Abstract
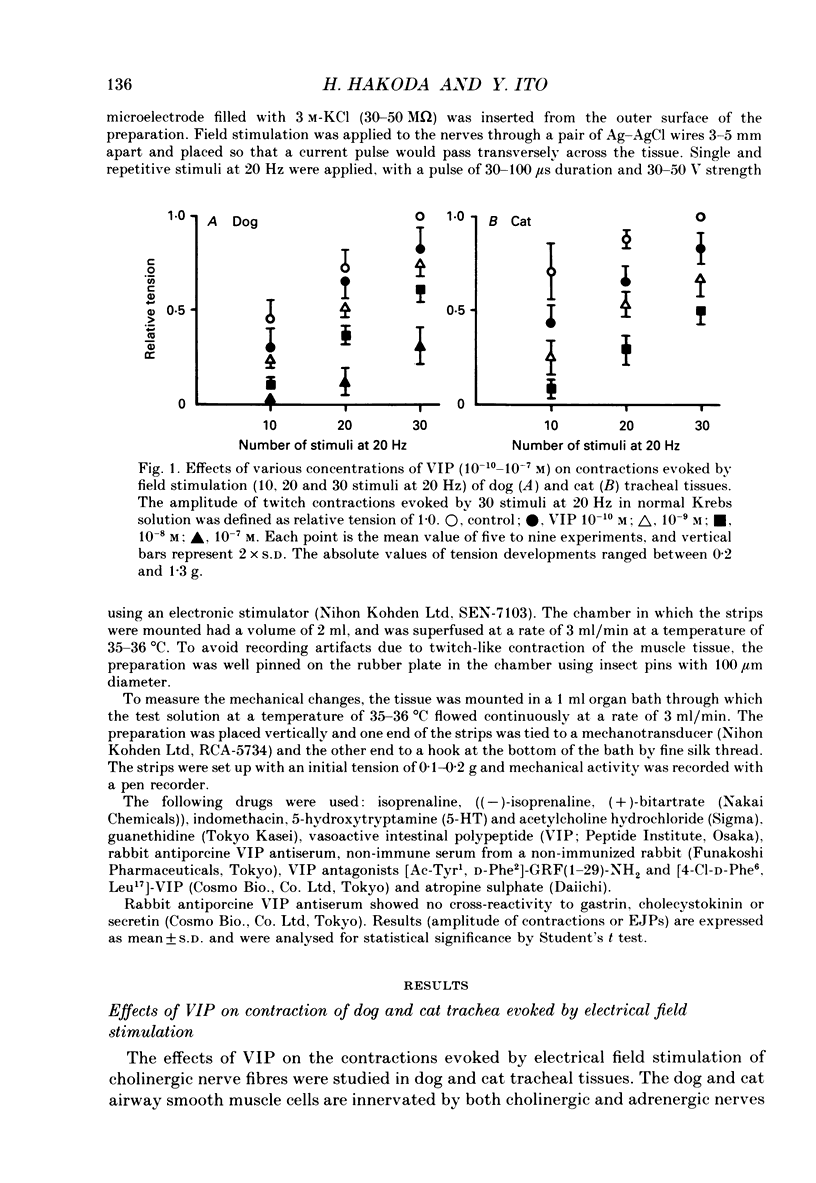
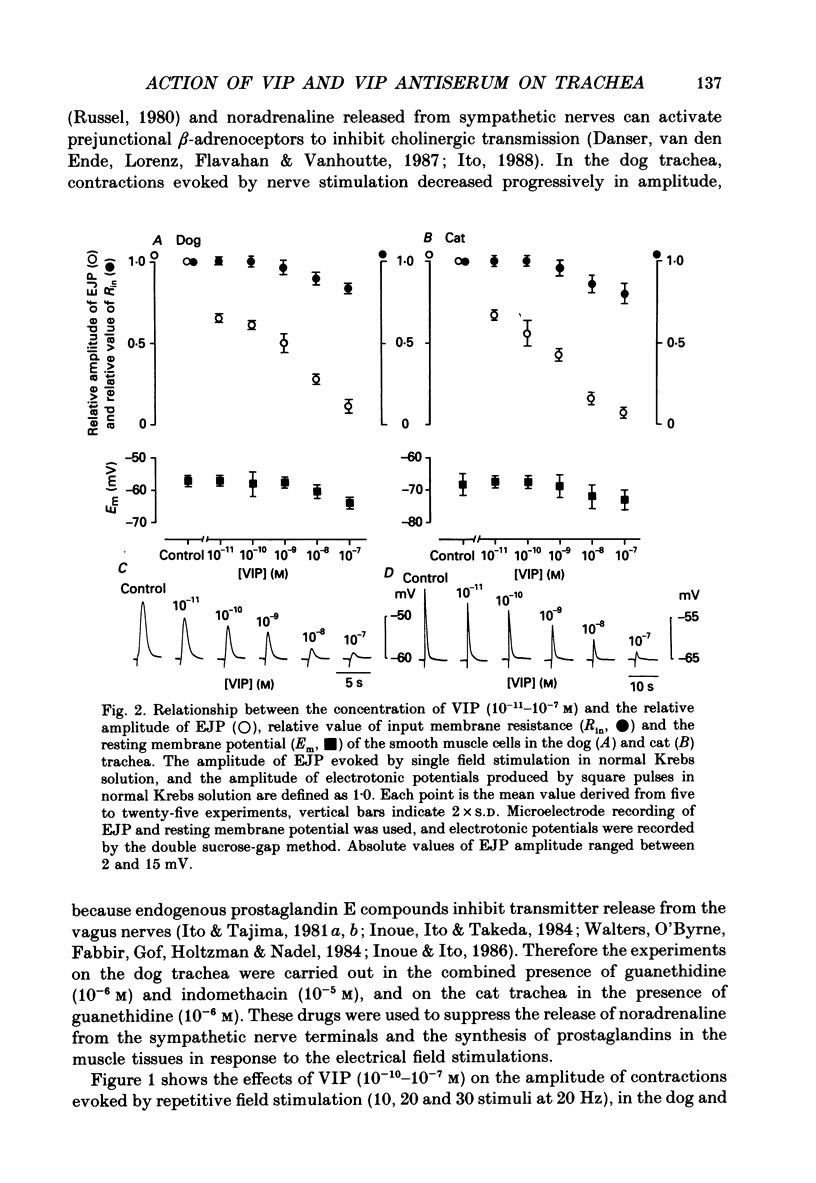
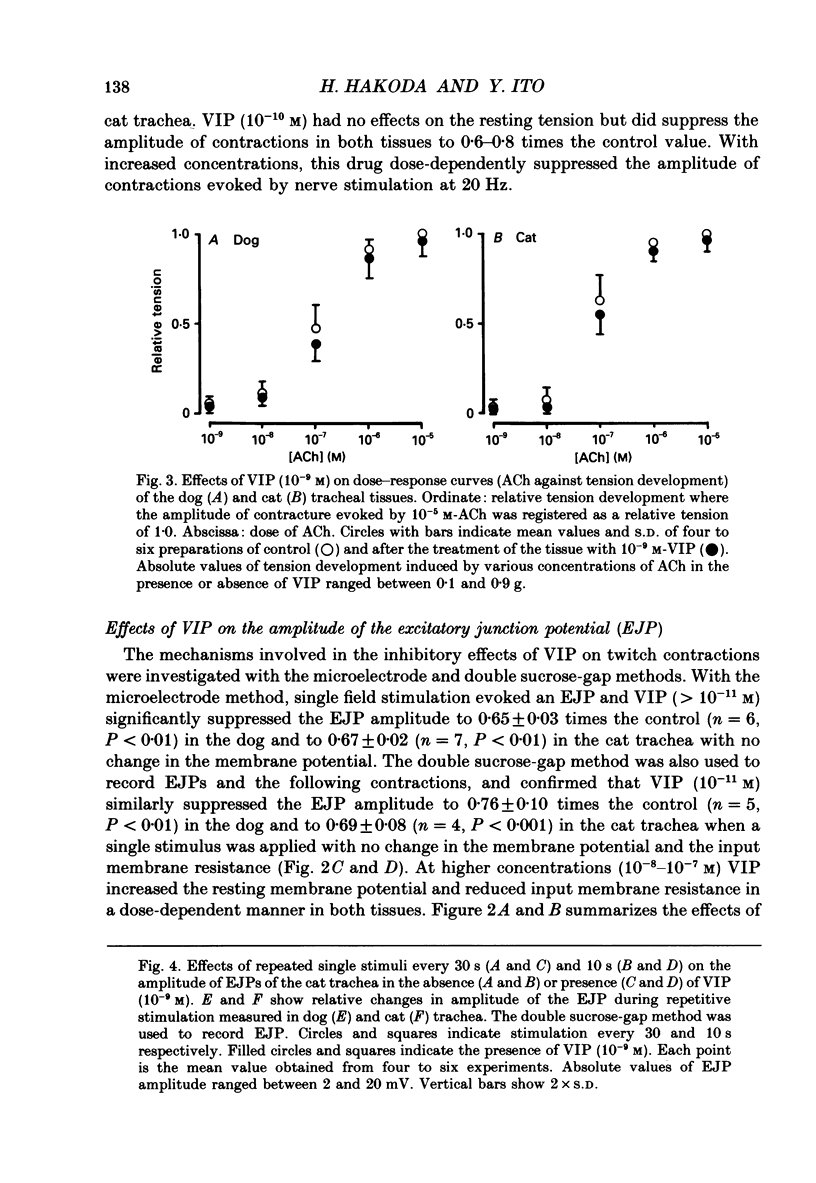
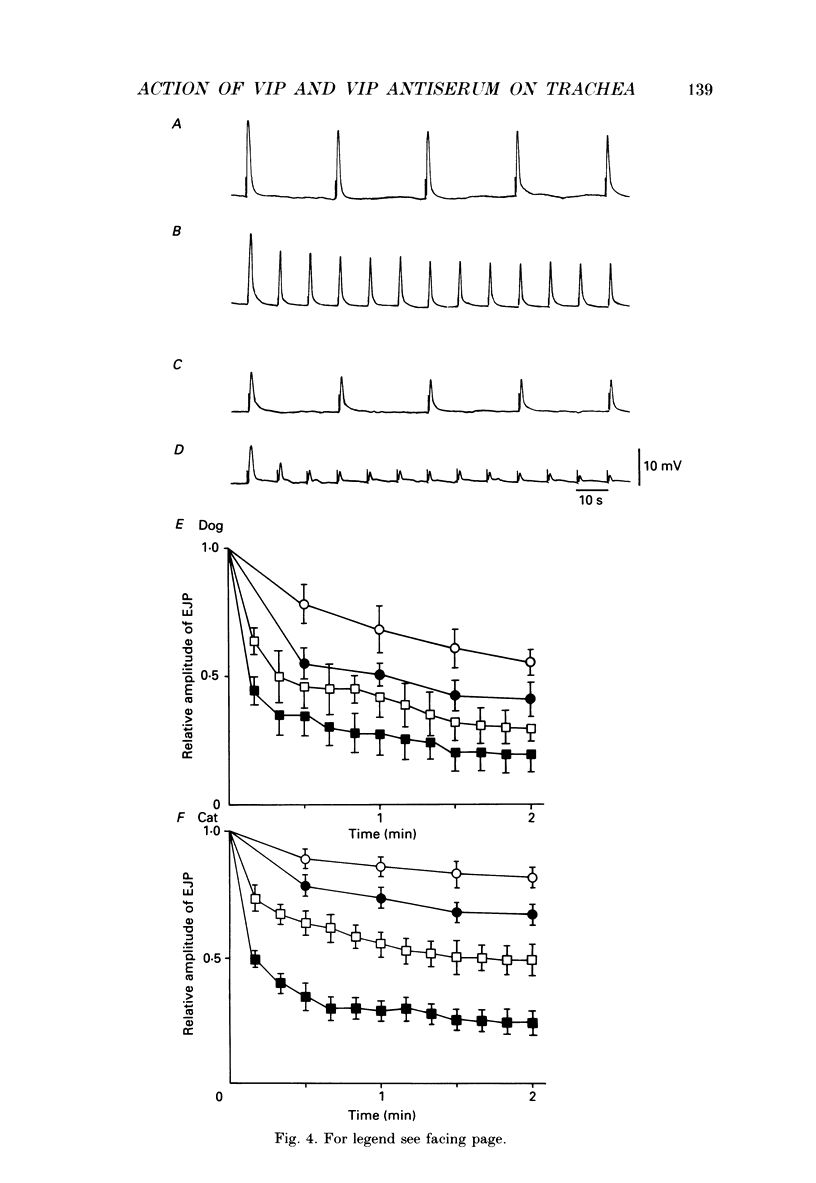
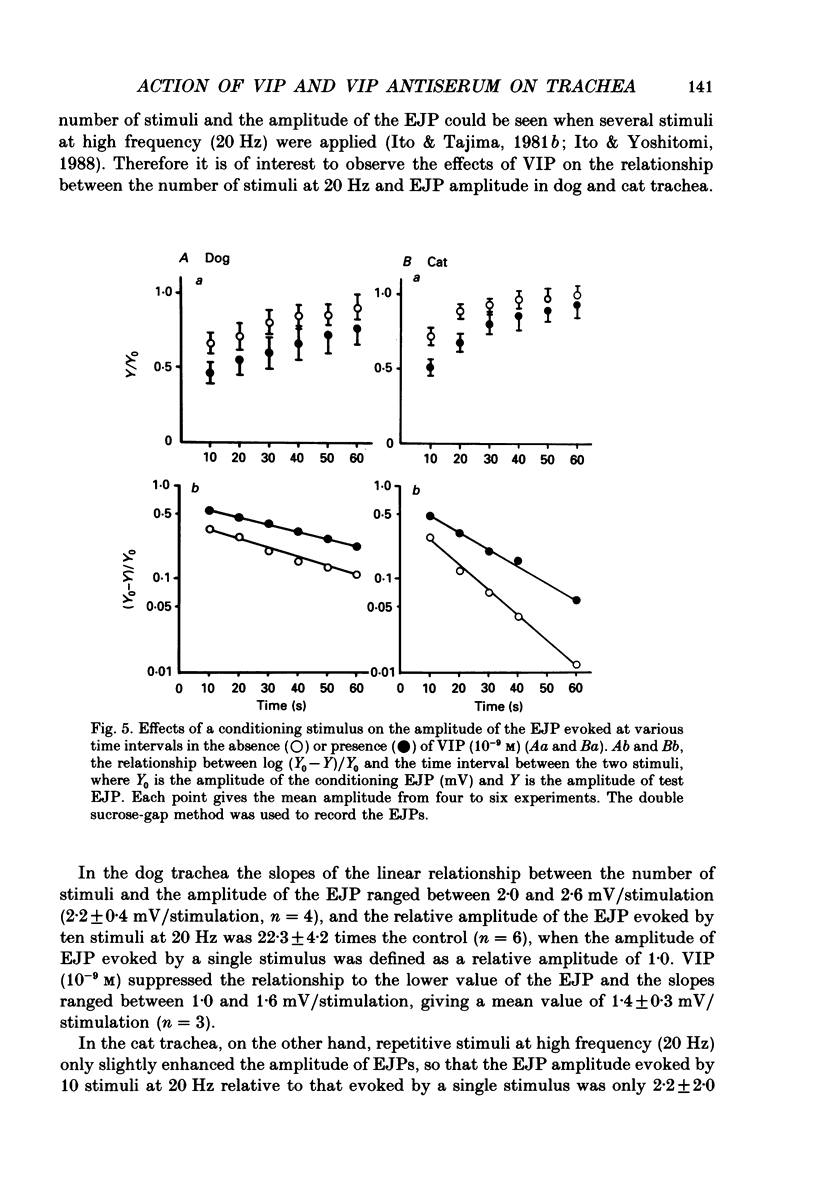
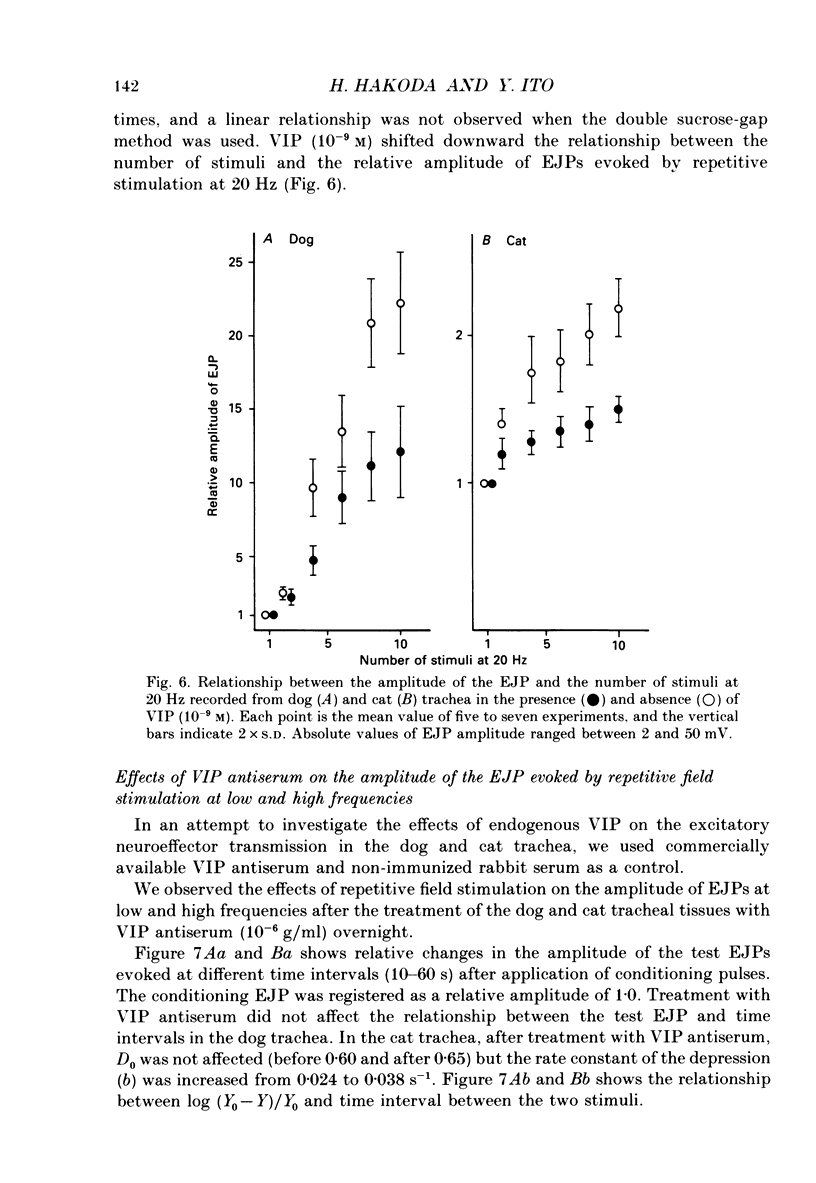
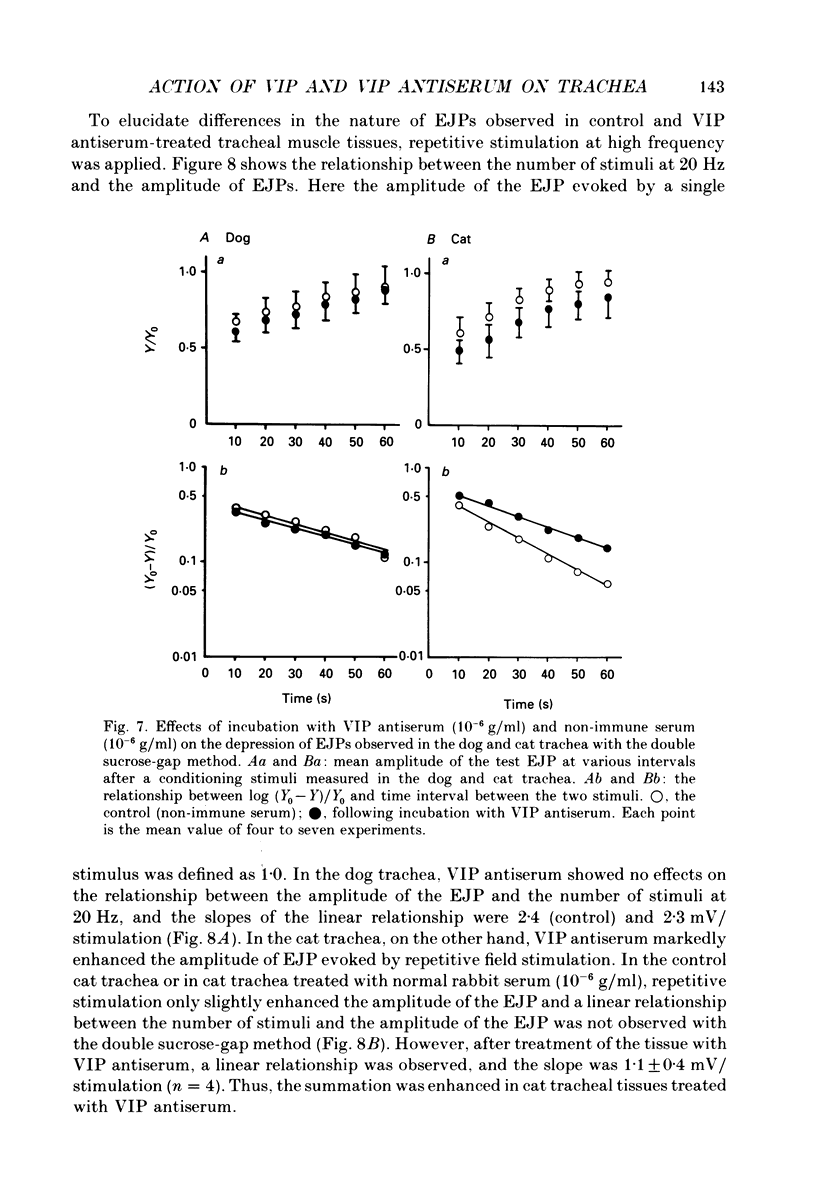
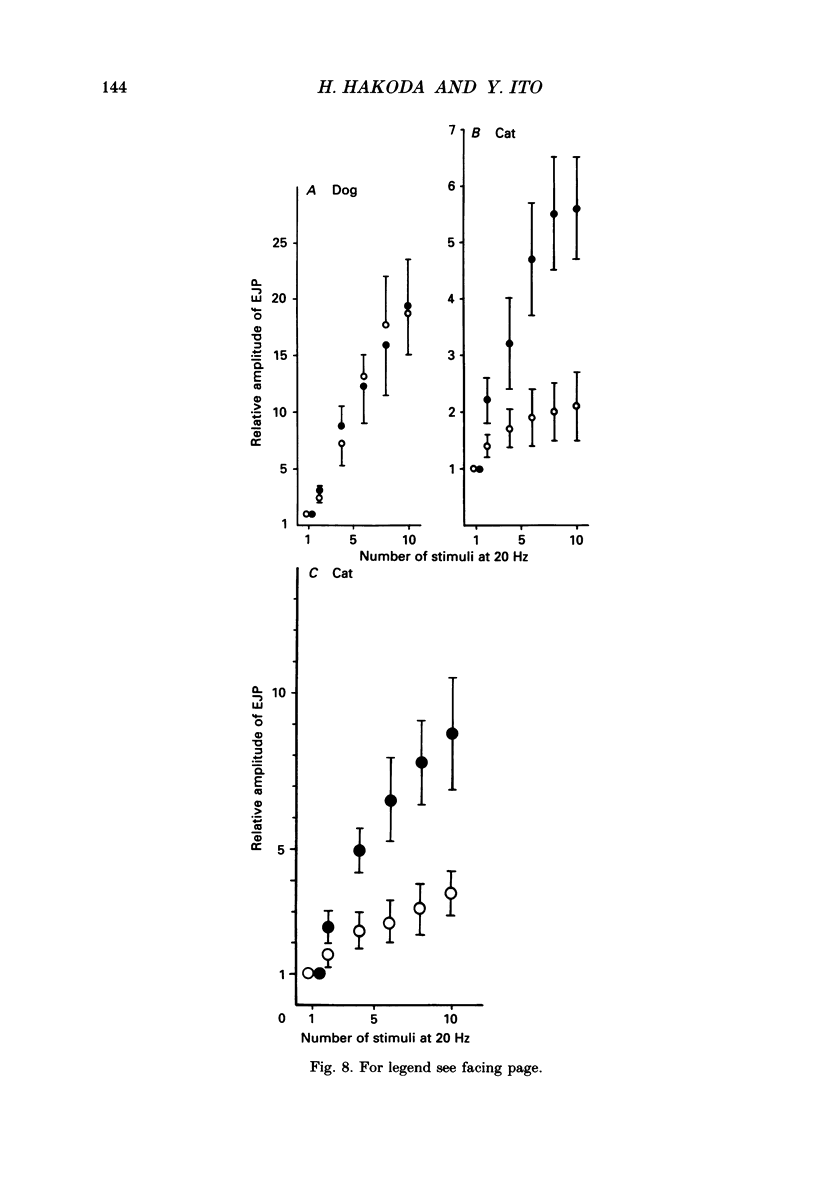
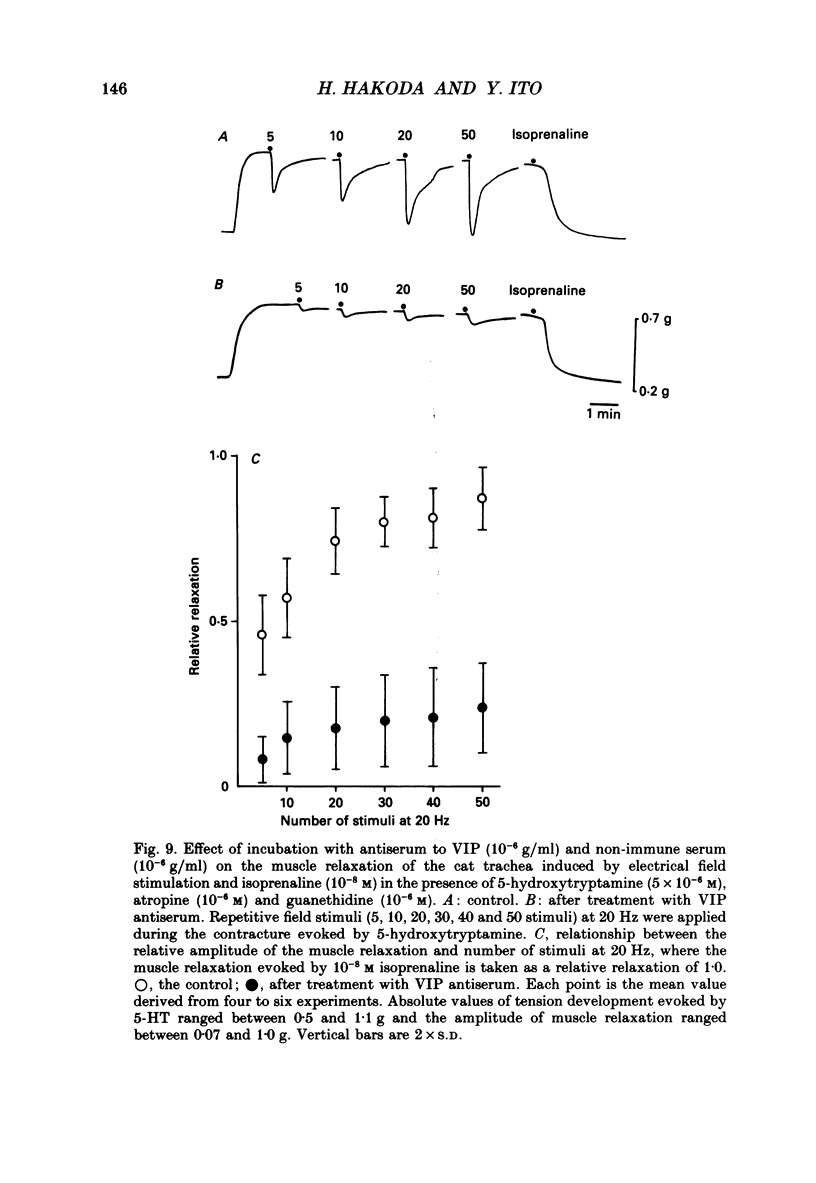
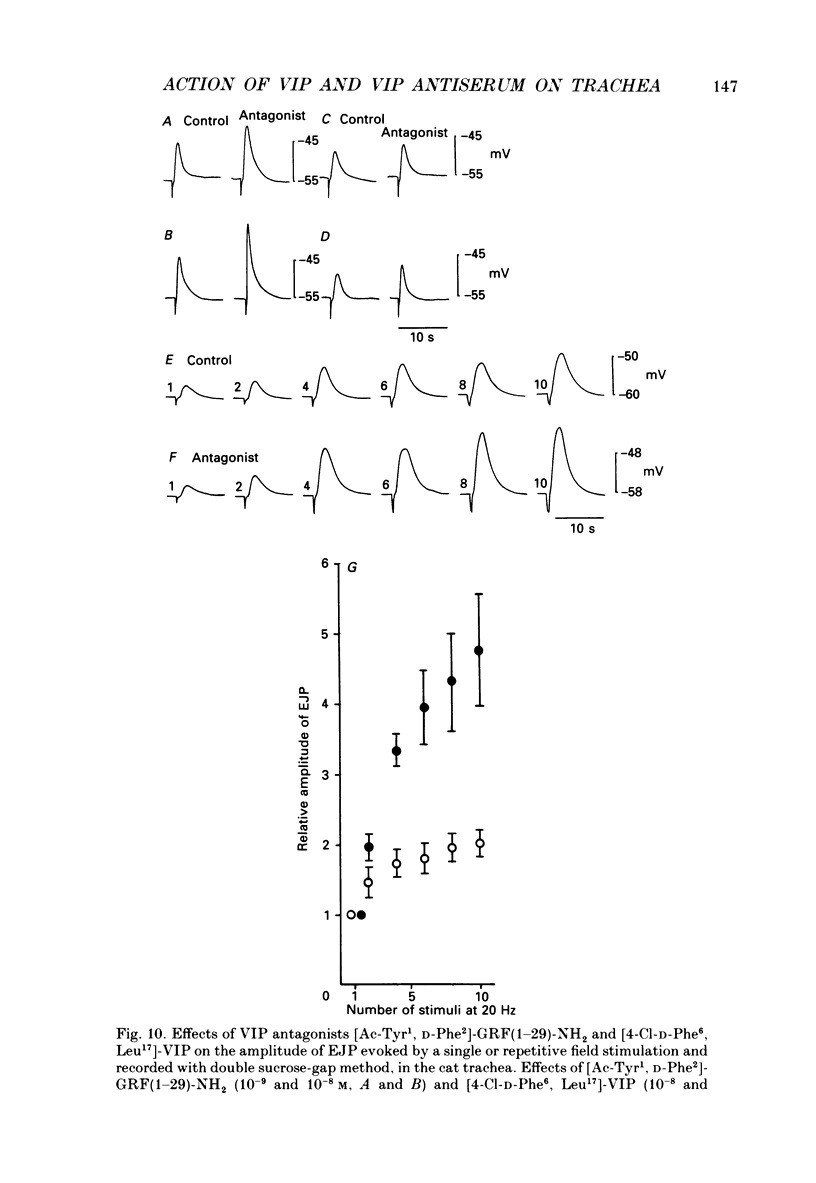
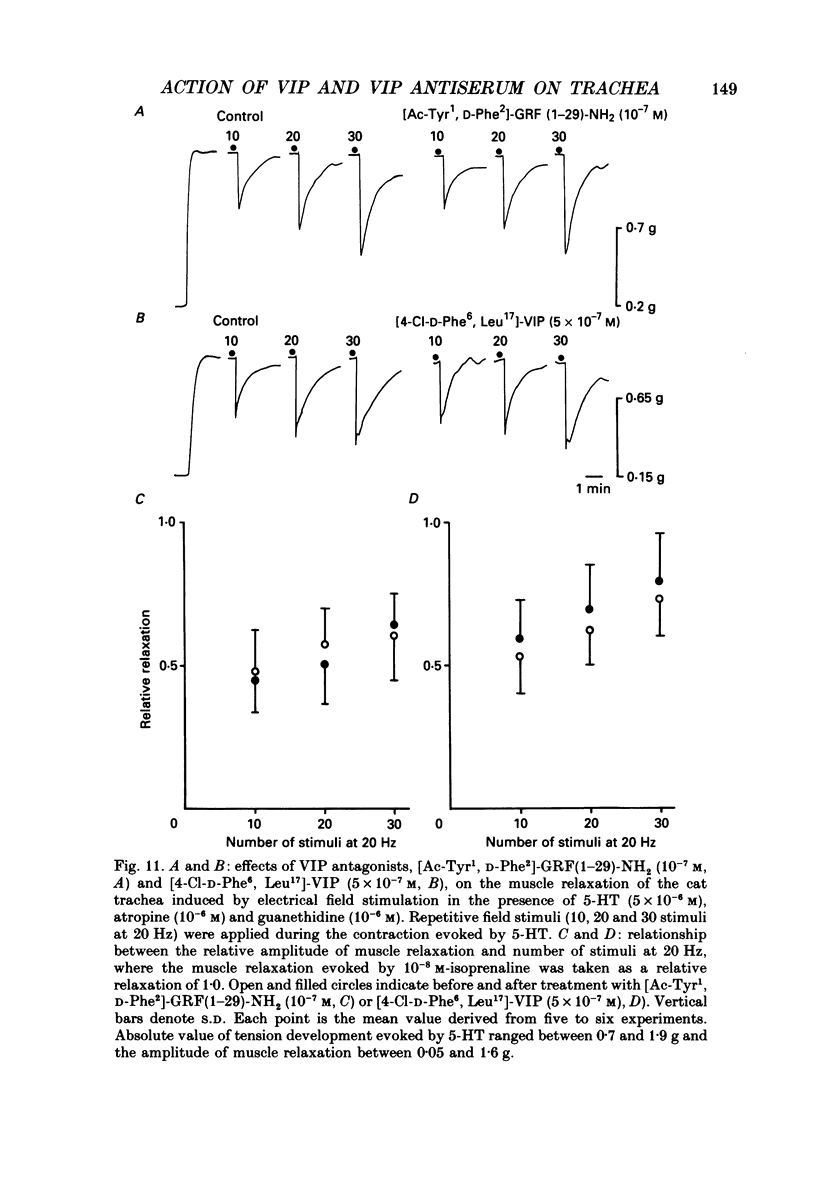
1. Comparative studies on the effects of vasoactive intestinal polypeptide (VIP), commercially available VIP antiserum or VIP antagonists [Ac-Tyr1, D-Phe2]-GRF(1-29)-NH2 and [4-Cl-D-Phe6, Leu17]-VIP on excitatory neuroeffector transmission in the dog and cat trachea were performed with microelectrode, double sucrose-gap, and tension recording methods. 2. VIP (10(-11)-10(-9) M) had no effect on the resting membrane potential or on the input resistance of the smooth muscle cells of dog and cat trachea. However, with increased concentrations (greater than 10(-8) M) VIP hyperpolarized the membrane and decreased the input resistance of the membrane in both tissues. 3. VIP (10(-10)-10(-7) M) dose-dependently reduced the amplitude of the contractions evoked through the nervous structure excited by field stimulation in the combined presence of indomethacin (10(-5) M) and guanethidine (10(-6) M) in the dog, and in the presence of guanethidine (10(-6) M) in cat trachea. In parallel with actions on twitch contractions, VIP (10(-11)-10(-7) M) reduced the amplitude of the excitatory junction potentials (EJPs) evoked through the nervous structure excited by single pulse field stimulation in both tissues. 