsed in uninfected)] × 100}.
Intracellular cytokine staining.
The number of LCMV-specific cytokine-producing CD8 T cells in the spleen was quantitated by staining for intracellular gamma interferon (IFN-γ) as described previously (32). Freshly explanted single-cell suspensions of splenocytes were cultured for 5 h with or without the LCMV CTL epitope peptides in the presence of brefeldin A and interleukin-2. After culture, the cells were stained with allophycocyanin-labeled anti-CD8 and fluorescein isothiocyanate-labeled anti-IFN-γ using the Cytofix/Cytoperm kit (BD PharMingen). After staining, cells were fixed in 2% paraformaldehyde, and samples were acquired and analyzed as described above.
Measurement of BrdU incorporation in vivo.
The proliferation of CD8 T cells was monitored in vivo by feeding mice with 5-bromo-2′-deoxyuridine (BrdU) in drinking water (0.8 mg/ml) (32). At the end of BrdU treatment, splenocytes or hepatic mononuclear cells were stained with anti-CD8, anti-CD44, and MHC-I tetramers. Following surface staining, cells were stained for intracellular BrdU using anti-BrdU antibodies. The number of BrdU-positive cells was determined by flow cytometry as described above.
Statistical analysis.
Statistical analyses of data were performed using Systat (Chicago, IL) version 10.2 software program. Groups were compared by the Student's t test, and significance was defined as a P value of ≤0.05.
RESULTS
Role of thymic output in regulating primary antigen-specific CD8 T-cell responses to an acute lymphocytic choriomeningitis virus infection.
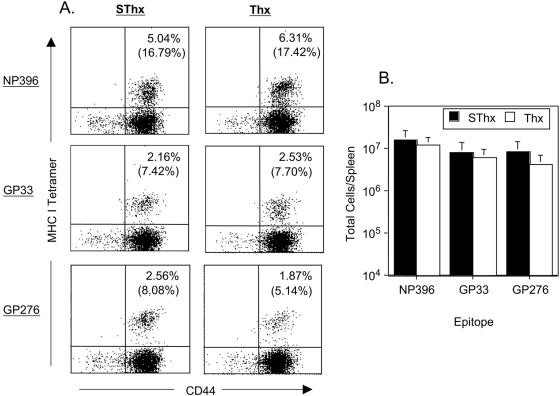
Here, we determined the effect of thymic deficiency on CD8 T-cell responses to an acute viral infection by infecting control SThx and Thx mice with LCMV-Arm. LCMV-specific CD8 T cells were enumerated in the spleen using MHC-I tetramers (Db) specific to the CTL epitopes NP396, GP33, and GP276. Data in Fig. 1A and B show that on day 8 postinfection (p.i.), the frequencies and total number of LCMV-specific CD8 T cells in the spleen of SThx and Thx mice were comparable. These data indicated that thymectomy at ∼6 weeks of age did not have any detectable adverse effect on the primary expansion of CD8 T cells during an acute LCMV infection. Consistent with elicitation of strong virus-specific CD8 T-cell responses, both SThx and Thx mice resolved LCMV-Arm by day 8 p.i. (data not shown). These data are consistent with previous work which showed that primary CD8 T-cell responses are normal in Thx mice at least until 4 to 5 months after thymectomy (11).
FIG. 1.
Effect of thymectomy on virus-specific CD8 T-cell responses during an acute LCMV infection. Groups of SThx and Thx mice were infected with LCMV-Arm. On the eighth day after infection, the number of CD8 T cells specific to the CTL epitopes NP396, GP33, and GP276 was determined by staining splenocytes with anti-CD8, anti-CD44, and MHC-I tetramers. The dot plots in panel A are gated on total CD8 T cells, and the numbers are the percentages of tetramer-binding CD8 T cells of total splenocytes or total CD8 T cells (parentheses). The data in panel B represent absolute numbers of epitope-specific CD8 T cells in the spleen. The data are the means of three mice/group ± standard deviations (SD) and are representative of two independent experiments.
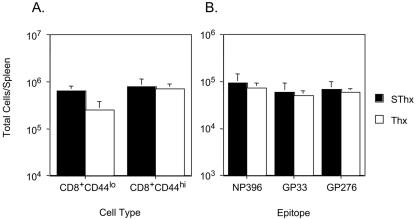
Effect of thymic deficiency on CD8 T-cell memory and homeostatic turnover of naive and antigen-specific memory CD8 T cells in an acute LCMV infection.
Recovery from an acute LCMV infection is associated with the development of potent CD8 T-cell memory and lifelong immunity to reinfection (32). Forty days after infection with LCMV-Arm, we investigated the effect of thymectomy on the generation and maintenance of memory CD8 T cells in immune mice. As shown in Fig. 2A, during the memory phase of the CD8 T-cell response, the total number of naive (CD44lo) CD8 T cells in the spleens of Thx mice was lower than that in SThx mice, which confirms that the thymus is the dominant source of naive T cells in mice. In contrast to reduced naive T-cell numbers, the total number of activated/memory (CD44hi) CD8 T cells in the spleens of Thx mice was comparable to those in SThx mice. These data suggested that the lack of thymic output influenced the homeostasis of only naive T cells but not activated/memory T cells in the periphery. To examine the effect of thymic deficiency on the homeostasis of antigen-specific memory T cells, we quantitated LCMV-specific memory CD8 T cells in the spleen of SThx and Thx mice using MHC-I tetramers. As illustrated in Fig. 2B, the number of LCMV-specific memory CD8 T cells in the spleen of Thx mice was similar to that in SThx mice. Similar findings were noted when LCMV-specific CD8 T-cell memory was assessed 160 days later (data not shown). Taken together, thymectomy at 6 weeks of age did not lead to appreciable alterations in the number of memory CD8 T cells generated following an acute LCMV infection.
