Abstract
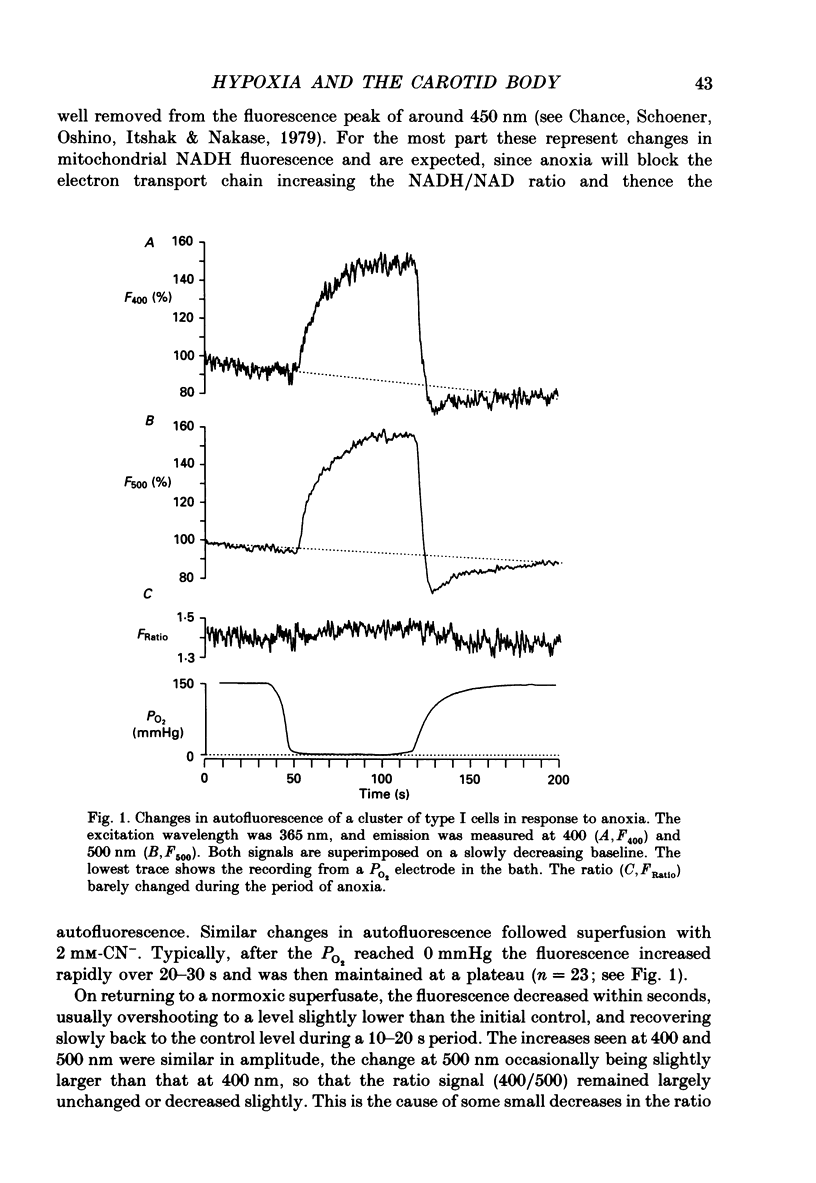
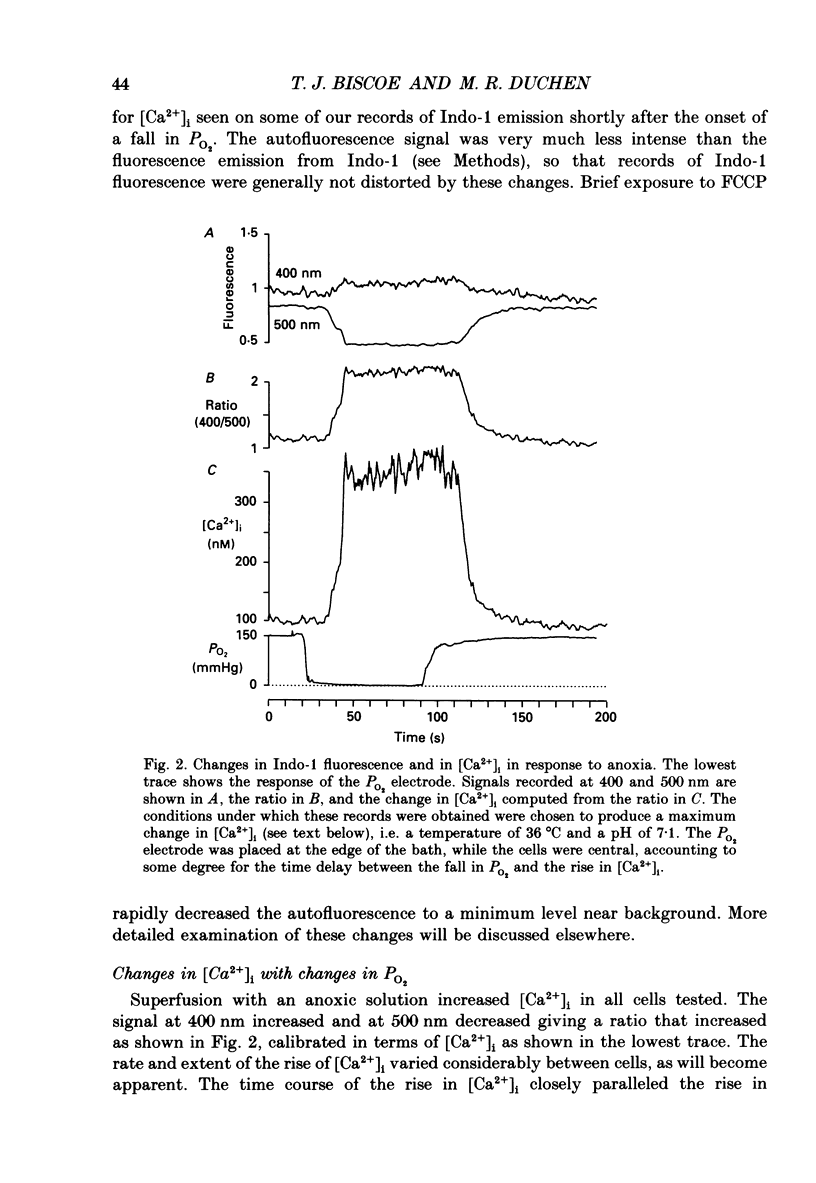
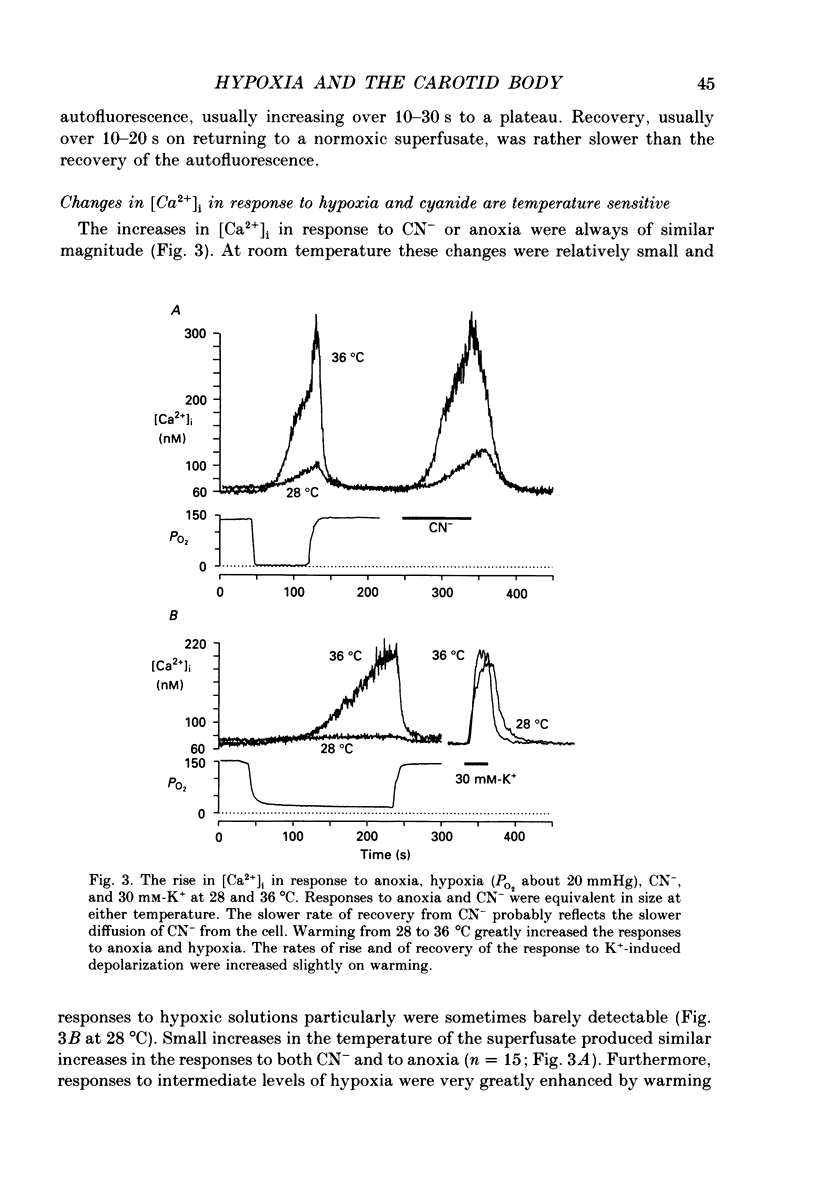
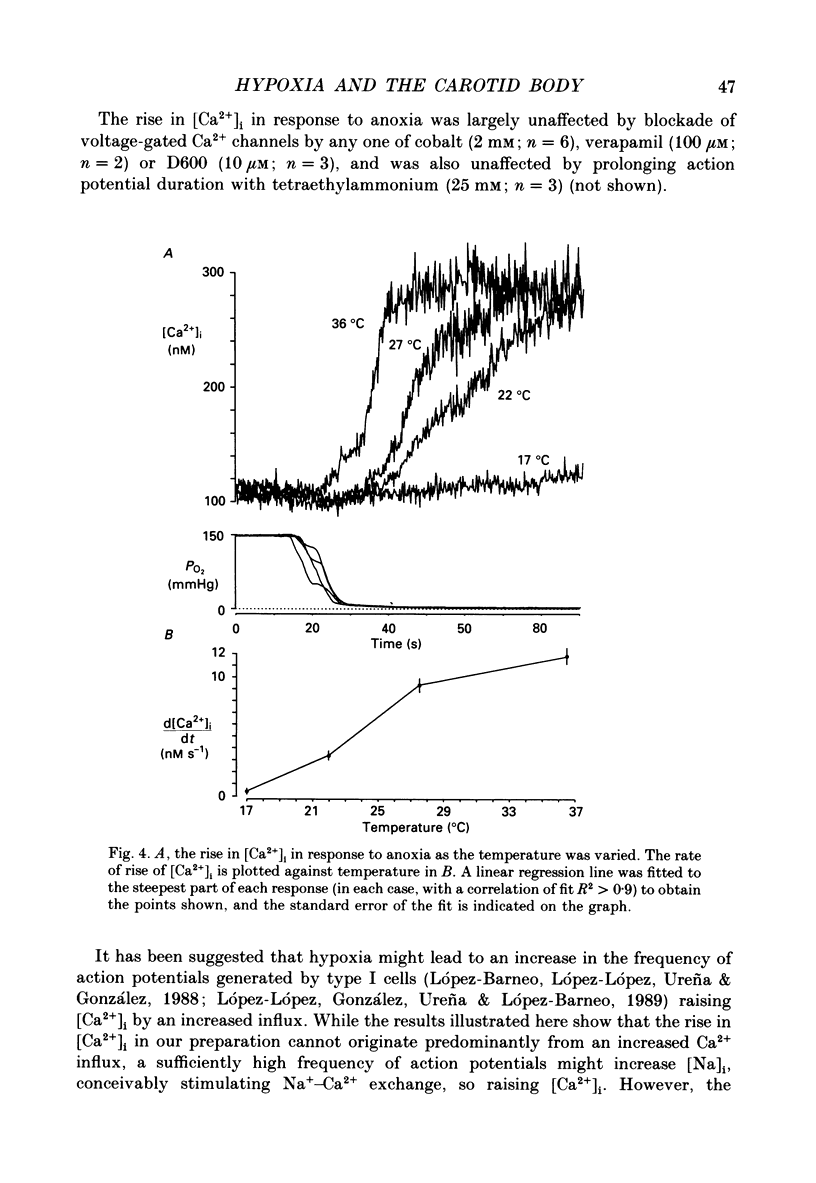
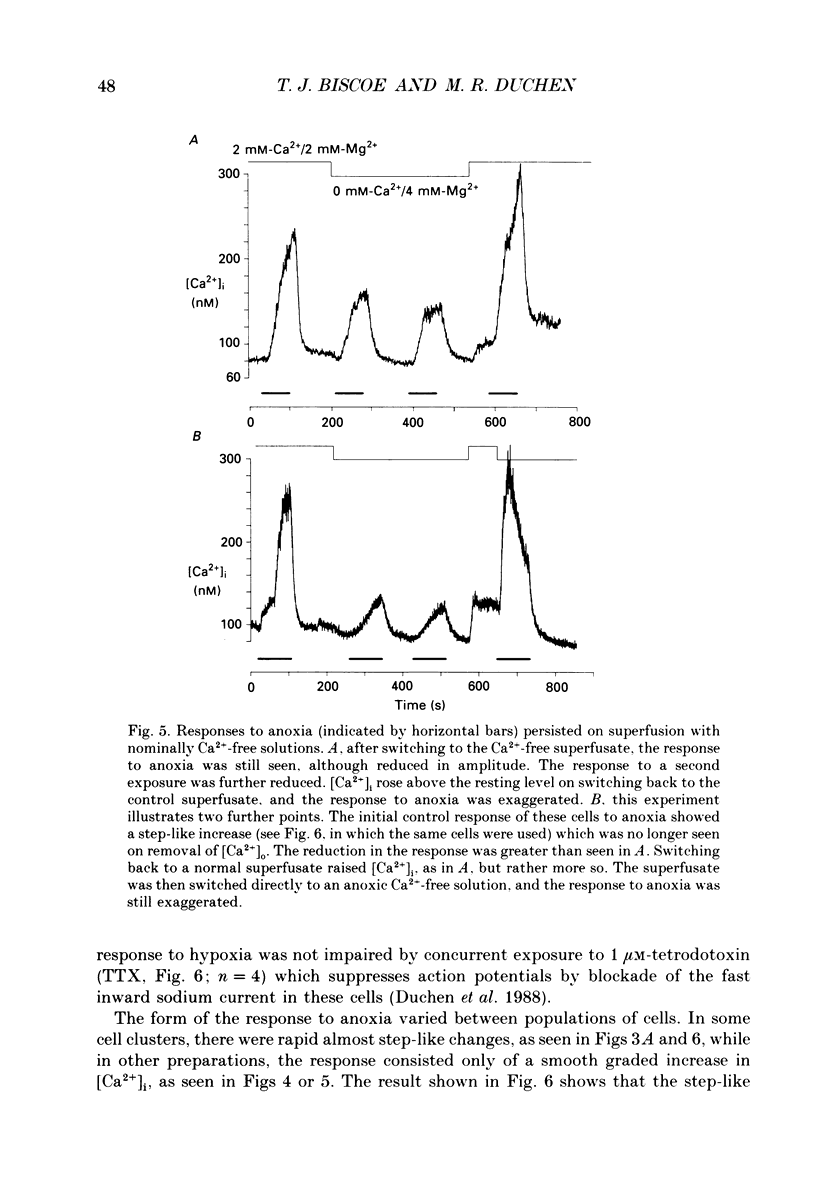
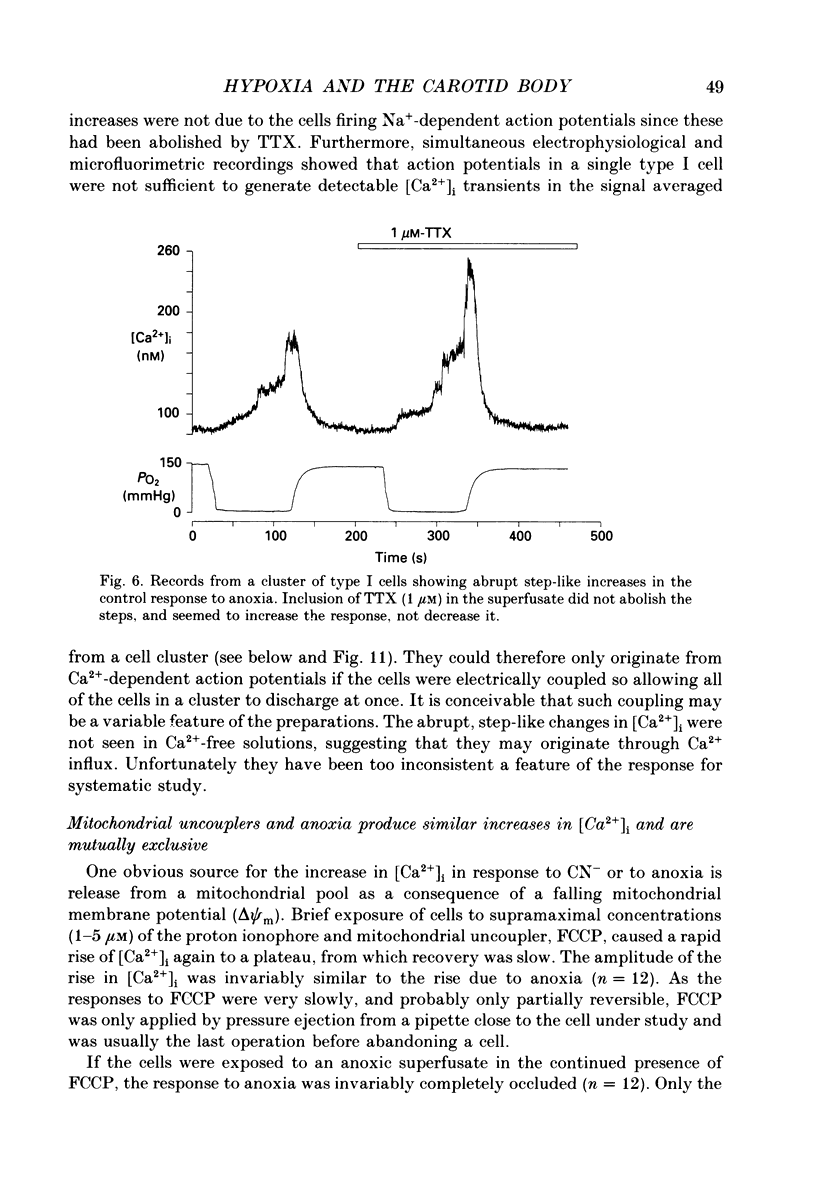
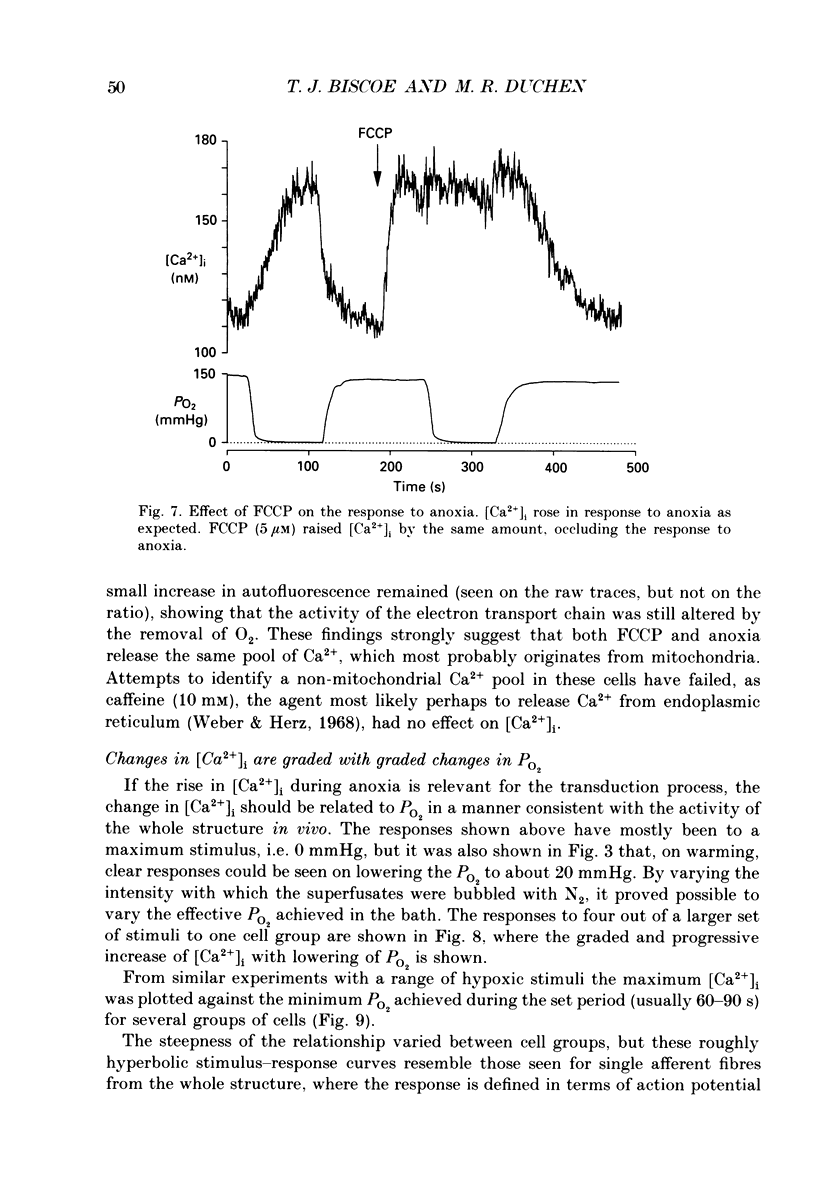
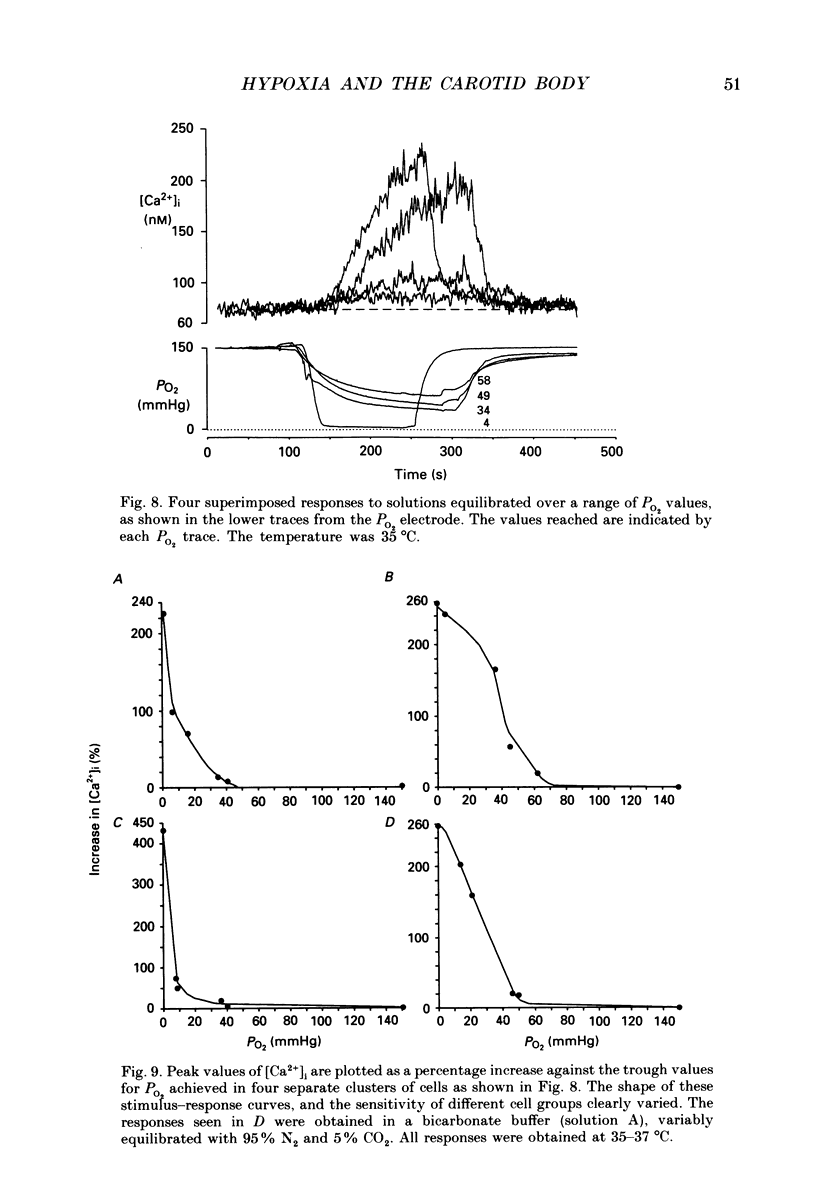
1. The carotid body chemoreceptors are stimulated in situ by hypoxia. We have studied type I cells freshly dissociated from the carotid body of the rabbit. We have used microfluorimetric and patch clamp techniques to examine the responses to hypoxia, to anoxia, and to metabolic inhibition. 2. NADH autofluorescence measured at both 400 and 500 nm increased rapidly and reversibly in response to anoxia or to cyanide (CN-), reflecting a change in mitochondrial metabolism. 3. Indo-1 was used to measure changes in intracellular calcium, [Ca2+]i. Anoxia reversibly increased [Ca2+]i from approximately 50-100 to approximately 200-450 nM in all cells tested. The response showed a striking temperature sensitivity. Responses to hypoxic stimuli were barely detectable at 17-20 degrees C, and were dramatically increased on warming to 36 degrees C. In contrast, responses to K(+)-induced depolarization were only slightly increased in rate of onset and recovery by warming. 