4. VIP (10(-9) M) had no effect on the post-junctional response of smooth muscle cells to exogenous acetylcholine (ACh) (10(-9)-10(-5) M). 5. During repetitive field stimulation at the stimulus frequency of 0.033-0.1 Hz, the amplitude of the EJPs was gradually reduced, and VIP (10(-9) M) enhanced this depression phenomenon in the dog and cat trachea. 6. EJPs also showed summation when repetitive field stimulation was applied at high frequency (20 Hz) in the dog trachea. The slope of the relationship between the relative amplitude of the EJP and number of stimuli at 20 Hz was 2.2 +/- 0.4 mV/stimulation (n = 4) in the dog trachea. However, in the cat trachea, summation of EJPs was not prominent, giving a mean slope of 0.6 +/- 0.2 mV/stimulation (n = 6) measured by the microelectrode method. VIP (10(-9) M) shifted downward the relationship between the relative amplitude of the EJP and the number of stimuli at 20 Hz in both tissues. 7. Overnight incubation with VIP antiserum (10(-6) g/ml) had little effect on the depression of the EJP in the dog and cat trachea, or the summation of the EJP observed in the dog trachea.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altiere R. J., Diamond L. Relaxation of cat tracheobronchial and pulmonary arterial smooth muscle by vasoactive intestinal peptide: lack of influence by peptidase inhibitors. Br J Pharmacol. 