FIG. 2.
Effect of thymic deficiency on CD8 T-cell memory in an acute viral infection. Groups of SThx and Thx mice were infected with LCMV-Arm, and on day 40 postinfection, the number of CD8 T cells in the spleen that are specific to the indicated epitopes was determined by staining with anti-CD8, anti-CD44, and MHC-I tetramers. The data in panel A are the total number of naive (CD8+ CD44lo) and activated/memory (CD8+ CD44hi) CD8 T cells in the spleen. The data in panel B are the total numbers of epitope-specific CD8 T cells in the spleen. The data shown are the means of three mice/group ± SD and are representative of two independent experiments.
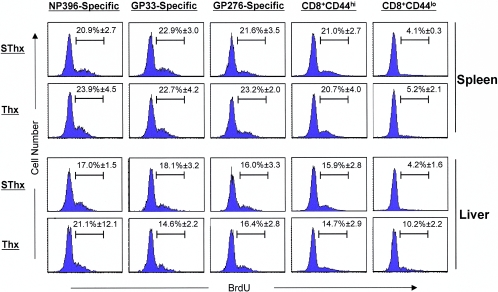
In the absence of thymic output, the peripheral naive T-cell pool is maintained by expansion and/or extended longevity of the preexisting T cells and T-cell development in extrathymic locations (17, 41). On the other hand, memory T cells are maintained by slow proliferation, termed proliferative renewal, under the influence of cytokines (36). Under normal homeostatic conditions, it is believed that naive and memory T cells belong to separate niches and are subject to independent regulatory mechanisms (12). However, it was of interest to examine the effect of thymic deficiency on the proliferation of naive and antigen-specific memory CD8 T cells during the memory phase of the CD8 T-cell response to an acute viral infection. About 200 days after LCMV infection, we compared the proliferation of naive CD8 T cells and LCMV-specific memory CD8 T cells in vivo in both lymphoid and nonlymphoid organs between SThx and Thx mice. As illustrated in Fig. 3, in SThx mice, as expected, the naive CD8 T cells (CD44lo) in both spleen and liver showed very little proliferation during a period of 8 days. Interestingly, in the Thx mice, while the rate of naive CD8 T-cell turnover in the spleen was comparable to that in SThx mice, the proliferation of naive CD8 T cells in the liver of Thx mice was higher than that in SThx mice. The turnover rate of activated/memory (CD44hi) CD8 T cells in both SThx and Thx mice was higher than that of the naive CD8 T cells. However, the relative proportions of proliferating cells among activated/memory CD8 T cells in both spleen and liver were comparable between SThx and Thx mice. Consistent with these findings, thymic deficiency had a minimal impact on the turnover of LCMV-specific memory CD8 T cells in both lymphoid and nonlymphoid organs.
FIG. 3.
Effect of thymic deficiency on the proliferative renewal of LCMV-specific memory CD8 T cells following an acute viral infection. About 200 days after infection with LCMV-Arm, groups of SThx and Thx mice were treated with BrdU for 8 days in drinking water. At the end of BrdU pulse, splenocytes or mononuclear cells isolated from the liver were stained with anti-CD8, anti-CD44, MHC-I tetramers, and anti-BrdU antibodies and analyzed by flow cytometry. The histograms are gated on the indicated subsets of CD8 T cells, and the numbers are the percentages of BrdU-positive cells among the gated population. The data are the means of three mice/group ± SD.
Role of thymic output in regulating the kinetics of antigen-specific CD8 T-cell responses during a chronic lymphocytic choriomeningitis virus infection.
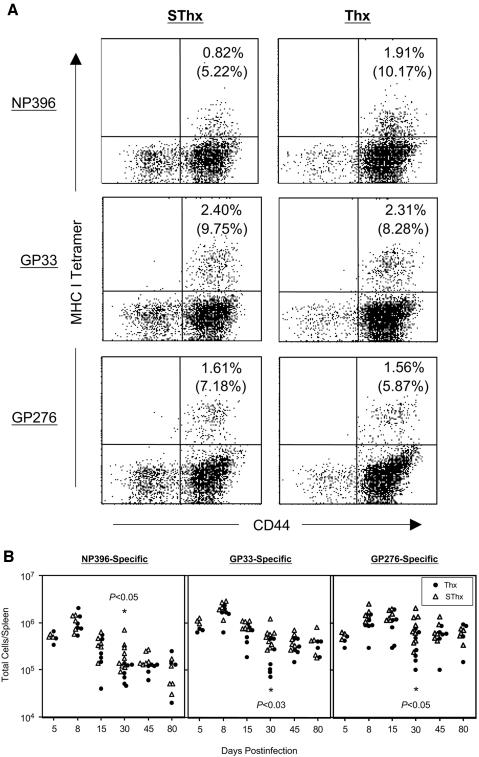
Next, we determined whether a lack of thymic output would affect the kinetics of the virus-specific CD8 T-cell response during a chronic LCMV infection. Groups of SThx and Thx mice were infected with LCMV-clone 13, and the kinetics of the CD8 T-cell response was studied using MHC-I tetramers (Fig. 4). As shown in Fig. 4, at the peak of the CD8 T-cell response (day 8 p.i.), the frequencies and total number of LCMV-specific CD8 T cells in SThx and Thx mice were similar. These data suggested that thymectomy did not significantly affect the expansion phase of the CD8 T-cell response to LCMV-clone 13 infection. Following the expansion phase, the CD8 T-cell response in both SThx and Thx mice underwent a phase of programmed contraction. In the SThx mice, between days 8 and 30 p.i., there was an approximately 4.3-, 4.0-, and 1.8-fold drop in the total number of NP396-specific, GP33-specific, and GP276-specific CD8 T cells, respectively. Notably, the contraction phase of the CD8 T-cell response was more accentuated in the Thx mice than that in the SThx mice; between days 8 and 30 p.i., the total number of NP396-, GP33-, and GP276-specific CD8 T cells dropped by ∼12-, 7.4-, and 2.5-fold, respectively, in the Thx mice. On day 30 p.i., the total number of LCMV-specific CD8 T cells in the spleens of Thx mice was significantly lower than that in SThx mice. However, after day 30 p.i., the number of LCMV-specific CD8 T cells in Thx mice rebounded to levels comparable to those in SThx mice.
