Abstract
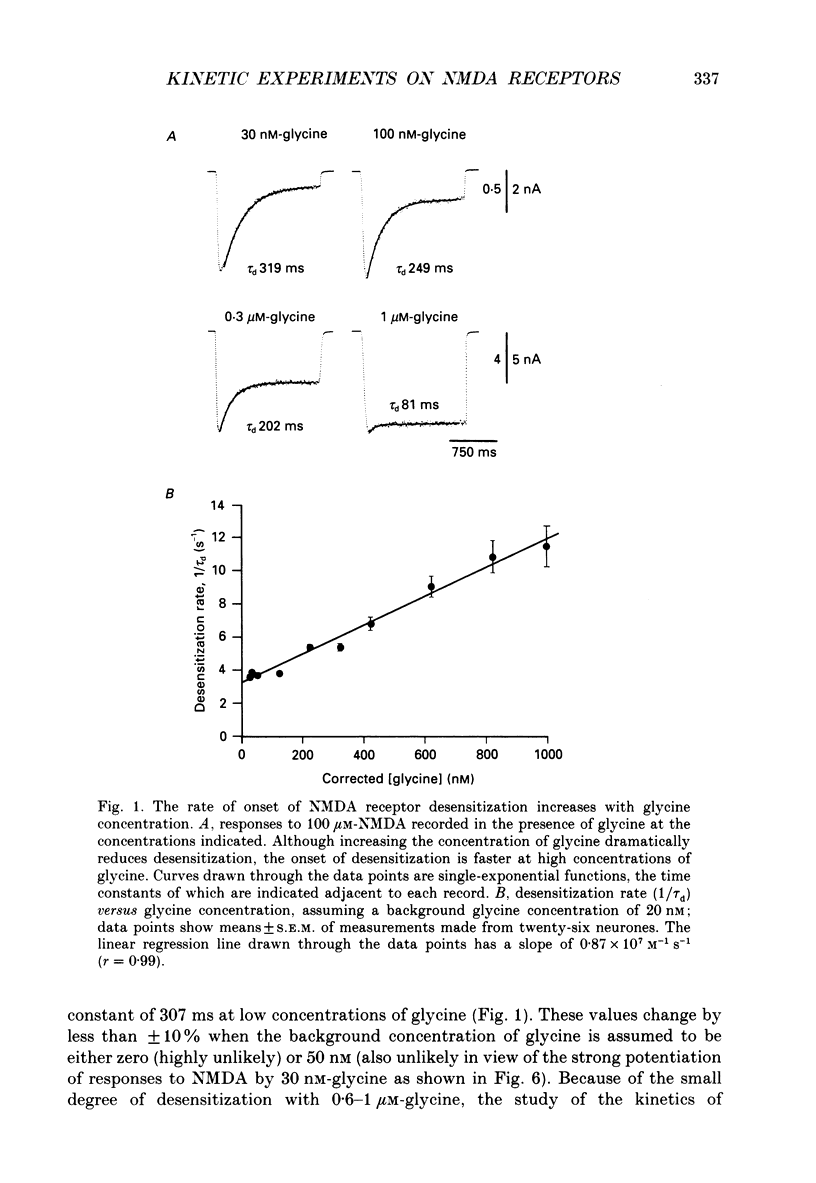
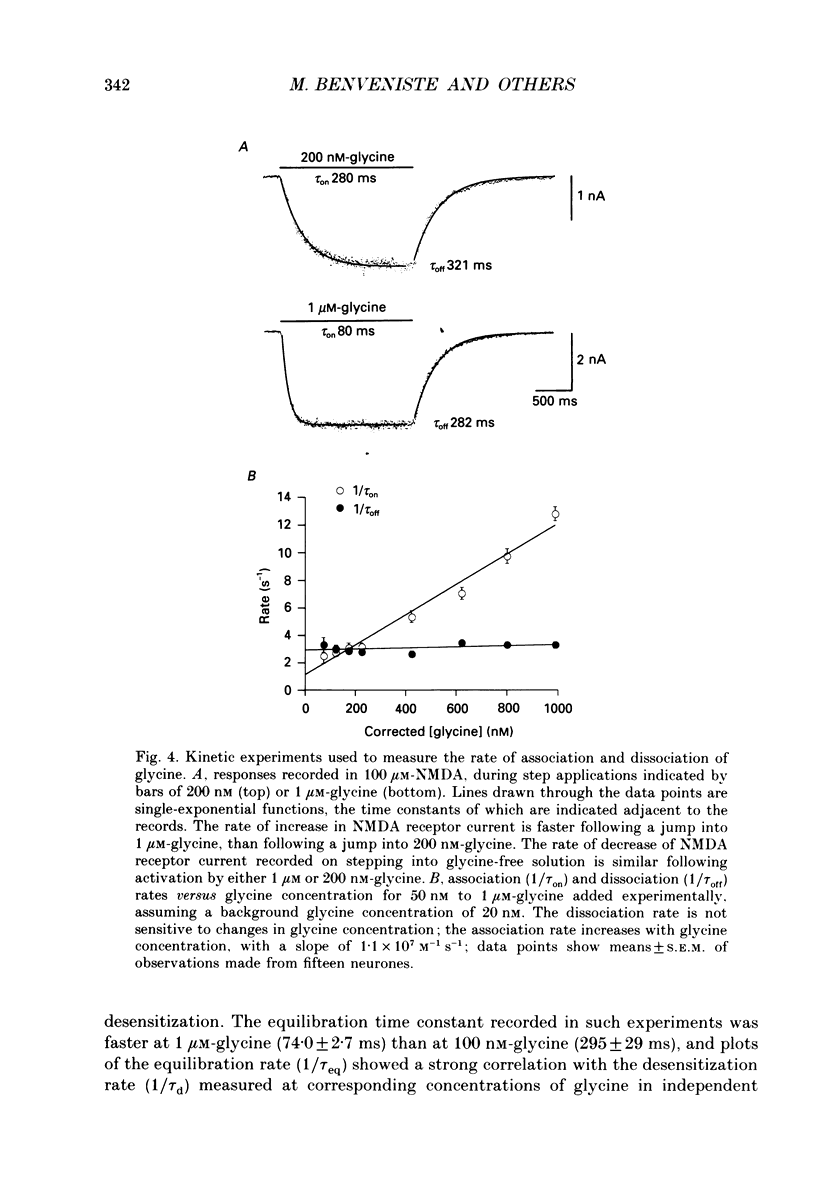
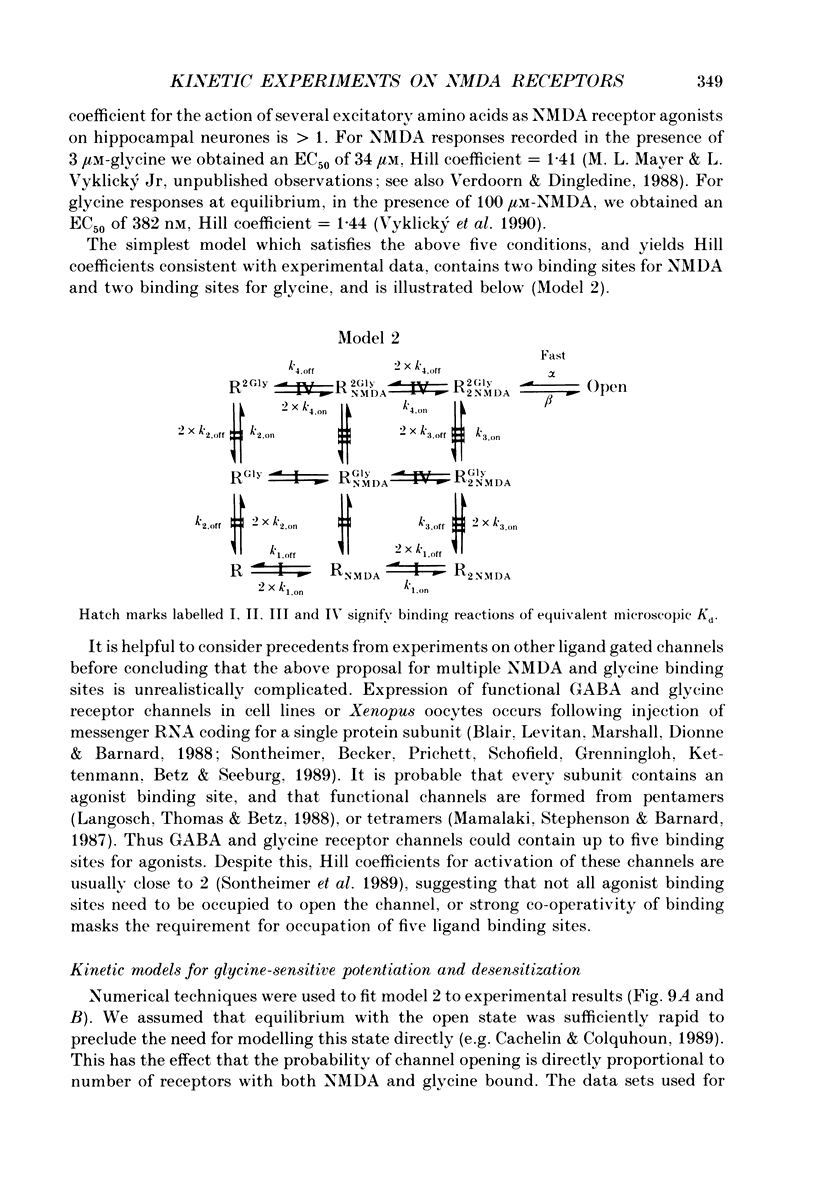
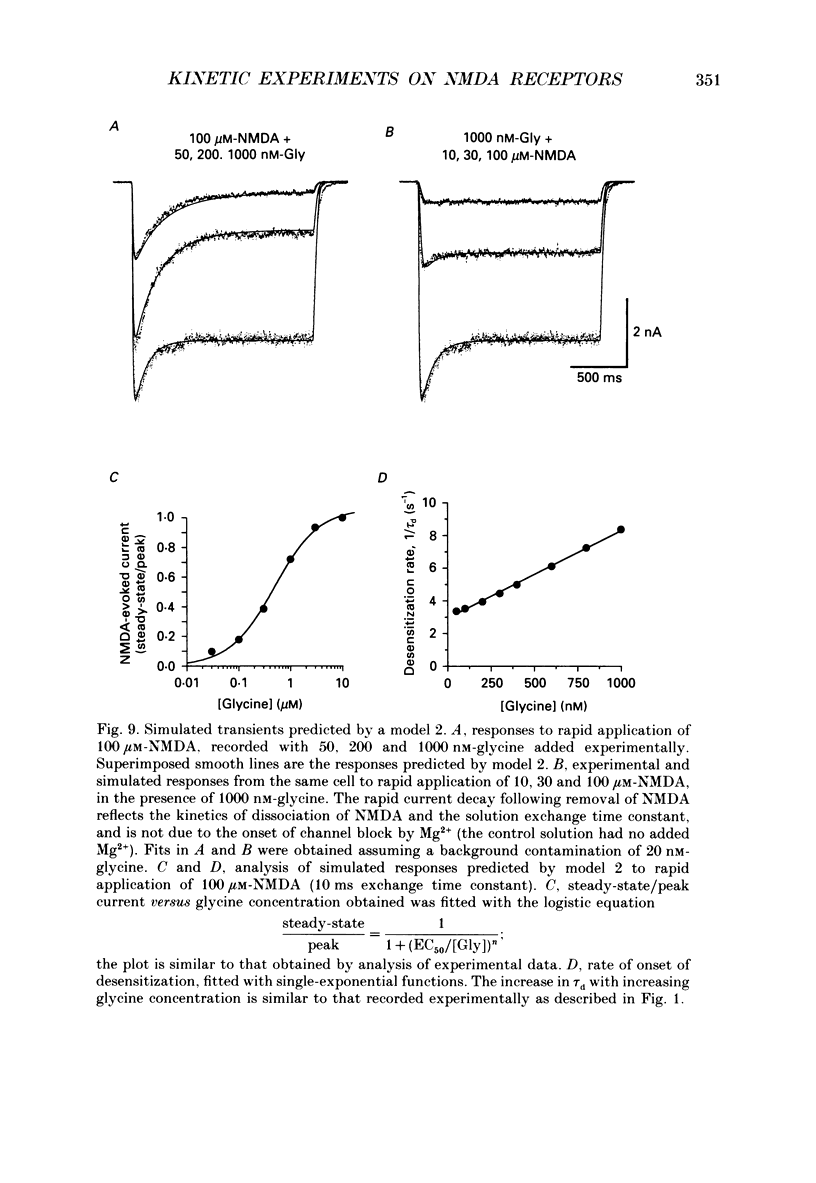
1. Responses to N-methyl-D-aspartic acid (NMDA) were recorded from mouse embryonic hippocampal neurones in dissociated culture, using whole-cell patch-clamp recording. A fast perfusion system, with an exchange time constant of less than 10 ms, was used to study modulation of NMDA receptor desensitization by glycine. 2. The onset of NMDA receptor desensitization was well fitted by a single-exponential function; with 30 nM-glycine the time constant was 250 ms, corresponding to a rate of 4 s-1. The rate of onset of desensitization became faster with increasing glycine concentration, with a slope of 0.87 x 10(7) M-1 s-1. Recovery from desensitization, studied with a twin-pulse technique, was also well fitted by a single-exponential function; with 30 nM-glycine the time constant of recovery was 1.95 s-1. The rate of recovery from desensitization became faster with increasing glycine concentration, with a slope of 0.76 x 10(7) M-1 s-1. These results are consistent with a model in which the effect of glycine occurs via an increase in the rate constant for recovery from desensitization, with little effect on the rate constant for onset of desensitization. Over the range 30-300 nM-glycine, the ratio of the rate constants calculated for recovery and onset of desensitization was a good predictor of the degree of desensitization recorded at equilibrium. 3. Concentration jump experiments with glycine were performed with 100 microM-NMDA present continuously, and for a single binding site model gave estimates of the association (1.1 x 10(7) M-1 s-1) and dissociation (3.1 s-1) rate constants for interaction of glycine with the NMDA receptor. In the presence of NMDA, concentration jumps from 3 microM-glycine to lower concentrations gave relaxations which became slower with decreasing glycine concentration over the range 1 microM-30 nM. A similar slowing of desensitization occurred when the glycine concentration was altered over the same range. 