Abstract
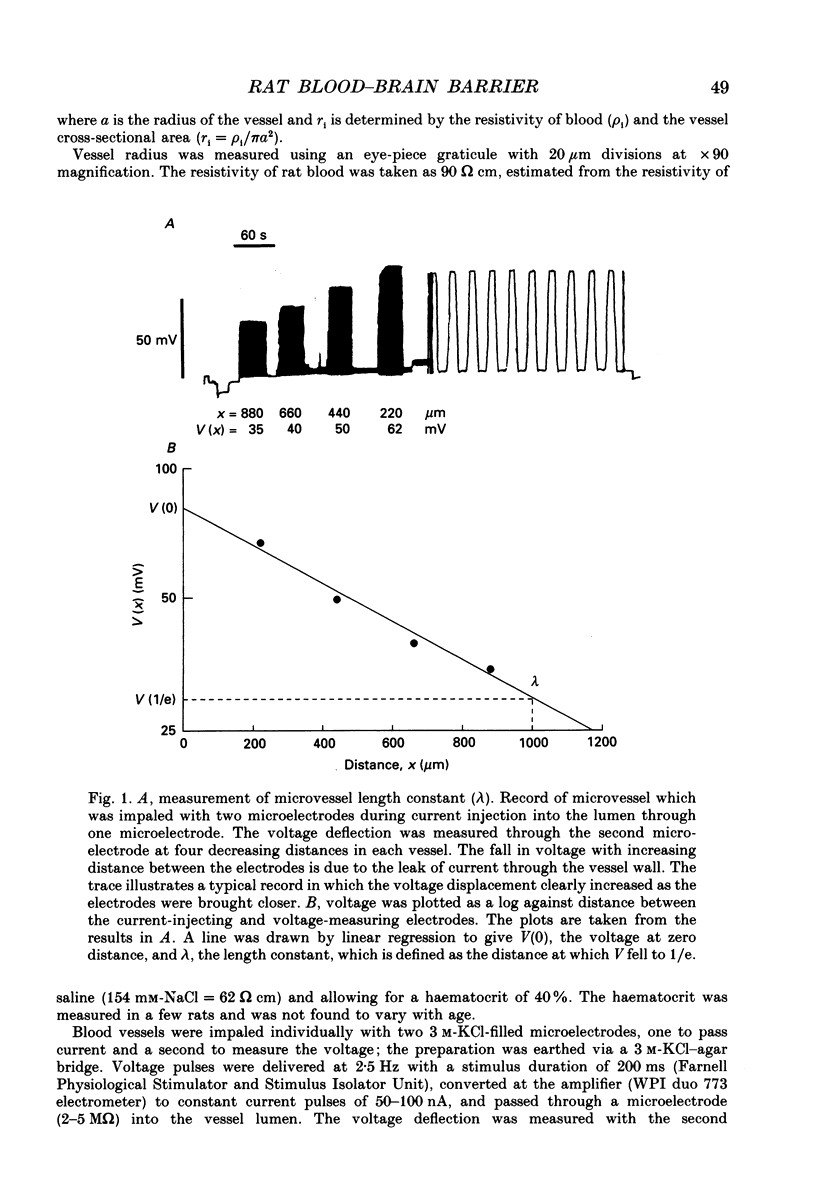
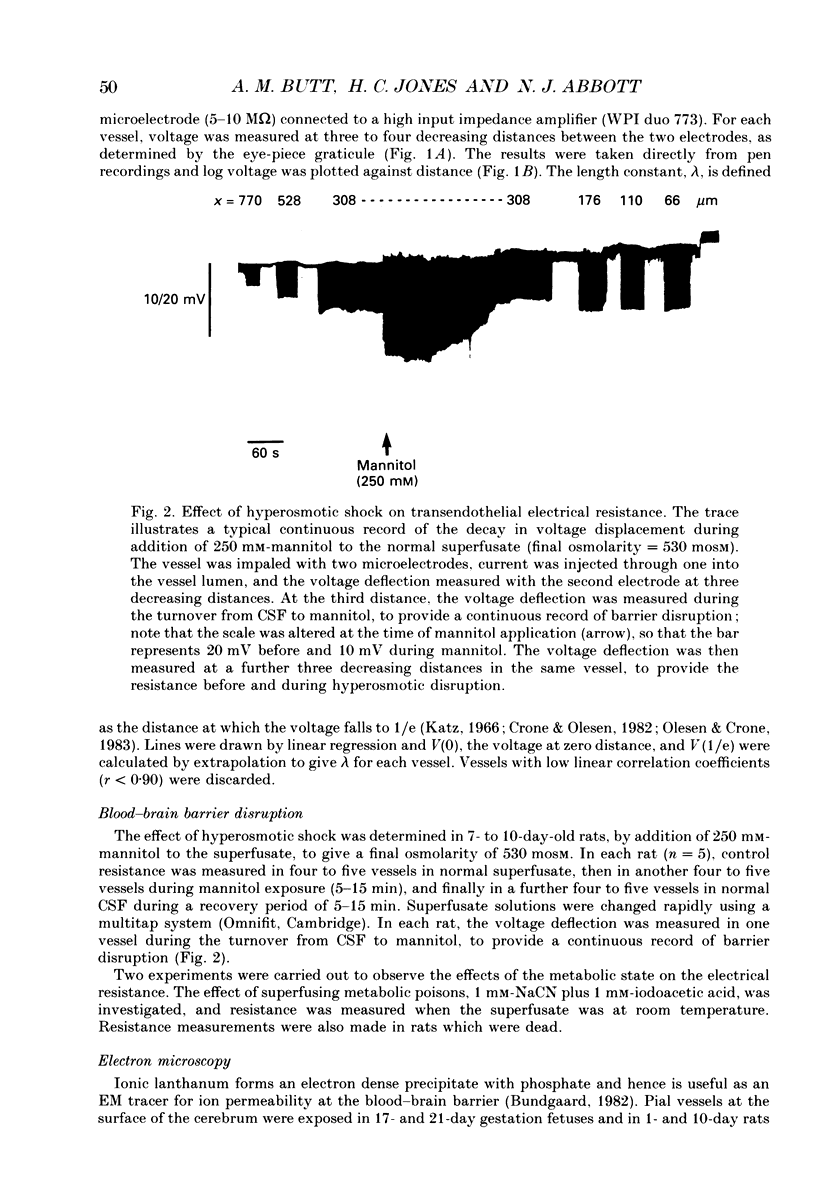
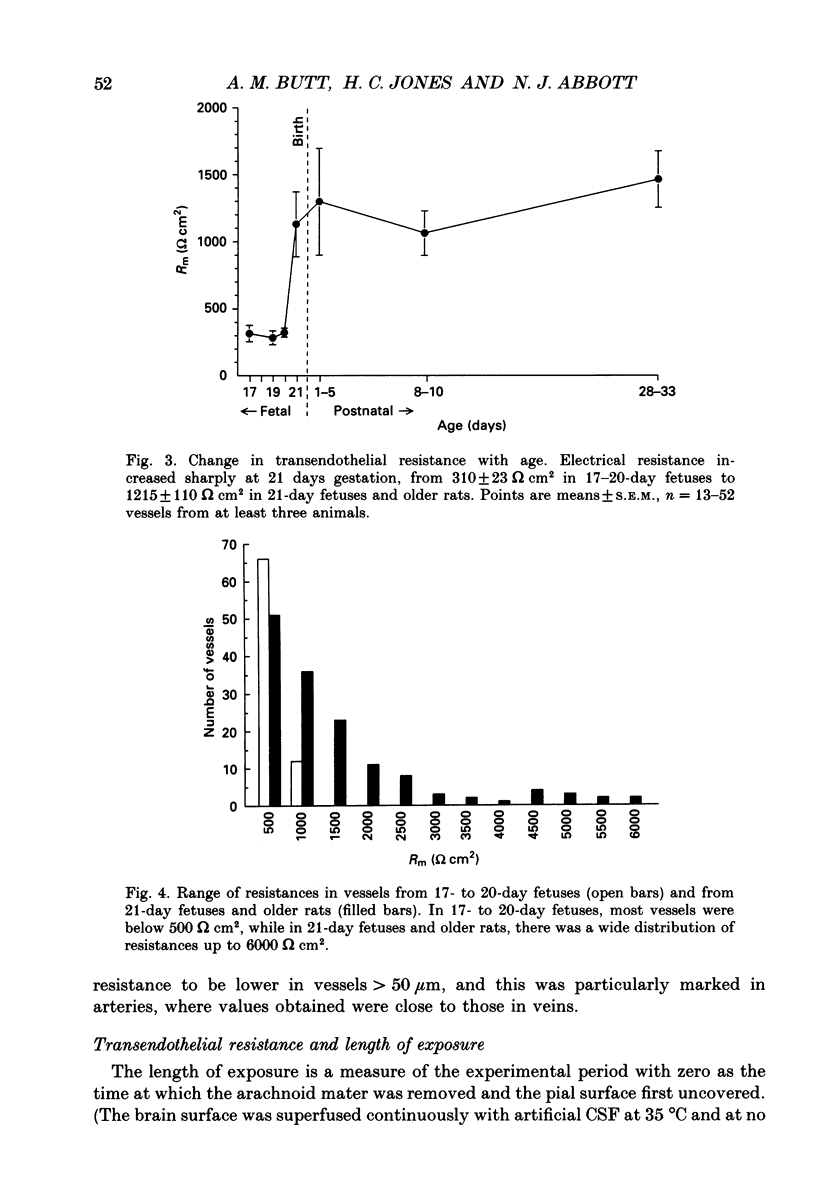
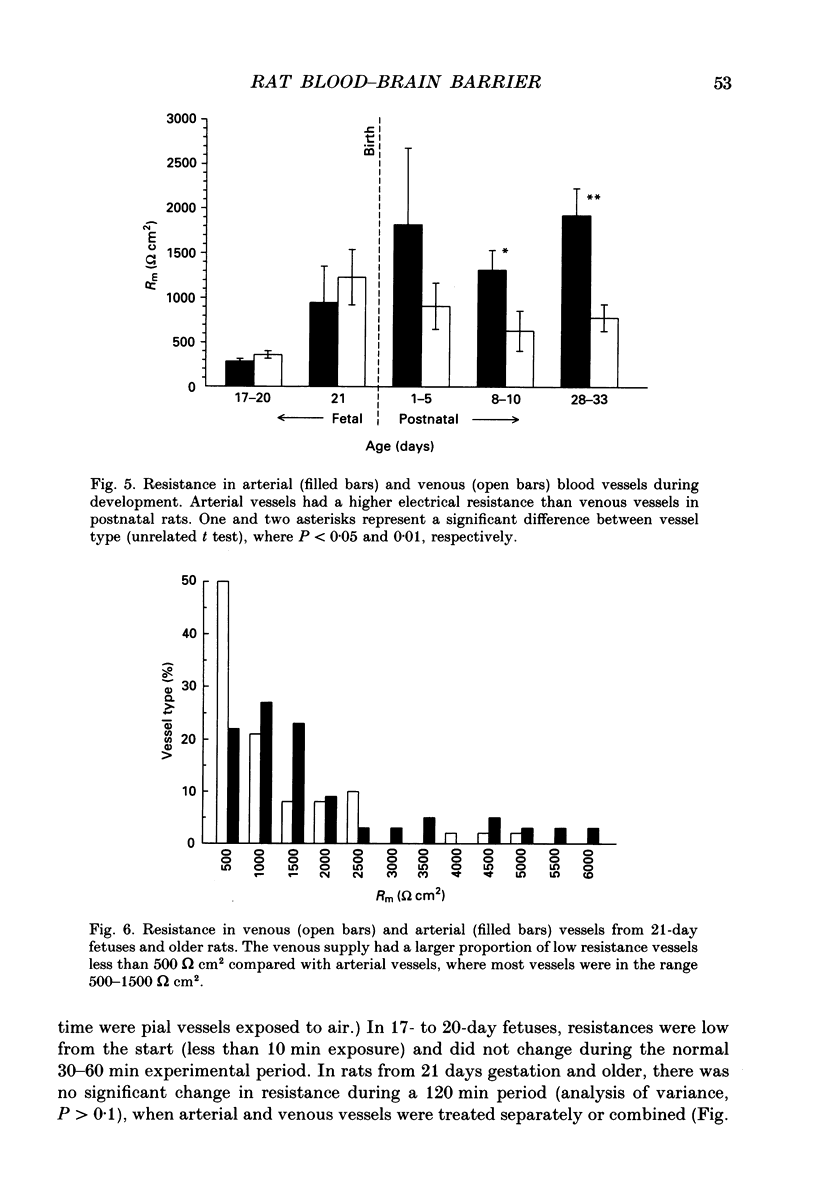
1. Ion permeability of the blood-brain barrier was studied by in situ measurement of transendothelial electrical resistance in anaesthetized rats aged between 17 days gestation and 33 days after birth, and by electron microscopic examination of lanthanum permeability in fetal and neonatal rats aged up to 10 days old. 2. The blood-brain barrier in 17- to 20-day fetuses had a resistance of 310 omega cm2 but was impermeable to lanthanum, and therefore had properties intermediate between leaky and tight epithelia. 3. From 21 days gestation, the resistance was 1128 omega cm2, indicating a tight blood-brain barrier and low ion permeability. There was little further change in barrier resistance after birth, and in 28- to 33-day rats, when the brain barrier systems are mature in other ways, vessels had a mean resistance of 1462 omega cm2. 4. In the tight blood-brain barrier, arterial vessels had a significantly higher resistance than venous vessels, 1490 and 918 omega cm2 respectively. In vessels less than 50 microns diameter and within the normal 60 min experimental period, there was no significant variation in vessel resistance. 