4. The rise in [Ca2+]i originated largely from an intracellular store which was slowly depleted by exposure to nominally Ca2(+)-free solutions. Responses were unaffected by blockade of Ca2+ channels with organic (D600, verapamil) or inorganic (Co2+) blockers, by blockade of Na+ channels with tetrodotoxin (TTX), or by increasing action potential duration with tetraethylammonium (TEA). Responses to anoxia were increased by the increased [Ca2+]i loading that follows prior exposure to Ca2(+)-free solutions. 5. Responses to anoxia, to blockade of electron transport by CN-, and to the mitochondrial uncoupler, carbonyl cyanide p-trifluoromethoxy-phenylhydrazone (FCCP), were equivalent in amplitude. The response to anoxia was occluded by concurrent application of FCCP, suggesting that the Ca2+ originates from the same pool in each case. 6. At 35-36 degrees C, responses to graded levels of PO2 were also graded. Thresholds varied between cells, but were typically 30-50 mmHg. Stimulus-responses curves were essentially hyperbolic, increasing dramatically as the PO2 approached 0 mmHg. 7. The sensitivity of cells to hypoxic solutions was increased by acidification of the superfusate over the pH range from 7.3 to 6.85. 8. Cell-attached patch clamp recordings showed depression of spontaneous action potentials associated with a rise in [Ca2+]i during exposure to anoxic solutions. Whole-cell recordings showed that anoxia increased a voltage-gated gK as described previously for CN-, while producing no change in resting conductance. 9. These data suggest that the rise in [Ca2+]i originates largely from Ca2+ efflux from a mitochondrial pool.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alnaes E., Rahamimoff R. On the role of mitochondria in transmitter release from motor nerve terminals. J Physiol. 1975 Jun;248(2):285–306. doi: 10.1113/jphysiol.1975.sp010974. [DOI] [PMC free article] [PubMed] [Google Scholar]
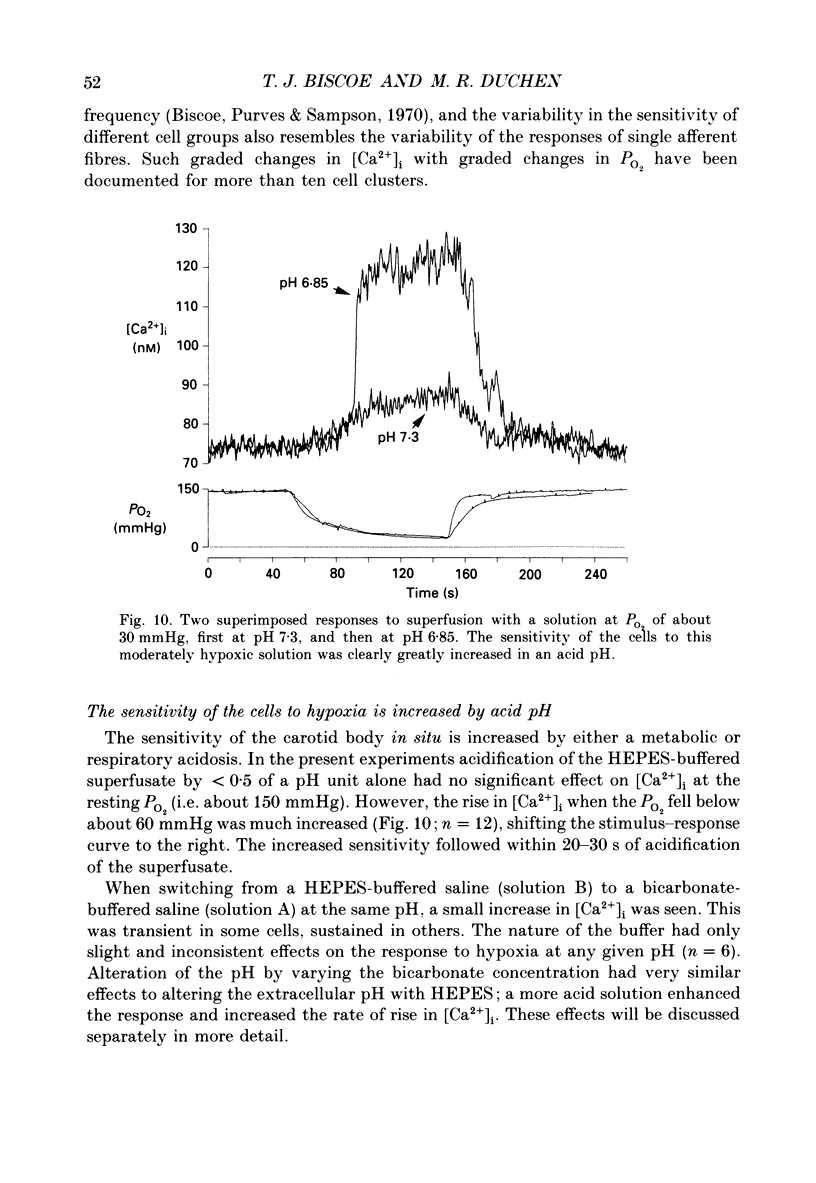
- Bers D. M., Bridge J. H., Spitzer K. W. Intracellular Ca2+ transients during rapid cooling contractures in guinea-pig ventricular myocytes. J Physiol. 1989 Oct;417:537–553. doi: 10.1113/jphysiol.1989.sp017817. [DOI] [PMC free article] [PubMed] [Google Scholar]