4. Glycine analogues of lower affinity produced desensitization with faster kinetics. D-Alanine, 150 nM, produced desensitization with a time constant of 175 ms, faster than recorded with an equipotent concentration of glycine (50 nM, time constant 259 ms). Responses of similar peak amplitude, recorded with 60 microM-L-alanine, and 500 microM-D,L-homoserine, did not produce strong desensitization, consistent with desensitization too rapid to resolve in our experiments.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascher P., Bregestovski P., Nowak L. N-methyl-D-aspartate-activated channels of mouse central neurones in magnesium-free solutions. J Physiol. 1988 May;399:207–226. doi: 10.1113/jphysiol.1988.sp017076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair L. A., Levitan E. S., Marshall J., Dionne V. E., Barnard E. A. Single subunits of the GABAA receptor form ion channels with properties of the native receptor. Science. 1988 Oct 28;242(4878):577–579. doi: 10.1126/science.2845583. [DOI] [PubMed] [Google Scholar]
- Bonhaus D. W., Yeh G. C., Skaryak L., McNamara J. O. Glycine regulation of the N-methyl-D-aspartate receptor-gated ion channel in hippocampal membranes. Mol Pharmacol. 1989 Aug;36(2):273–279. [PubMed] [Google Scholar]
- Bristow D. R., Bowery N. G., Woodruff G. N. Light microscopic autoradiographic localisation of [3H]glycine and [3H]strychnine binding sites in rat brain. Eur J Pharmacol. 1986 Jul 31;126(3):303–307. doi: 10.1016/0014-2999(86)90062-2. [DOI] [PubMed] [Google Scholar]
- Burgen A. S. The drug-receptor complex. J Pharm Pharmacol. 1966 Mar;18(3):137–149. doi: 10.1111/j.2042-7158.1966.tb07840.x. [DOI] [PubMed] [Google Scholar]