5. Hyperosmotic shock caused a rapid decay in resistance (maximal within 5 min), and after disruption of the blood-brain barrier, vessel resistance was 100-300 omega cm2 in both arterial and venous vessels, and the effect was reversible. After the application of metabolic poisons (NaCN plus iodoacetate) and low temperature there was a similarly low electrical resistance. 6. It is concluded that the increase in electrical resistance at birth indicates a decrease in paracellular ion permeability at the blood-brain barrier and is required for effective brain interstitial fluid ion regulation.
Full text
PDF


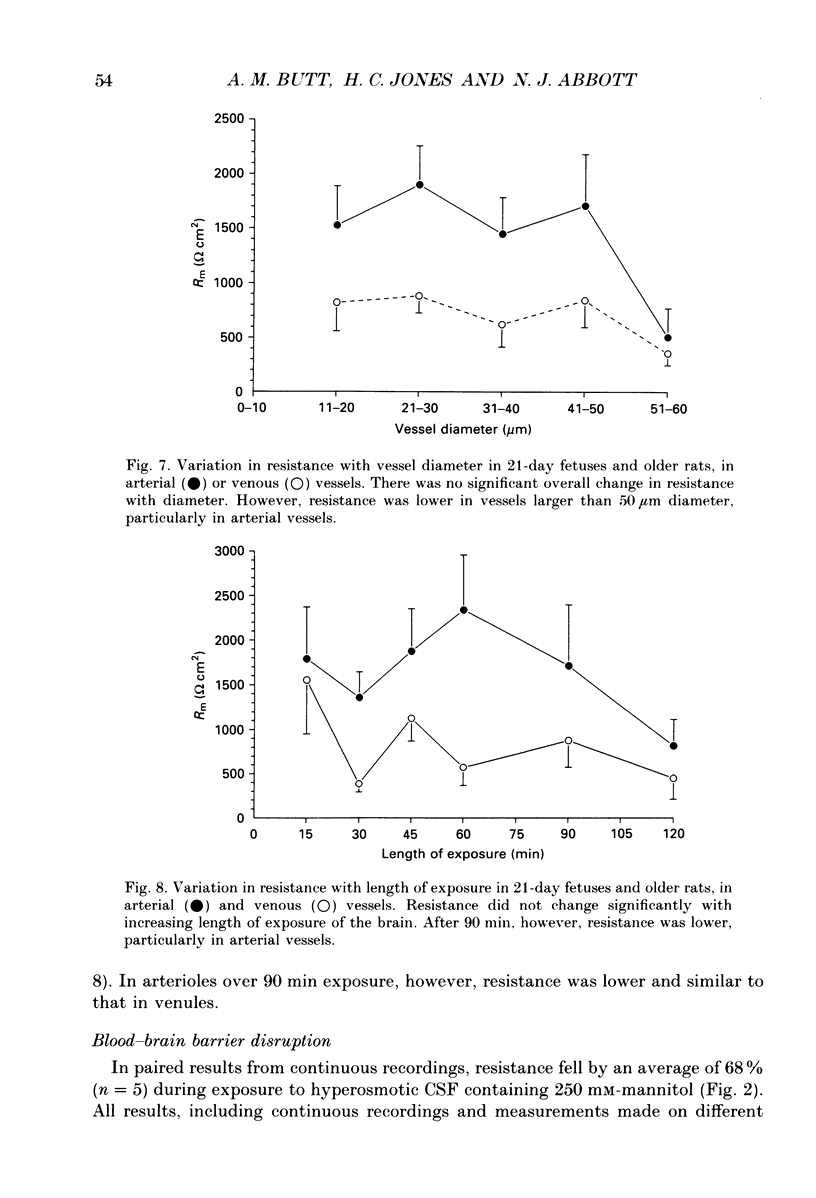







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betz A. L., Goldstein G. W. Developmental changes in metabolism and transport properties of capillaries isolated from rat brain. J Physiol. 1981 Mar;312:365–376. doi: 10.1113/jphysiol.1981.sp013633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouldin T. W., Krigman M. R. Differential permeability of cerebral capillary and choroid plexus to lanthanum ion. Brain Res. 1975 Dec 5;99(2):444–448. doi: 10.1016/0006-8993(75)90053-0. [DOI] [PubMed] [Google Scholar]
- Brightman M. W., Hori M., Rapoport S. I., Reese T. S., Westergaard E. Osmotic opening of tight junctions in cerebral endothelium. J Comp Neurol. 1973 Dec 15;152(4):317–325. doi: 10.1002/cne.901520402. [DOI] [PubMed] [Google Scholar]