1984 Jun;82(2):321–328. doi: 10.1111/j.1476-5381.1984.tb10766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. J. Neural control of human airways in health and disease. Am Rev Respir Dis. 1986 Dec;134(6):1289–1314. doi: 10.1164/arrd.1986.134.5.1289. [DOI] [PubMed] [Google Scholar]
- Coburn R. F., Tomita T. Evidence for nonadrenergic inhibitory nerves in the guinea pig trachealis muscle. Am J Physiol. 1973 May;224(5):1072–1080. doi: 10.1152/ajplegacy.1973.224.5.1072. [DOI] [PubMed] [Google Scholar]
- Danser A. H., van den Ende R., Lorenz R. R., Flavahan N. A., Vanhoutte P. M. Prejunctional beta 1-adrenoceptors inhibit cholinergic transmission in canine bronchi. J Appl Physiol (1985) 1987 Feb;62(2):785–790. doi: 10.1152/jappl.1987.62.2.785. [DOI] [PubMed] [Google Scholar]
- Dey R. D., Shannon W. A., Jr, Said S. I. Localization of VIP-immunoreactive nerves in airways and pulmonary vessels of dogs, cat, and human subjects. Cell Tissue Res. 1981;220(2):231–238. doi: 10.1007/BF00210505. [DOI] [PubMed] [Google Scholar]
- Diamond L., O'Donnell M. A nonadrenergic vagal inhibitory pathway to feline airways. Science. 1980 Apr 11;208(4440):185–188. doi: 10.1126/science.7361114. [DOI] [PubMed] [Google Scholar]
- Ellis J. L., Farmer S. G. Effects of peptidases on non-adrenergic, non-cholinergic inhibitory responses of tracheal smooth muscle: a comparison with effects on VIP- and PHI-induced relaxation. Br J Pharmacol. 