FIG. 4.
CD8 T-cell responses to a chronic LCMV infection in thymectomized mice. Groups of SThx and Thx mice were infected with LCMV-clone 13, and the number of virus-specific CD8 T cells in the spleen was determined by staining with anti-CD8, anti-CD44, and MHC-I tetramers. The dot plots represent staining for LCMV-specific CD8 T cells on day 8 postinfection, and the numbers are the percentages of epitope-specific CD8 T cells among splenocytes or total CD8 T cells (parentheses). Data in the bottom panel are the total number of CD8 T cells in the spleen that are specific to the indicated epitopes on various days after infection. Each data point represents the cell numbers of an individual mouse.
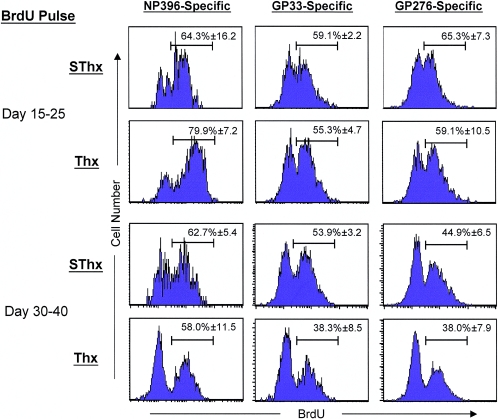
The transient nature of the CD8 T-cell defect in Thx mice was not due to incomplete thymectomy, because (i) careful postmortem visual inspection revealed no evidence of thymic remnants in any of the Thx mice and (ii) thymectomy resulted in a significant reduction in the number of naive CD8 T cells (Fig. 2A) over time, which is in complete agreement with previously published data (11). The differences in the kinetics of the CD8 T-cell response between SThx and Thx mice could be a sequel to alterations in the in vivo rate of proliferation of LCMV-specific CD8 T cells. First, we investigated whether deficits in the proliferation of LCMV-specific CD8 T cells accounted for the exaggerated loss of CD8 T cells in Thx mice (between days 15 and 30 p.i.). Groups of SThx and Thx mice were infected with LCMV-clone 13 and treated with BrdU between days 15 and 25 p.i., and BrdU incorporation by LCMV-specific CD8 T cells was assessed by flow cytometry. A large proportion of LCMV-specific CD8 T cells were actively proliferating in vivo in both SThx and Thx mice during the BrdU pulse (Fig. 5). However, data in Fig. 5 show that similar proportions of LCMV-specific CD8 T cells incorporated BrdU in SThx and Thx mice between days 15 and 25 p.i. Next, we assessed the proliferation of LCMV-specific CD8 T cells between days 30 and 40 p.i. in SThx and Thx mice. Data in Fig. 5 show that between days 30 and 40 p.i., comparable numbers of LCMV-specific CD8 T cells incorporated BrdU in SThx and Thx mice. Taken together, these data suggested that a transient deficit in the number of LCMV-specific CD8 T cells in Thx mice might not be a consequence of an in vivo proliferative defect.
FIG. 5.
Proliferation of virus-specific CD8 T cells in thymectomized mice during a chronic LCMV infection. SThx and Thx mice were infected with LCMV-clone 13. Between days 15 and 25 and 30 to 40 after infection, mice were treated with BrdU in drinking water. At the end of each pulse, splenocytes were stained with anti-CD8, MHC-I tetramers, and anti-BrdU and analyzed by flow cytometry. The histograms showing BrdU staining are gated on tetramer-binding CD8 T cells. The numbers are the percentages of BrdU-positive cells among the tetramer-binding CD8 T cells ± SD (3 mice/group).
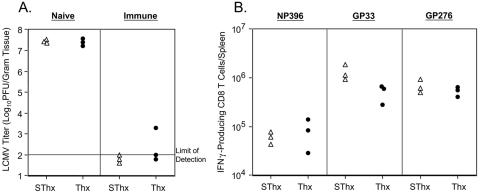
Resolution of a chronic LCMV infection in the absence of thymic output.