- Brightman M. W., Reese T. S. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969 Mar;40(3):648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundgaard M. Ultrastructure of frog cerebral and pial microvessels and their impermeability to lanthanum ions. Brain Res. 1982 Jun 3;241(1):57–65. doi: 10.1016/0006-8993(82)91228-8. [DOI] [PubMed] [Google Scholar]
- Caley D. W., Maxwell D. S. Development of the blood vessels and extracellular spaces during postnatal maturation of rat cerebral cortex. J Comp Neurol. 1970 Jan;138(1):31–47. doi: 10.1002/cne.901380104. [DOI] [PubMed] [Google Scholar]
- Crone C., Christensen O. Electrical resistance of a capillary endothelium. J Gen Physiol. 1981 Apr;77(4):349–371. doi: 10.1085/jgp.77.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C. Lack of selectivity to small ions in paracellular pathways in cerebral and muscle capillaries of the frog. J Physiol. 1984 Aug;353:317–337. doi: 10.1113/jphysiol.1984.sp015338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C., Olesen S. P. Electrical resistance of brain microvascular endothelium. Brain Res. 1982 Jun 3;241(1):49–55. doi: 10.1016/0006-8993(82)91227-6. [DOI] [PubMed] [Google Scholar]
- Cserr H. F., DePasquale M., Patlak C. S. Volume regulatory influx of electrolytes from plasma to brain during acute hyperosmolality. Am J Physiol. 1987 Sep;253(3 Pt 2):F530–F537. doi: 10.1152/ajprenal.1987.253.3.F530. [DOI] [PubMed] [Google Scholar]