1989 Mar;96(3):521–526. doi: 10.1111/j.1476-5381.1989.tb11848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelbaum J., Tapia-Arancibia L., Besson J., Rotsztejn W. H., Kordon C. Vasoactive intestinal peptide inhibits release of somatostatin from hypothalamus in vitro. Eur J Pharmacol. 1979 Oct 15;58(4):493–495. doi: 10.1016/0014-2999(79)90323-6. [DOI] [PubMed] [Google Scholar]
- Frawley L. S., Neill J. D. Stimulation of prolactin secretion in rhesus monkeys by vasoactive intestinal polypeptide. Neuroendocrinology. 1981 Aug;33(2):79–83. doi: 10.1159/000123206. [DOI] [PubMed] [Google Scholar]
- Håkanson R., Sundler F., Moghimzadeh E., Leander S. Peptide-containing nerve fibres in the airways: distribution and functional implications. Eur J Respir Dis Suppl. 1983;131:115–140. [PubMed] [Google Scholar]
- Inoue T., Ito Y. Characteristics of neuro-effector transmission in the smooth muscle layer of dog bronchiole and modifications by autacoids. J Physiol. 1986 Jan;370:551–565. doi: 10.1113/jphysiol.1986.sp015950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T., Ito Y. Pre- and post-junctional actions of prostaglandin I2, carbocyclic thromboxane A2 and leukotriene C4 in dog tracheal tissue. Br J Pharmacol. 1985 Feb;84(2):289–298. doi: 10.1111/j.1476-5381.1985.tb12913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T., Ito Y., Takeda K. Prostaglandin-induced inhibition of acetylcholine release from neuronal elements of dog tracheal tissue. J Physiol. 