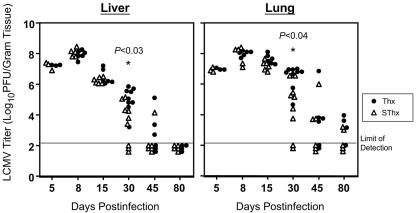
It was of interest to determine whether the accelerated loss of LCMV-specific CD8 T cells in Thx mice affected clearance of LCMV-clone 13 from the tissues. To address this issue, we quantitated infectious LCMV levels in liver and lung on various days after infection (Fig. 6). Compared to SThx mice, Thx mice exhibited delayed clearance of LCMV-clone 13 from the tissues. On days 5, 8, and 15 p.i., the viral load in the tissues of Thx and SThx mice were comparable. However, notably, on day 30 p.i., the viral titers in the liver and lung of Thx mice were 50- to 100-fold higher than in SThx mice. On day 45 p.i., while five of six SThx mice resolved LCMV in the liver, three of six livers from Thx mice contained relatively high levels of infectious virus. By day 80 p.i., while both SThx and Thx mice resolved LCMV from the liver, the viral titers in the lungs of Thx mice were slightly higher than those in SThx mice. Taken together, these data show that the absence of thymic output might delay normal viral clearance during a chronic LCMV infection.
FIG. 6.
Effect of thymectomy on viral clearance during a chronic LCMV infection. Following LCMV-clone 13 infection, the viral titers in the liver and lung of sham-thymectomized (▵) and thymectomized (•) mice were quantitated by a plaque assay using Vero cells. Each data point represents the viral titer of an individual mouse.
CD8 T-cell function during a chronic LCMV infection in the absence of thymic output.
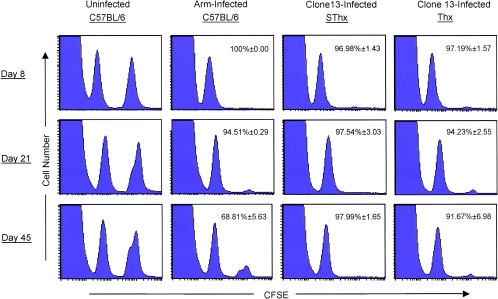
The control of LCMV-clone 13 infection is dependent upon the presence of functional virus-specific CD8 T cells (27, 45, 47). CD8+ T cells exert their effector function via cell-mediated cytotoxicity and/or producing cytokines like IFN-γ and tumor necrosis factor alpha. First, we assessed the class I-restricted cytotoxic activity of LCMV-specific CD8 T cells using an in vivo cytotoxicity assay. As shown in Fig. 7, as expected, uninfected mice showed no detectable CTL activity, while mice infected with LCMV-Arm showed potent in vivo CTL activity. Importantly, LCMV-specific CD8 T cells present in both SThx and Thx mice exhibited strong cytotoxic activity in vivo on days 8, 21, and 45 p.i.; GP33-specific CD8 T cells in both SThx and Thx mice promptly eliminated the peptide-pulsed target cells within 5 h after adoptive transfer (Fig. 7). These data suggested that delayed clearance of LCMV in Thx mice cannot be attributed to a defect in the ability of effector CD8 T cells to perform cytotoxicity in vivo.
FIG. 7.
In vivo cytotoxic activity in thymectomized mice in a chronic LCMV infection. On days 8, 21, and 45 after infection with LCMV-clone 13, the cytotoxic activity in the spleens of SThx and Thx mice was assessed in vivo as described in Materials and Methods. Uninfected and LCMV-Arm-infected C57BL/6 mice were used as negative and positive controls, respectively. The numbers represent percent specific cytotoxicity ± SD, and the data are the means of four mice/group.
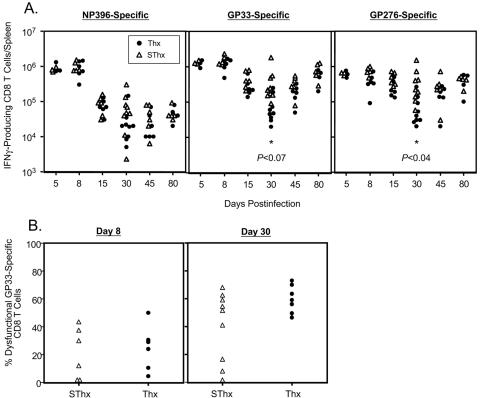
Previous work has shown that cytokines might be important in the resolution of an LCMV infection (25, 39). Therefore, we examined the effect of thymectomy on the maintenance of cytokine-producing virus-specific CD8 T cells in mice during a chronic infection with LCMV-clone 13. The number of cytokine-producing LCMV-specific CD8 T cells was enumerated using intracellular staining for IFN-γ following antigenic stimulation ex vivo. As shown in Fig. 8A, on days 5 and 8 p.i., the spleens of SThx and Thx mice contained comparable numbers of LCMV-specific IFN-γ-producing CD8 T cells. In both SThx and Thx mice, after day 8 p.i., there was a marked drop in the number of IFN-γ-producing NP396-specific CD8 T cells, and they were barely detectable by day 30 p.i. Between days 8 and 15 p.i., the number of IFN-γ-producing GP33- and GP276-specific CD8 T cells dropped by ∼4- and 2-fold, respectively, in the SThx mice and showed minimal change thereafter. Strikingly, substantially greater numbers of IFN-γ-producing CD8 T cells were lost in Thx mice between days 8 and 30 p.i. compared to SThx mice (Fig. 8A). In Thx mice, the magnitude of reduction in the number of GP33- and GP276-specific IFN-γ-producing CD8 T cells between days 8 and 30 