- Frömter E., Diamond J. Route of passive ion permeation in epithelia. Nat New Biol. 1972 Jan 5;235(53):9–13. doi: 10.1038/newbio235009a0. [DOI] [PubMed] [Google Scholar]
- Jones H. C., Keep R. F. Brain fluid calcium concentration and response to acute hypercalcaemia during development in the rat. J Physiol. 1988 Aug;402:579–593. doi: 10.1113/jphysiol.1988.sp017223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. C., Keep R. F. The control of potassium concentration in the cerebrospinal fluid and brain interstitial fluid of developing rats. J Physiol. 1987 Feb;383:441–453. doi: 10.1113/jphysiol.1987.sp016419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhan W. G., Heistad D. D. Permeability of blood-brain barrier to various sized molecules. Am J Physiol. 1985 May;248(5 Pt 2):H712–H718. doi: 10.1152/ajpheart.1985.248.5.H712. [DOI] [PubMed] [Google Scholar]
- Møllgård K., Saunders N. R. The development of the human blood-brain and blood-CSF barriers. Neuropathol Appl Neurobiol. 1986 Jul-Aug;12(4):337–358. doi: 10.1111/j.1365-2990.1986.tb00146.x. [DOI] [PubMed] [Google Scholar]
- Nabeshima S., Reese T. S., Landis D. M., Brightman M. W. Junctions in the meninges and marginal glia. J Comp Neurol. 1975 Nov 15;164(2):127–169. doi: 10.1002/cne.901640202. [DOI] [PubMed] [Google Scholar]
- Nagy Z., Peters H., Hüttner I. Fracture faces of cell junctions in cerebral endothelium during normal and hyperosmotic conditions. Lab Invest. 1984 Mar;50(3):313–322. [PubMed] [Google Scholar]