1984 Apr;349:553–570. doi: 10.1113/jphysiol.1984.sp015173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin C. G., Martin R. R., Macklem P. T. Nonpurinergic nature and efficacy of nonadrenergic bronchodilation. J Appl Physiol Respir Environ Exerc Physiol. 1982 Mar;52(3):562–569. doi: 10.1152/jappl.1982.52.3.562. [DOI] [PubMed] [Google Scholar]
- Ito Y., Itoh T. The roles of stored calcium in contractions of cat tracheal smooth muscle produced by electrical stimulation, acetylcholine and high K+. Br J Pharmacol. 1984 Nov;83(3):667–676. doi: 10.1111/j.1476-5381.1984.tb16220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y. Pre- and post-junctional actions of procaterol, a beta 2-adrenoceptor stimulant, on dog tracheal tissue. Br J Pharmacol. 1988 Sep;95(1):268–274. doi: 10.1111/j.1476-5381.1988.tb16573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Tajima K. Actions of indomethacin and prostaglandins on neuro-effector transmission in the dog trachea. J Physiol. 1981;319:379–392. doi: 10.1113/jphysiol.1981.sp013915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Tajima K. Spontaneous activity in the trachea of dogs treated with indomethacin: an experimental model for aspirin-related asthma. Br J Pharmacol. 1981 Jun;73(2):563–571. doi: 10.1111/j.1476-5381.1981.tb10456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Takeda K. Non-adrenergic inhibitory nerves and putative transmitters in the smooth muscle of cat trachea. J Physiol. 