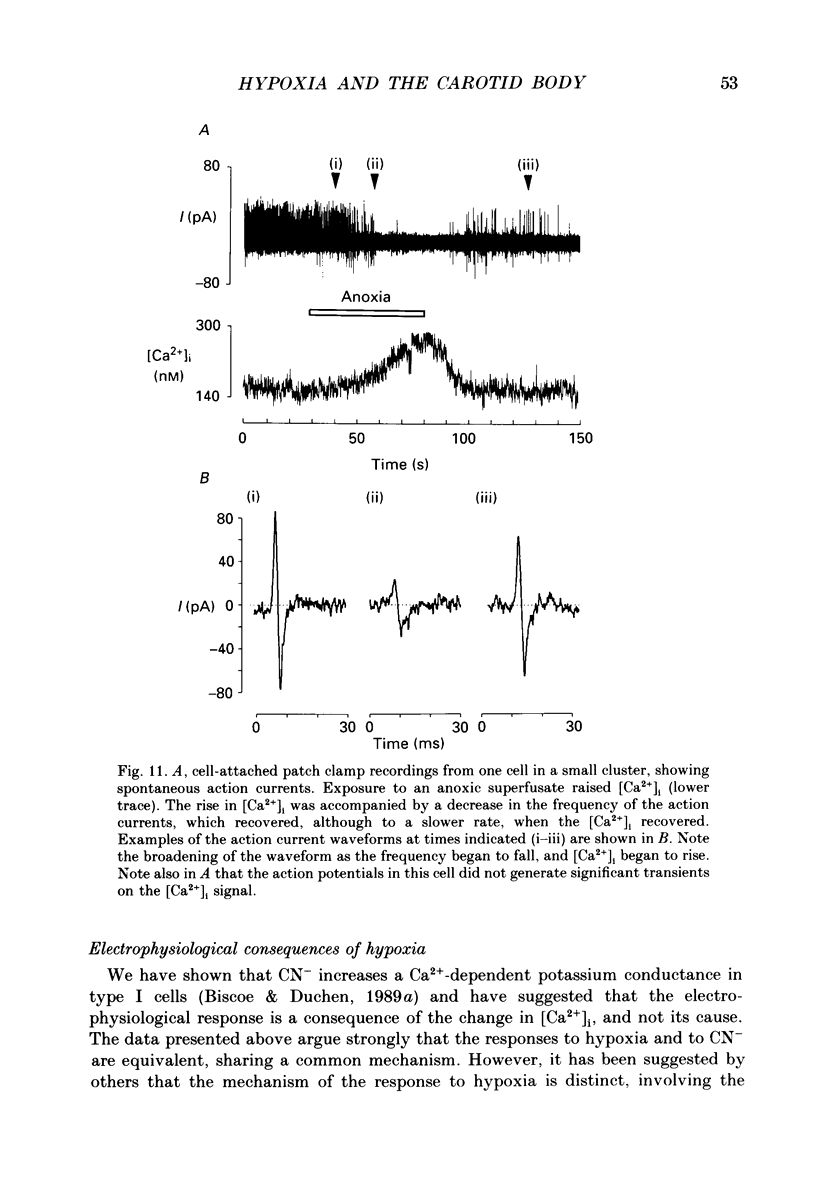
- Biscoe T. J. Carotid body: structure and function. Physiol Rev. 1971 Jul;51(3):437–495. doi: 10.1152/physrev.1971.51.3.437. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Duchen M. R., Eisner D. A., O'Neill S. C., Valdeolmillos M. Measurements of intracellular Ca2+ in dissociated type I cells of the rabbit carotid body. J Physiol. 1989 Sep;416:421–434. doi: 10.1113/jphysiol.1989.sp017769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Duchen M. R. Electrophysiological responses of dissociated type I cells of the rabbit carotid body to cyanide. J Physiol. 1989 Jun;413:447–468. doi: 10.1113/jphysiol.1989.sp017663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Purves M. J. Observations on the rhythmic variation in the cat carotid body chemoreceptor activity which has the same period as respiration. J Physiol. 1967 Jun;190(3):389–412. doi: 10.1113/jphysiol.1967.sp008217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Purves M. J., Sampson S. R. The frequency of nerve impulses in single carotid body chemoreceptor afferent fibres recorded in vivo with intact circulation. J Physiol. 