- Oldendorf W. H., Cornford M. E., Brown W. J. The large apparent work capability of the blood-brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann Neurol. 1977 May;1(5):409–417. doi: 10.1002/ana.410010502. [DOI] [PubMed] [Google Scholar]
- Olesen S. P., Crone C. Electrical resistance of muscle capillary endothelium. Biophys J. 1983 Apr;42(1):31–41. doi: 10.1016/S0006-3495(83)84366-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen S. P., Davies P. F., Clapham D. E. Muscarinic-activated K+ current in bovine aortic endothelial cells. Circ Res. 1988 Jun;62(6):1059–1064. doi: 10.1161/01.res.62.6.1059. [DOI] [PubMed] [Google Scholar]
- Olesen S. P. Leakiness of rat brain microvessels to fluorescent probes following craniotomy. Acta Physiol Scand. 1987 May;130(1):63–68. doi: 10.1111/j.1748-1716.1987.tb08112.x. [DOI] [PubMed] [Google Scholar]
- Olesen S. P. Rapid increase in blood-brain barrier permeability during severe hypoxia and metabolic inhibition. Brain Res. 1986 Mar 12;368(1):24–29. doi: 10.1016/0006-8993(86)91038-3. [DOI] [PubMed] [Google Scholar]
- Olsson Y., Klatzo I., Sourander P., Steinwall O. Blood-brain barrier to albumin in embryonic new born and adult rats. Acta Neuropathol. 1968 Mar 4;10(2):117–122. doi: 10.1007/BF00691305. [DOI] [PubMed] [Google Scholar]