p.i. was ∼14- and 5-fold, respectively. As a result, on day 30 p.i., there were approximately fivefold fewer functional LCMV-specific CD8 T cells in the spleens of Thx mice than in those of SThx mice. The striking reduction in the number of functional LCMV-specific CD8 T cells on day 30 p.i. in Thx mice is at least partly due to clonal deletion (Fig. 4, bottom). However, functional unresponsiveness of LCMV-specific CD8 T cells on day 30 p.i. was also a contributing factor, because simultaneous enumeration of LCMV-specific CD8 T cells by both MHC-I tetramers (phenotypic) and intracellular cytokine staining (functional) indicated that a slightly greater proportion of GP33-specific CD8 T cells in Thx mice were unable to produce IFN-γ (dysfunctional) upon stimulation ex vivo (Fig. 8B). Nonetheless, in the ensuing 50 days, the number of IFN-γ-producing CD8 T cells in Thx mice reached levels similar to those in SThx mice. Taken together, these data suggested that delayed virus clearance in Thx mice correlates with the transient reduction in the number of functional cytokine-producing CD8 T cells in vivo. By 45 days p.i., most of the SThx and Thx mice had resolved LCMV-clone 13 from the liver (Fig. 6). We were interested to examine whether CD8 T cells in LCMV-clone 13-infected SThx and Thx mice differed in their ability to protect against reinfection in the liver. To this end, we rechallenged naive and LCMV-immune SThx and Thx mice with LCMV-clone 13 and examined them for protection 5 days later (Fig. 9). As expected, the viral titers in the liver of LCMV-clone 13-infected naive SThx and Thx mice were very high (Fig. 9A). In striking contrast, viral titers in the livers of LCMV-immune SThx and Thx mice were extremely low or below the level of detection. We also quantitated the number of LCMV-specific CD8 T cells in the spleens of rechallenged LCMV-immune SThx and Thx mice (Fig. 9B). The numbers of NP396- and GP276-specific CD8 T cells in the spleens of rechallenged SThx and Thx mice were comparable (Fig. 9B). However, the spleens of SThx mice contained ∼3-fold more GP33-specific CD8 T cells than those in Thx mice. Thus, thymectomy did not preclude the development of protective immunity to reinfection in LCMV-clone 13-immune mice.
FIG. 8.
Virus-specific IFN-γ-producing CD8 T cells in the spleen of thymectomized mice during a chronic LCMV infection. (A) On the indicated days after LCMV-clone 13 infection, the number of epitope-specific IFN-γ-producing CD8 T cells in the spleen of SThx (▵) and Thx (•) mice was quantitated ex vivo by intracellular staining. Each data point represents the total number of epitope-specific IFN-γ-producing CD8 T-cell numbers in an individual mouse. (B) On days 8 and 30 after LCMV-clone 13 infection, the number of GP33-specific CD8 T cells in the spleen was enumerated by tetramer staining and intracellular IFN-γ staining. The data are the percentages of GP33-specific tetramer-binding CD8 T cells that failed to produce IFN-γ. Each data point represents the percentage of dysfunctional GP33-specific CD8 T cells in individual mice.
FIG. 9.
Protective immunity against rechallenge in LCMV-immune SThx and Thx mice. Approximately 45 days after LCMV-clone 13 infection, Thx and SThx mice were rechallenged with LCMV-clone 13. Naive SThx and Thx mice were infected as controls. Five days after rechallenge, the viral titers in the liver were quantitated by plaque assay (A), and the numbers of LCMV-specific CD8 T cells in the spleen were enumerated by intracellular cytokine staining (B). The plotted data are derived from individual mice.
DISCUSSION
A thorough understanding of the mechanisms underlying the regulation of CD8 T-cell responses during chronic viral infections is imperative for the development of effective prophylactic and immunotherapeutic strategies. In this study, we have investigated an important aspect of CD8 T-cell responses during a chronic LCMV infection: the relative importance of the peripheral T-cell repertoire versus recent thymic emigrants in the long-term maintenance of virus-specific T-cell responses. We show that thymic deficiency in adults can lead to transient T-cell deficits and delayed viral clearance with minimal impact on the long-term maintenance of T-cell responses during a chronic viral infection.