1970 May;208(1):121–131. doi: 10.1113/jphysiol.1970.sp009109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Chance B., Schoener B., Oshino R., Itshak F., Nakase Y. Oxidation-reduction ratio studies of mitochondria in freeze-trapped samples. NADH and flavoprotein fluorescence signals. J Biol Chem. 1979 Jun 10;254(11):4764–4771. [PubMed] [Google Scholar]
- Delpiano M. A., Hescheler J. Evidence for a PO2-sensitive K+ channel in the type-I cell of the rabbit carotid body. FEBS Lett. 1989 Jun 5;249(2):195–198. doi: 10.1016/0014-5793(89)80623-4. [DOI] [PubMed] [Google Scholar]
- Duchen M. R., Caddy K. W., Kirby G. C., Patterson D. L., Ponte J., Biscoe T. J. Biophysical studies of the cellular elements of the rabbit carotid body. Neuroscience. 1988 Jul;26(1):291–311. doi: 10.1016/0306-4522(88)90146-7. [DOI] [PubMed] [Google Scholar]
- Duchen M. R. Effects of metabolic inhibition on the membrane properties of isolated mouse primary sensory neurones. J Physiol. 1990 May;424:387–409. doi: 10.1113/jphysiol.1990.sp018073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen M. R., Valdeolmillos M., O'Neill S. C., Eisner D. A. Effects of metabolic blockade on the regulation of intracellular calcium in dissociated mouse sensory neurones. J Physiol. 