- Ransom B. R., Carlini W. G., Connors B. W. Brain extracellular space: developmental studies in rat optic nerve. Ann N Y Acad Sci. 1986;481:87–105. doi: 10.1111/j.1749-6632.1986.tb27141.x. [DOI] [PubMed] [Google Scholar]
- Rapoport S. I., Hori M., Klatzo I. Testing of a hypothesis for osmotic opening of the blood-brain barrier. Am J Physiol. 1972 Aug;223(2):323–331. doi: 10.1152/ajplegacy.1972.223.2.323. [DOI] [PubMed] [Google Scholar]
- Rechthand E., Rapoport S. I. Regulation of the microenvironment of peripheral nerve: role of the blood-nerve barrier. Prog Neurobiol. 1987;28(4):303–343. doi: 10.1016/0301-0082(87)90006-2. [DOI] [PubMed] [Google Scholar]
- Robinson P. J., Rapoport S. I. Size selectivity of blood-brain barrier permeability at various times after osmotic opening. Am J Physiol. 1987 Sep;253(3 Pt 2):R459–R466. doi: 10.1152/ajpregu.1987.253.3.R459. [DOI] [PubMed] [Google Scholar]
- Smith Q. R., Rapoport S. I. Cerebrovascular permeability coefficients to sodium, potassium, and chloride. J Neurochem. 1986 Jun;46(6):1732–1742. doi: 10.1111/j.1471-4159.1986.tb08491.x. [DOI] [PubMed] [Google Scholar]
- USSING H. H., WINDHAGER E. E. NATURE OF SHUNT PATH AND ACTIVE SODIUM TRANSPORT PATH THROUGH FROG SKIN EPITHELIUM. Acta Physiol Scand. 1964 Aug;61:484–504. [PubMed] [Google Scholar]
- Zeuthen T., Wright E. M. Epithelial potassium transport: tracer and electrophysiological studies in choroid plexus. J Membr Biol. 1981;60(2):105–128. doi: 10.1007/BF01870414. [DOI] [PubMed] [Google Scholar]