- Cachelin A. B., Colquhoun D. Desensitization of the acetylcholine receptor of frog end-plates measured in a Vaseline-gap voltage clamp. J Physiol. 1989 Aug;415:159–188. doi: 10.1113/jphysiol.1989.sp017717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. On the multiple-conductance single channels activated by excitatory amino acids in large cerebellar neurones of the rat. J Physiol. 1989 Aug;415:555–582. doi: 10.1113/jphysiol.1989.sp017736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadda E., Danysz W., Wroblewski J. T., Costa E. Glycine and D-serine increase the affinity of N-methyl-D-aspartate sensitive glutamate binding sites in rat brain synaptic membranes. Neuropharmacology. 1988 Nov;27(11):1183–1185. doi: 10.1016/0028-3908(88)90015-9. [DOI] [PubMed] [Google Scholar]
- Howe J. R., Colquhoun D., Cull-Candy S. G. On the kinetics of large-conductance glutamate-receptor ion channels in rat cerebellar granule neurons. Proc R Soc Lond B Biol Sci. 1988 May 23;233(1273):407–422. doi: 10.1098/rspb.1988.0030. [DOI] [PubMed] [Google Scholar]
- Huettner J. E. Indole-2-carboxylic acid: a competitive antagonist of potentiation by glycine at the NMDA receptor. Science. 1989 Mar 24;243(4898):1611–1613. doi: 10.1126/science.2467381. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987 Feb 5;325(6104):522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M., Terramani T., Lynch G., Baudry M. A glycine site associated with N-methyl-D-aspartic acid receptors: characterization and identification of a new class of antagonists. J Neurochem. 1989 Apr;52(4):1319–1328. doi: 10.1111/j.1471-4159.1989.tb01881.x. [DOI] [PubMed] [Google Scholar]
- Kiskin N. I., Krishtal O. A., Tsyndrenko AYa Excitatory amino acid receptors in hippocampal neurons: kainate fails to desensitize them. Neurosci Lett. 1986 Jan 30;63(3):225–230. doi: 10.1016/0304-3940(86)90360-5. [DOI] [PubMed] [Google Scholar]