1990 May;424:411–426. doi: 10.1113/jphysiol.1990.sp018074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyzaguirre C., Koyano H. Effects of some pharmacological agents on chemoreceptor discharges. J Physiol. 1965 Jun;178(3):410–437. doi: 10.1113/jphysiol.1965.sp007635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidone S., Gonzalez C., Yoshizaki K. Effects of low oxygen on the release of dopamine from the rabbit carotid body in vitro. J Physiol. 1982 Dec;333:93–110. doi: 10.1113/jphysiol.1982.sp014441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman M. C., Greene W. L., Platika D. Oxygen chemoreception by carotid body cells in culture. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1448–1450. doi: 10.1073/pnas.82.5.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Jacobs L., Comroe J. H., Jr Stimulation of the carotid chemoreceptors of the dog by dopamine. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1187–1193. doi: 10.1073/pnas.59.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Barneo J., López-López J. R., Ureña J., González C. Chemotransduction in the carotid body: K+ current modulated by PO2 in type I chemoreceptor cells. Science. 1988 Jul 29;241(4865):580–582. doi: 10.1126/science.2456613. [DOI] [PubMed] [Google Scholar]
- López-López J., González C., Ureña J., López-Barneo J. Low pO2 selectively inhibits K channel activity in chemoreceptor cells of the mammalian carotid body. J Gen Physiol. 1989 May;93(5):1001–1015. doi: 10.1085/jgp.93.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills E., Jöbsis F. F. Mitochondrial respiratory chain of carotid body and chemoreceptor response to changes in oxygen tension. J Neurophysiol. 1972 Jul;35(4):405–428. doi: 10.1152/jn.1972.35.4.405. [DOI] [PubMed] [Google Scholar]
- Mills E., Jöbsis F. F. Simultaneous measurement of cytochrome a3 reduction and chemoreceptor afferent activity in the carotid body. Nature. 1970 Mar 21;225(5238):1147–1149. doi: 10.1038/2251147a0. [DOI] [PubMed] [Google Scholar]
- Nair P. K., Buerk D. G., Whalen W. J. Cat carotid body oxygen metabolism and chemoreception described by a two-cytochrome model. Am J Physiol. 1986 Feb;250(2 Pt 2):H202–H207. doi: 10.1152/ajpheart.1986.250.2.H202. [DOI] [PubMed] [Google Scholar]