1982 Sep;330:497–511. doi: 10.1113/jphysiol.1982.sp014355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Yoshitomi T. Autoregulation of acetylcholine release from vagus nerve terminals through activation of muscarinic receptors in the dog trachea. Br J Pharmacol. 1988 Mar;93(3):636–646. doi: 10.1111/j.1476-5381.1988.tb10321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen A., Partanen M., Hervonen A., Laitinen L. A. Electron microscopic study on the innervation of the human lower respiratory tract: evidence of adrenergic nerves. Eur J Respir Dis. 1985 Sep;67(3):209–215. [PubMed] [Google Scholar]
- Laitinen A., Partanen M., Hervonen A., Pelto-Huikko M., Laitinen L. A. VIP like immunoreactive nerves in human respiratory tract. Light and electron microscopic study. Histochemistry. 1985;82(4):313–319. doi: 10.1007/BF00494059. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M. Evidence for coexistence of vasoactive intestinal polypeptide (VIP) and acetylcholine in neurons of cat exocrine glands. Morphological, biochemical and functional studies. Acta Physiol Scand Suppl. 1981;496:1–57. [PubMed] [Google Scholar]
- Lundberg J. M., Fahrenkrug J., Hökfelt T., Martling C. R., Larsson O., Tatemoto K., Anggård A. Co-existence of peptide HI (PHI) and VIP in nerves regulating blood flow and bronchial smooth muscle tone in various mammals including man. Peptides. 1984 May-Jun;5(3):593–606. doi: 10.1016/0196-9781(84)90090-1. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Hedlund B., Bartfai T. Vasoactive intestinal polypeptide enhances muscarinic ligand binding in cat submandibular salivary gland. Nature. 1982 Jan 14;295(5845):147–149. doi: 10.1038/295147a0. [DOI] [PubMed] [Google Scholar]