- Kleckner N. W., Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988 Aug 12;241(4867):835–837. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- Kushner L., Lerma J., Zukin R. S., Bennett M. V. Coexpression of N-methyl-D-aspartate and phencyclidine receptors in Xenopus oocytes injected with rat brain mRNA. Proc Natl Acad Sci U S A. 1988 May;85(9):3250–3254. doi: 10.1073/pnas.85.9.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langosch D., Thomas L., Betz H. Conserved quaternary structure of ligand-gated ion channels: the postsynaptic glycine receptor is a pentamer. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7394–7398. doi: 10.1073/pnas.85.19.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamalaki C., Stephenson F. A., Barnard E. A. The GABAA/benzodiazepine receptor is a heterotetramer of homologous alpha and beta subunits. EMBO J. 1987 Mar;6(3):561–565. doi: 10.1002/j.1460-2075.1987.tb04791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr, Clements J. Regulation of NMDA receptor desensitization in mouse hippocampal neurons by glycine. Nature. 1989 Mar 30;338(6214):425–427. doi: 10.1038/338425a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr Concanavalin A selectively reduces desensitization of mammalian neuronal quisqualate receptors. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1411–1415. doi: 10.1073/pnas.86.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan D. T., Olverman H. J., Nguyen L., Watkins J. C., Cotman C. W. Two classes of N-methyl-D-aspartate recognition sites: differential distribution and differential regulation by glycine. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9836–9840. doi: 10.1073/pnas.85.24.9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds I. J., Murphy S. N., Miller R. J. 3H-labeled MK-801 binding to the excitatory amino acid receptor complex from rat brain is enhanced by glycine. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7744–7748. doi: 10.1073/pnas.84.21.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell L. D., Morter R. S., Johnson K. M. Structural requirements for activation of the glycine receptor that modulates the N-methyl-D-aspartate operated ion channel. Eur J Pharmacol. 1988 Oct 26;156(1):105–110. doi: 10.1016/0014-2999(88)90152-5. [DOI] [PubMed] [Google Scholar]
- Sontheimer H., Becker C. M., Pritchett D. B., Schofield P. R., Grenningloh G., Kettenmann H., Betz H., Seeburg P. H. Functional chloride channels by mammalian cell expression of rat glycine receptor subunit. Neuron. 1989 May;2(5):1491–1497. doi: 10.1016/0896-6273(89)90195-5. [DOI] [PubMed] [Google Scholar]
- Tang C. M., Dichter M., Morad M. Quisqualate activates a rapidly inactivating high conductance ionic channel in hippocampal neurons. Science. 1989 Mar 17;243(4897):1474–1477. doi: 10.1126/science.2467378. [DOI] [PubMed] [Google Scholar]
- Thedinga K. H., Benedict M. S., Fagg G. E. The N-methyl-D-aspartate (NMDA) receptor complex: a stoichiometric analysis of radioligand binding domains. Neurosci Lett. 1989 Sep 25;104(1-2):217–222. doi: 10.1016/0304-3940(89)90357-1. [DOI] [PubMed] [Google Scholar]
- Trussell L. O., Fischbach G. D. Glutamate receptor desensitization and its role in synaptic transmission. Neuron. 1989 Aug;3(2):209–218. doi: 10.1016/0896-6273(89)90034-2. [DOI] [PubMed] [Google Scholar]
- Verdoorn T. A., Dingledine R. Excitatory amino acid receptors expressed in Xenopus oocytes: agonist pharmacology. Mol Pharmacol. 1988 Sep;34(3):298–307. [PubMed] [Google Scholar]
- Vyklický L., Jr, Benveniste M., Mayer M. L. Modulation of N-methyl-D-aspartic acid receptor desensitization by glycine in mouse cultured hippocampal neurones. J Physiol. 1990 Sep;428:313–331. doi: 10.1113/jphysiol.1990.sp018214. [DOI] [PMC free article] [PubMed] [Google Scholar]