It is well established that progressive disease with HIV and HCV is associated with a loss of CTL responses (7, 22, 23, 28, 43, 44). Therefore, it makes sense to develop therapeutic strategies to enhance and/or maintain strong CD8 T-cell responses during chronic viral infections. However, mechanisms underlying the maintenance of T-cell responses during chronic viral infections are not well characterized. Chronic viral infections are associated with high rates of T-cell turnover over an extended period of time (4, 5, 10, 26, 30, 31, 37). Despite the continuing proliferation, the number of activated/memory T cells does not increase over time in chronic infections like HIV, which suggests a scenario where cellular apoptotic rates exceed the rate of proliferation (2, 18, 19, 28, 29, 30). Extended periods of proliferation will likely predispose CD8 T cells to activation-induced cell death and possibly replicative senescence (6, 8, 9, 33, 45). It is not known whether recruitment of naive CD8 T cells is needed as “reinforcement” to maintain CD8 T-cell numbers during extended periods of high T-cell turnover. In this study, we asked the question of whether the peripheral T-cell compartment is capable of sustaining CD8 T-cell responses in the absence of thymic output during chronic infection of mice with LCMV. The CD8 T-cell response during a chronic LCMV infection can be divided into three phases: the expansion phase (days 0 to 8 p.i.), the contraction phase (days 8 to 30 p.i.), and the phase of stability. Consistent with previously published findings, in LCMV-clone 13-infected SThx mice, the phase of CD8 T-cell expansion was followed by the contraction phase (3). By contrast, in LCMV-clone 13-infected Thx mice, following a normal expansion phase, the contraction phase was characterized by an exaggerated loss of virus-specific CD8 T cells between days 8 and 30 p.i. which was followed by a strong rebound in CD8 T-cell numbers between days 30 and 45 p.i. The mechanism(s) underlying the pronounced contraction and rebound of CD8 T cells in Thx mice is unclear. In vivo BrdU incorporation studies showed that similar percentages of LCMV-specific CD8 T cells incorporated BrdU in SThx and Thx mice between days 15 and 25 p.i., which suggested that the increased loss of virus-specific CD8 T cells in Thx mice between days 8 and 30 p.i. might not be due to “sluggish” proliferation of CD8 T cells. Furthermore, we did not find correlations between the in vivo proliferation rate and the observed rebound in the number of LCMV-specific CD8 T cells in the spleen of Thx mice after day 30 p.i. However, it is worth pointing out that BrdU incorporation studies quantitated only the percentages of LCMV-specific CD8 T cells that incorporated BrdU in a 10-day period and not the number of cell divisions. Although precise measurements of the number of cell divisions in a given time interval should resolve this issue, current technologies preclude such in vivo assessments during an ongoing immune response. If indeed the proliferation rates of LCMV-specific CD8 T cells are unaffected by thymic deficiency, perhaps the apoptotic rates of LCMV-specific CD8 T cells are different between SThx and Thx mice at different stages of the T-cell response. It should be noted that on day 30 p.i., viral load in the nonlymphoid tissues of LCMV-clone 13-infected Thx mice was greater than that in SThx mice. Therefore, it is possible that a reduced number of LCMV-specific CD8 T cells in the spleen of Thx mice on day 30 p.i. might be due to sequestration of CD8 T cells in the infected peripheral tissues. After day 30 p.i., with reducing viral load in the peripheral tissues, LCMV-specific CD8 T cells in Thx mice might relocate to the spleen.
In comparison to SThx mice, clearance of LCMV-clone 13 is slightly delayed in Thx mice. The viral burden in Thx mice, particularly on day 30 p.i., was higher than that in SThx mice. It is likely that perforin-dependent cytotoxicity and noncytolytic cytokine-dependent antiviral mechanisms are both important in the CD8 T-cell-dependent resolution of a chronic LCMV infection. GP33-specific CD8 T cells in both SThx and Thx mice exhibited very good CTL activity in vivo on days 8, 21, and 45 p.i., which suggested that the inefficient control of LCMV-clone 13 in Thx mice might not be due to a defect in cell-mediated cytotoxicity. On day 30 p.i., higher viral titers in Thx mice correlated with a reduced number of cytokine-producing CD8 T cells in the spleen. However, in the ensuing 2 weeks, virus-specific CD8 T cells in Thx mice regained their cytokine-producing ability and cleared LCMV-clone 13, albeit with a slight delay. In summary, a slight delay in LCMV clearance in Thx mice might be related to a transient deficit in cytokine production by virus-specific CD8 T cells.
In summary, first, we have documented that thymic deficiency has a minimal impact on the expansion phase of the CD8 T-cell response and regulation of memory CD8 T-cell homeostasis during an acute LCMV infection. Second, we show that during a chronic LCMV infection, thymic deficiency led to only a transient deficit in CD8 T-cell function and a slight delay in viral clearance. Importantly, we show that the peripheral T-cell repertoire is extremely robust and competent in sustaining T-cell responses during a protracted infection in the absence of thymic output. In conclusion, the findings presented in this paper should further our understanding of the dynamics of T-cell responses in chronic viral infections and also may have implications in immune therapy of human viral infections.
Acknowledgments
This work was supported by Public Health Service grants AI48785 and AI59804 to M.S. from the NIH. J.R.B. was supported by a Merck-Merial fellowship.