- Matsuzaki Y., Hamasaki Y., Said S. I. Vasoactive intestinal peptide: a possible transmitter of nonadrenergic relaxation of guinea pig airways. Science. 1980 Dec 12;210(4475):1252–1253. doi: 10.1126/science.6254154. [DOI] [PubMed] [Google Scholar]
- Oliva D., Nicosia S., Spada A., Giannattasio G. VIP stimulates ACTH release and adenylate cyclase in human ACTH-secreting pituitary adenomas. Eur J Pharmacol. 1982 Sep 10;83(1-2):101–105. doi: 10.1016/0014-2999(82)90291-6. [DOI] [PubMed] [Google Scholar]
- Ollerenshaw S., Jarvis D., Woolcock A., Sullivan C., Scheibner T. Absence of immunoreactive vasoactive intestinal polypeptide in tissue from the lungs of patients with asthma. N Engl J Med. 1989 May 11;320(19):1244–1248. doi: 10.1056/NEJM198905113201904. [DOI] [PubMed] [Google Scholar]
- Pandol S. J., Dharmsathaphorn K., Schoeffield M. S., Vale W., Rivier J. Vasoactive intestinal peptide receptor antagonist [4Cl-D-Phe6, Leu17] VIP. Am J Physiol. 1986 Apr;250(4 Pt 1):G553–G557. doi: 10.1152/ajpgi.1986.250.4.G553. [DOI] [PubMed] [Google Scholar]
- Polak J. M., Bloom S. R. Regulatory peptides and neuron-specific enolase in the respiratory tract of man and other mammals. Exp Lung Res. 1982 Nov;3(3-4):313–328. doi: 10.3109/01902148209069660. [DOI] [PubMed] [Google Scholar]
- Russell J. A. Noradrenergic inhibitory innervation of canine airways. J Appl Physiol Respir Environ Exerc Physiol. 1980 Jan;48(1):16–22. doi: 10.1152/jappl.1980.48.1.16. [DOI] [PubMed] [Google Scholar]