- Nishimura M. Factors influencing an increase in spontaneous transmitter release by hypoxia at the mouse neuromuscular junction. J Physiol. 1986 Mar;372:303–313. doi: 10.1113/jphysiol.1986.sp016010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paintal A. S. The responses of chemoreceptors with medullated and non-medullated fibres to chemical substances and the mechanical hypothesis. Prog Brain Res. 1988;74:161–168. doi: 10.1016/s0079-6123(08)63010-1. [DOI] [PubMed] [Google Scholar]
- Pallot D. J. The mammalian carotid body. Adv Anat Embryol Cell Biol. 1987;102:1–91. doi: 10.1007/978-3-642-71857-1. [DOI] [PubMed] [Google Scholar]
- Rottenberg H., Scarpa A. Calcium uptake and membrane potential in mitochondria. Biochemistry. 1974 Nov 5;13(23):4811–4817. doi: 10.1021/bi00720a020. [DOI] [PubMed] [Google Scholar]
- Weber A., Herz R. The relationship between caffeine contracture of intact muscle and the effect of caffeine on reticulum. J Gen Physiol. 1968 Nov;52(5):750–759. doi: 10.1085/jgp.52.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen W. J., Savoca J., Nair P. Oxygen tension measurements in carotid body of the cat. Am J Physiol. 1973 Oct;225(4):986–991. doi: 10.1152/ajplegacy.1973.225.4.986. [DOI] [PubMed] [Google Scholar]