We thank John D. Altman (Emory University) for providing the MHC-I tetramers. We appreciate the technical help by Katie Skell.
REFERENCES
- 1.Ahmed, R., A. Salmi, L. D. Butler, J. M. Chiller, and M. B. Oldstone. 1984. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160:521-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appay, V., L. Papagno, C. A. Spina, P. Hansasuta, A. King, L. Jones, G. S. Ogg, S. Little, A. J. McMichael, D. D. Richman, and S. L. Rowland-Jones. 2002. Dynamics of T cell responses in HIV infection. J. Immunol. 168:3660-3666. [DOI] [PubMed] [Google Scholar]
- 3.Badovinac, V. P., B. B. Porter, and J. T. Harty. 2002. Programmed contraction of CD8(+) T cells after infection. Nat. Immunol. 3:619-626. [DOI] [PubMed] [Google Scholar]
- 4.Barry, S. M., M. A. Johnson, and G. Janossy. 2003. Increased proportions of activated and proliferating memory CD8+ T lymphocytes in both blood and lung are associated with blood HIV viral load. J. Acquir. Immune Defic. Syndr. 34:351-357. [DOI] [PubMed] [Google Scholar]
- 5.Benlhassan-Chahour, K., C. Penit, V. Dioszeghy, F. Vasseur, G. Janvier, Y. Riviere, N. Dereuddre-Bosquet, D. Dormont, R. Le Grand, and B. Vaslin. 2003. Kinetics of lymphocyte proliferation during primary immune response in macaques infected with pathogenic simian immunodeficiency virus SIVmac251: preliminary report of the effect of early antiviral therapy. J. Virol. 77:12479-12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenchley, J. M., N. J. Karandikar, M. R. Betts, D. R. Ambrozak, B. J. Hill, L. E. Crotty, J. P. Casazza, J. Kuruppu, S. A. Migueles, M. Connors, M. Roederer, D. C. Douek, and R. A. Koup. 2003. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 101:2711-2720. [DOI] [PubMed] [Google Scholar]
- 7.Chisari, F. V., and C. Ferrari. 1995. Hepatitis B virus immunopathology. Springer Semin. Immunopathol. 17:261-281. [DOI] [PubMed] [Google Scholar]
- 8.Dagarag, M., H. Ng, R. Lubong, R. B. Effros, and O. O. Yang. 2003. Differential impairment of lytic and cytokine functions in senescent human immunodeficiency virus type 1-specific cytotoxic T lymphocytes. J. Virol. 77:3077-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davenport, M. P., C. Fazou, A. J. McMichael, and M. F. Callan. 2002. Clonal selection, clonal senescence, and clonal succession: the evolution of the T cell response to infection with a persistent virus. J. Immunol. 168:3309-3317. [DOI] [PubMed] [Google Scholar]
- 10.De Boer, R. J., H. Mohri, D. D. Ho, and A. S. Perelson. 2003. Turnover rates of B cells, T cells, and NK cells in simian immunodeficiency virus-infected and uninfected rhesus macaques. J. Immunol. 170:2479-2487. [DOI] [PubMed] [Google Scholar]
- 11.Di Rosa, F., S. Ramaswamy, J. P. Ridge, and P. Matzinger. 1999. On the lifespan of virgin T lymphocytes. J. Immunol. 163:1253-1257. [PubMed] [Google Scholar]
- 12.Freitas, A. A., and B. Rocha. 2000. Population biology of lymphocytes: the flight for survival. Annu. Rev. Immunol. 18:83-111. [DOI] [PubMed] [Google Scholar]
- 13.Fuller, M. J., A. Khanolkar, A. E. Tebo, and A. J. Zajac. 2004. Maintenance, loss, and resurgence of T cell responses during acute, protracted, and chronic viral infections. J. Immunol. 172:4204-4214. [DOI] [PubMed] [Google Scholar]
- 14.Goepfert, P. A., A. Bansal, B. H. Edwards, G. D. Ritter, Jr., I. Tellez, S. A. McPherson, S. Sabbaj, and M. J. Mulligan. 2000. A significant number of human immunodeficiency virus epitope-specific cytotoxic T lymphocytes detected by tetramer binding do not produce gamma interferon. J. Virol. 74:10249-10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grakoui, A., N. H. Shoukry, D. J. Woollard, J. Han, H. L. Hanson, J. Ghrayeb, K. K. Murthy, C. M. Rice, and C. M. Walker. 2003. HCV persistence and immune evasion in the absence of memory T cell help. Science 302:659-662. [DOI] [PubMed] [Google Scholar]
- 16.Gruener, N. H., F. Lechner, M. C. Jung, H. Diepolder, T. Gerlach, G. Lauer, B. Walker, J. Sullivan, R. Phillips, G. R. Pape, and P. Klenerman. 2001. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J. Virol. 75:5550-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haynes, B. F., M. L. Markert, G. D. Sempowski, D. D. Patel, and L. P. Hale. 2000. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu. Rev. Immunol. 18:529-560. [DOI] [PubMed] [Google Scholar]
- 18.Hazenberg, M. D., et al. 2000. Increased cell division but not thymic dysfunction rapidly affects the T-cell receptor excision circle content of the naive T cell population in HIV-1 infection. Nat. Med. 6:1036-1042. [DOI] [PubMed] [Google Scholar]
- 19.Hellerstein, M. K., R. A. Hoh, M. B. Hanley, D. Cesar, D. Lee, R. A. Neese, and J. M. McCune. 2003. Subpopulations of long-lived and short-lived T cells in advanced HIV-1 infection. J. Clin. Investig. 112:956-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kostense, S., G. S. Ogg, E. H. Manting, G. Gillespie, J. Joling, K. Vandenberghe, E. Z. Veenhof, D. van Baarle, S. Jurriaans, M. R. Klein, and F. Miedema. 2001. High viral burden in the presence of major HIV-specific CD8(+) T cell expansions: evidence for impaired CTL effector function. Eur. J. Immunol. 31:677-686. [DOI] [PubMed] [Google Scholar]
- 21.Lechner, F., D. K. H. Wong, P. R. Dunbar, R. Chapman, R. T. Chung, P. Dohrenwend, G. Robbins, R. Phillips, P. Klenerman, and B. D. Walker. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191:1499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lechner, F., N. H. Gruener, S. Urbani, J. Uggeri, T. Santantonio, A. R. Kammer, A. Cerny, R. Phillips, C. Ferrari, G. R. Pape, and P. Klenerman. 2000. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur. J. Immunol. 30:2479-2487. [DOI] [PubMed] [Google Scholar]