- Sekizawa K., Tamaoki J., Graf P. D., Nadel J. A. Modulation of cholinergic neurotransmission by vasoactive intestinal peptide in ferret trachea. J Appl Physiol (1985) 1988 Jun;64(6):2433–2437. doi: 10.1152/jappl.1988.64.6.2433. [DOI] [PubMed] [Google Scholar]
- Stjernquist M., Owman C. Vasoactive intestinal polypeptide (VIP) inhibits neurally evoked smooth muscle activity of rat uterine cervix in vitro. Regul Pept. 1984 Mar;8(2):161–167. doi: 10.1016/0167-0115(84)90171-x. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Morita K., Kuriyama H. Innervation and properties of the smooth muscle of the dog trachea. Jpn J Physiol. 1976;26(3):303–320. doi: 10.2170/jjphysiol.26.303. [DOI] [PubMed] [Google Scholar]
- Waelbroeck M., Robberecht P., Coy D. H., Camus J. C., De Neef P., Christophe J. Interaction of growth hormone-releasing factor (GRF) and 14 GRF analogs with vasoactive intestinal peptide (VIP) receptors of rat pancreas. Discovery of (N-Ac-Tyr1,D-Phe2)-GRF(1-29)-NH2 as a VIP antagonist. Endocrinology. 1985 Jun;116(6):2643–2649. doi: 10.1210/endo-116-6-2643. [DOI] [PubMed] [Google Scholar]
- Walters E. H., O'Byrne P. M., Fabbri L. M., Graf P. D., Holtzman M. J., Nadel J. A. Control of neurotransmission by prostaglandins in canine trachealis smooth muscle. J Appl Physiol Respir Environ Exerc Physiol. 1984 Jul;57(1):129–134. doi: 10.1152/jappl.1984.57.1.129. [DOI] [PubMed] [Google Scholar]