- 23.Lieberman, J., P. Shankar, N. Manjunath, and J. Andersson. 2001. Dressed to kill? A review of why antiviral CD8 T lymphocytes fail to prevent progressive immunodeficiency in HIV-1 infection. Blood 98:1667-1677. [DOI] [PubMed] [Google Scholar]
- 24.Linton, P. J., and K. Dorshkind. 2004. Age-related changes in lymphocyte development and function. Nat. Immunol. 2:133-139. [DOI] [PubMed] [Google Scholar]
- 25.Lohman, B. L., and R. M. Welsh. 1998. Apoptotic regulation of T cells and absence of immune deficiency in virus-infected gamma interferon receptor knockout mice. J. Virol. 72:7815-7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Macallan, D. C., D. L. Wallace, A. J. Irvine, B. Asquith, A. Worth, H. Ghattas, Y. Zhang, G. E. Griffin, D. F. Tough, and P. C. Beverley. 2003. Rapid turnover of T cells in acute infectious mononucleosis. Eur. J. Immunol. 33:2655-2665. [DOI] [PubMed] [Google Scholar]
- 27.Matloubian, M., R. J. Concepcion, and R. Ahmed. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68:8056-8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMichael, A. J., and S. L. Rowland-Jones. 2001. Cellular immune response to HIV. Nature 410:980-987. [DOI] [PubMed] [Google Scholar]
- 29.Meyaard, L., et al. 1992. Programmed death of T cells in HIV-1 infection. Science 257:217-219. [DOI] [PubMed] [Google Scholar]
- 30.Mohri, H., A. S. Perelson, K. Tung, R. M. Ribeiro, B. Ramratnam, M. Markowitz, R. Kost, A. Hurley, L. Weinberger, D. Cesar, M. K. Hellerstein, and D. D. Ho. 2001. Increased turnover of T lymphocytes in HIV-1 infection and its reduction by antiretroviral therapy. J. Exp. Med. 194:1277-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monceaux, V., R. H. T. Fang, M. C. Cumont, B. Hurtrel, and J. Estaquier. 2003. Distinct cycling CD4+- and CD8+-T-cell profiles during the asymptomatic phase of simian immunodeficiency virus SIVmac251 infection in rhesus macaques. J. Virol. 77:10047-10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murali-Krishna, K., J. D. Altman, M. Suresh, D. J. Sourdive, A. J. Zajac, J. D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8:177-187. [DOI] [PubMed] [Google Scholar]
- 33.Papagno, L., C. A. Spina, A. Marchant, M. Salio, N. Rufer, S. Little, T. Dong, G. Chesney, A. Waters, P. Easterbrook, P. R. Dunbar, D. Sheperd, V. Cerundolo, V. Emery, P. Griffiths, C. Conlon, A. J. McMichael, D. D. Richman, S. L. Rowland-Jones, and V. Appay. 2004. Immune activation and CD8(+) T cell differentiation towards senescence in HIV-1 infection. PLoS Biol. 2:E20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rickinson, A. B., and D. J. Moss. 1997. Human cytotoxic T lymphocyte responses to Epstein-Barr virus infection. Annu. Rev. Immunol. 15:405-431. [DOI] [PubMed] [Google Scholar]
- 35.Schlaak, J. F., G. Tully, H. F. Lohr, G. Gerken, and K. H. Meyer zum Buschenfelde. 1999. The presence of high amounts of HBV-DNA in serum is associated with suppressed costimulatory effects of interleukin 12 on HBV-induced immune response. J. Hepatol. 30:353-358. [DOI] [PubMed] [Google Scholar]
- 36.Schluns, K. S., and L. Lefrancois. 2003. Cytokine control of memory T-cell development and survival. Nat. Rev. Immunol. 3:269-279. [DOI] [PubMed] [Google Scholar]
- 37.Sopper, S., D. Nierwetberg, A. Halbach, U. Sauer, C. Scheller, C. Stahl-Hennig, K. Matz-Rensing, F. Schafer, T. Schneider, V. ter Meulen, and J. G. Muller. 2003. Impact of simian immunodeficiency virus (SIV) infection on lymphocyte numbers and T-cell turnover in different organs of rhesus monkeys. Blood 101:1213-1219. [DOI] [PubMed] [Google Scholar]
- 38.Spits, H. 2002. Development of αβ T cells in the human thymus. Nat. Rev. Immunol. 2:760-772. [DOI] [PubMed] [Google Scholar]
- 39.Suresh, M., X. Gao, C. Fischer, N. E. Miller, and K. Tewari. 2004. Dissection of antiviral and immune regulatory functions of tumor necrosis factor receptors in a chronic lymphocytic choriomeningitis virus infection. J. Virol. 78:3906-3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thimme, R., S. Wieland, C. Steiger, J. Ghrayeb, K. A. Reimann, R. H. Purcell, and F. V. Chisari. 2003. CD8+ T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J. Virol. 77:68-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van den Brink, M. R., O. Alpdogan, and R. L. Boyd. 2004. Strategies to enhance T-cell reconstitution in immunocompromised patients. Nat. Rev. Immunol. 4:856-867. [DOI] [PubMed] [Google Scholar]
- 42.Vogel, T. U., T. M. Allen, J. D. Altman, and D. I. Watkins. 2001. Functional impairment of simian immunodeficiency virus-specific CD8+ T cells during the chronic phase of infection. J. Virol. 75:2458-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward, S., G. Lauer, R. Isba, B. Walker, and P. Klenerman. 2002. Cellular immune responses against hepatitis C virus: the evidence base 2002. Clin. Exp. Immunol. 128:195-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welsh, R. M. 2001. Assessing CD8 T cell number and dysfunction in the presence of antigen. J. Exp. Med. 193:F19-F22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wherry, E. J., J. N. Blattman, K. Murali-Krishna, R. van der Most, and R. Ahmed. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiong, Y., M. A. Luscher, J. D. Altman, M. Hulsey, H. L. Robinson, M. Ostrowski, B. H. Barber, and K. S. MacDonald. 2001. Simian immunodeficiency virus (SIV) infection of a rhesus macaque induces SIV-specific CD8+ T cells with a defect in effector function that is reversible on extended interleukin-2 incubation. J. Virol. 75:3028-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]