Abstract
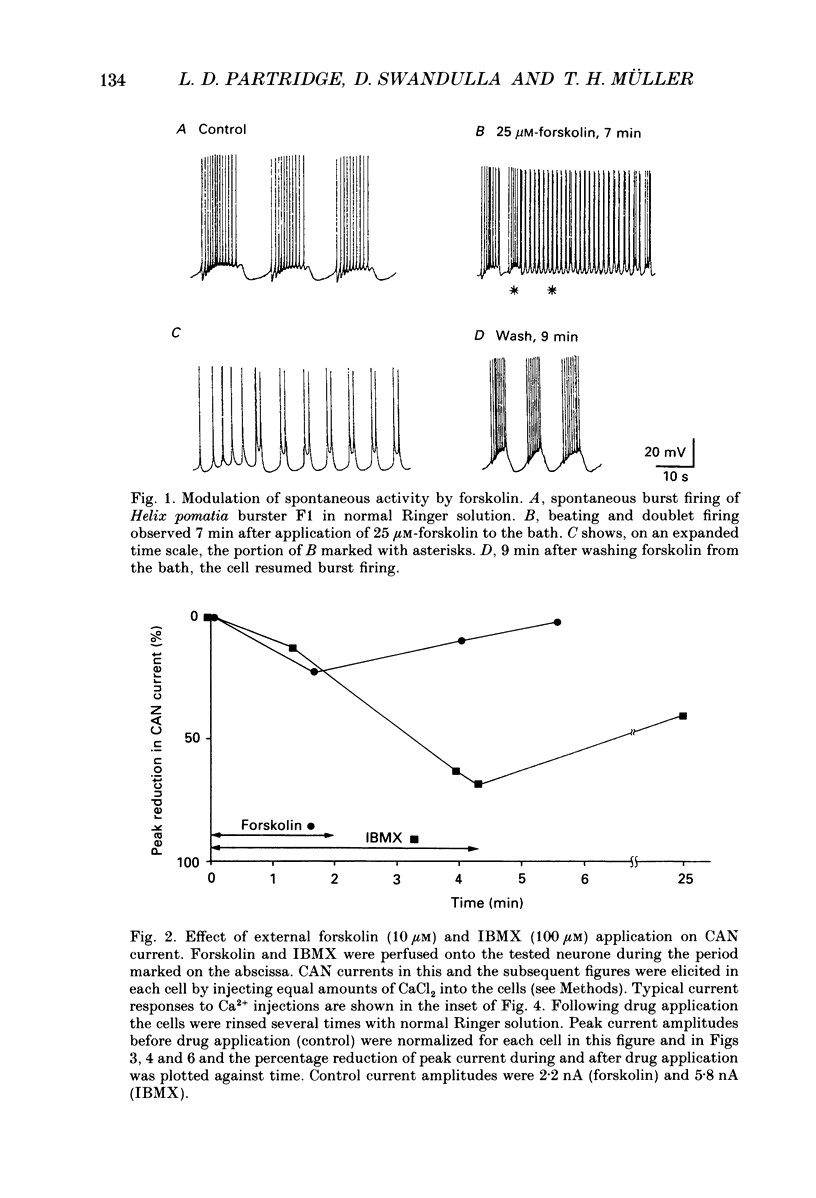
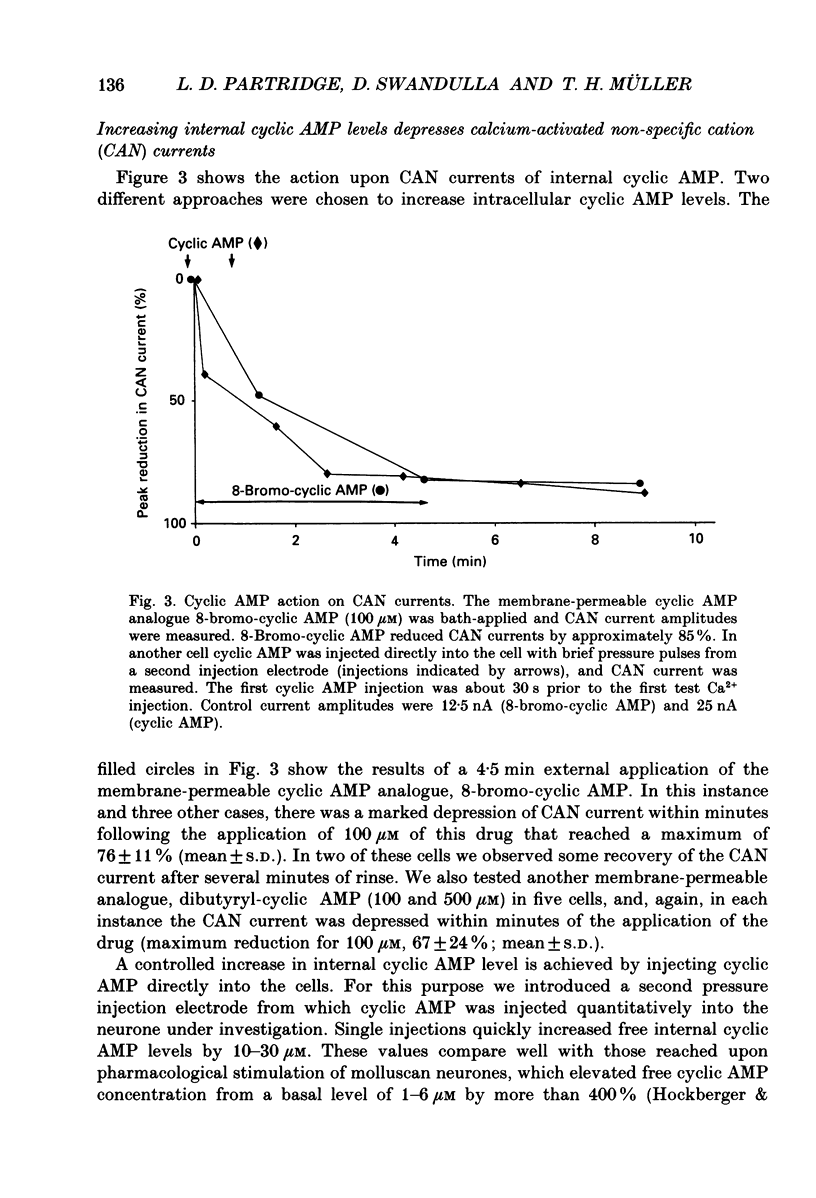
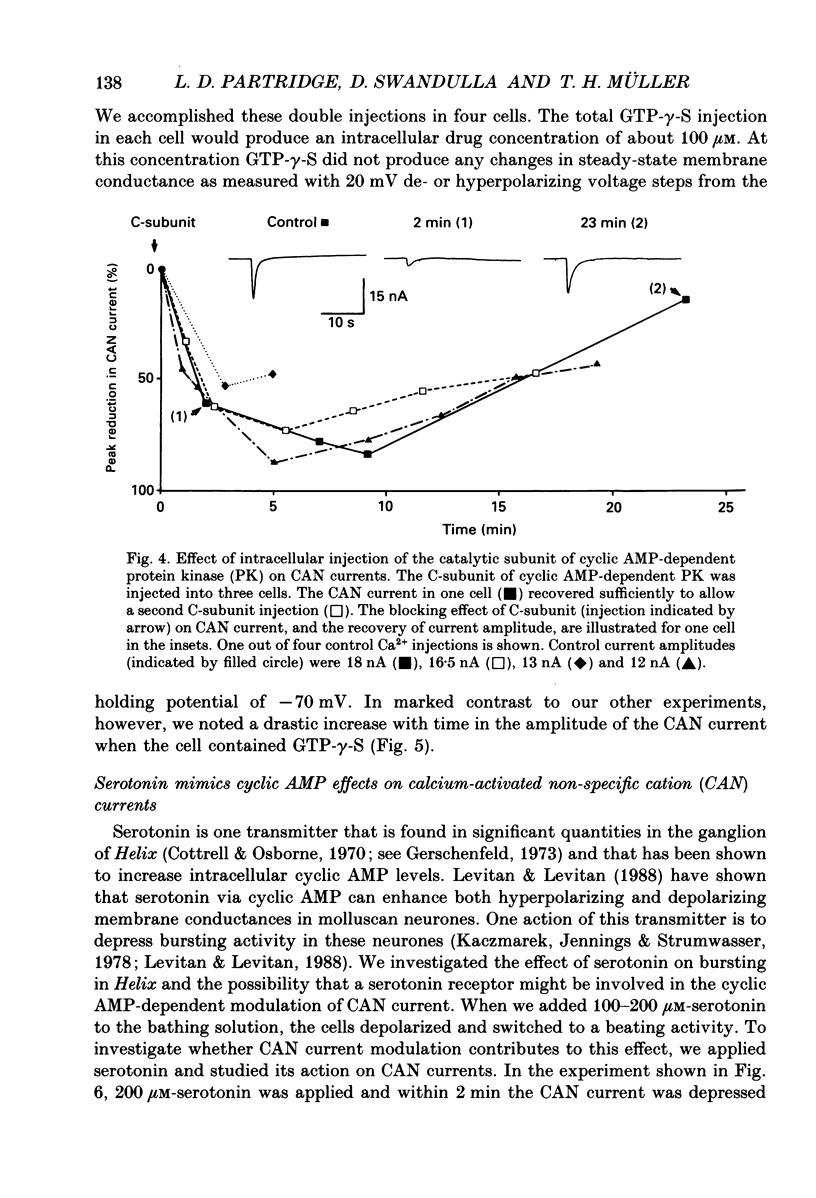
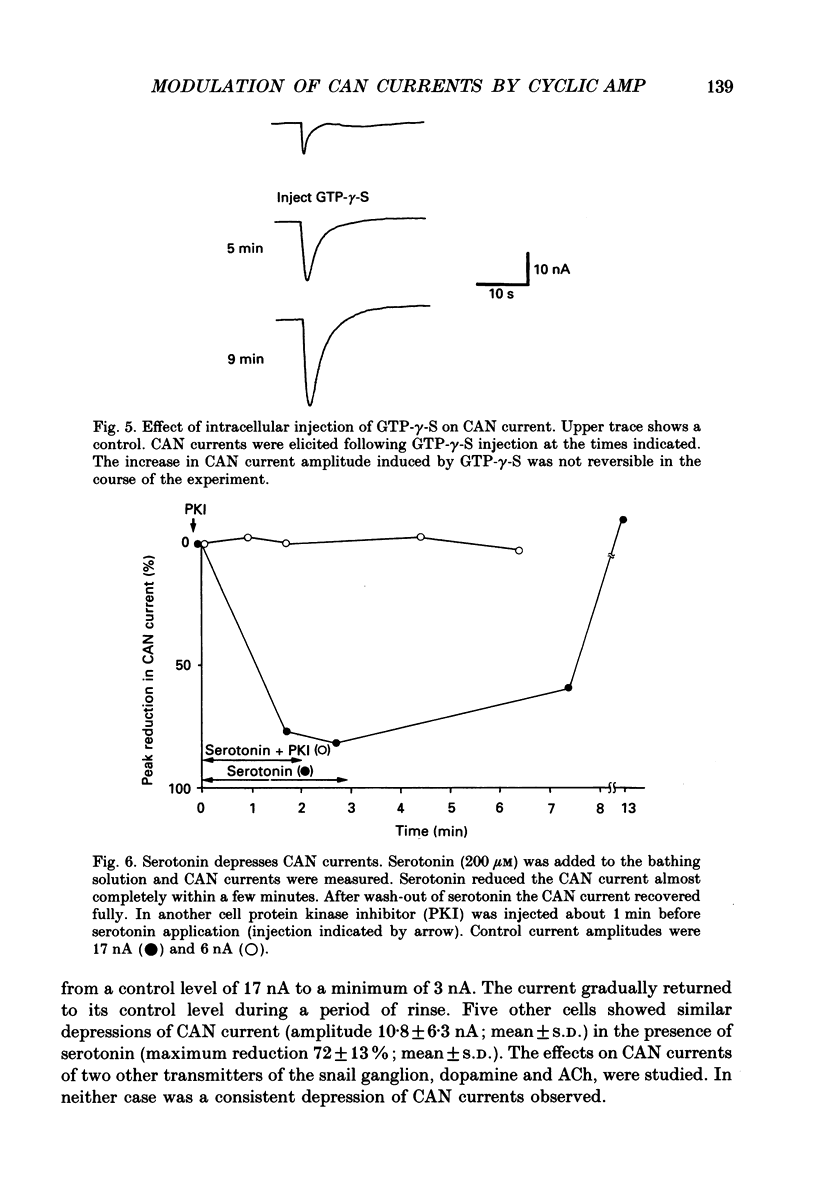
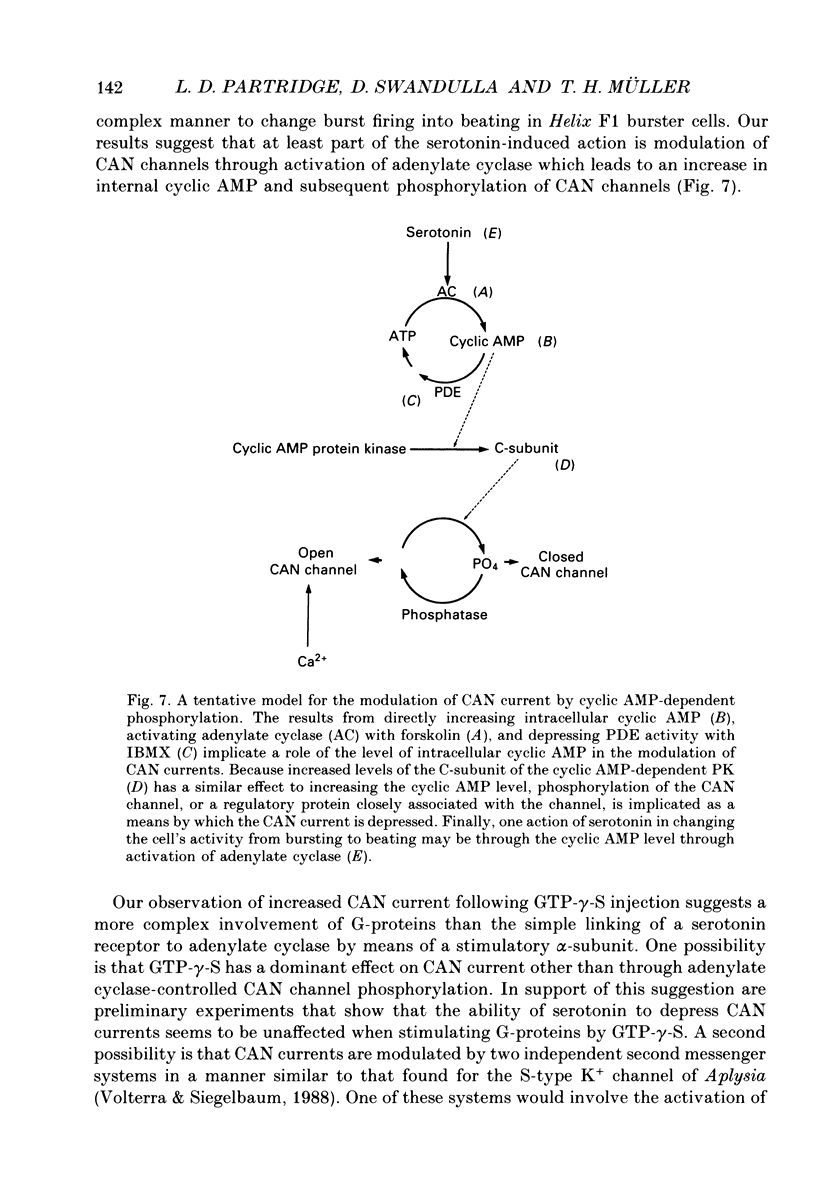
1. Currents through calcium-activated non-specific cation (CAN) channels were studied in the fast burster neurone of Helix aspersa and Helix pomatia. CAN currents were activated by reproducible intracellular injections of small quantities of Ca2+ utilizing a fast, quantitative pressure injection technique. 2. External application of forskolin (10-25 microM), an activator of adenylate cyclase, caused the endogenous bursting activity of the cells to be replaced by beating activity. These same concentrations of forskolin reduced CAN currents reversibly to about 50%. 3. External application of IBMX (3-isobutyl-1-methylxanthine, 100 microM), an inhibitor of phosphodiesterase, the enzyme which breaks down cyclic AMP, reduced CAN currents reversibly to about 40%. 4. External application of the membrane-permeable cyclic AMP analogues 8-bromo-cyclic AMP and dibutyryl-cyclic AMP (100 microM) caused almost complete block of the CAN current. A marked reduction in the CAN current was also observed following quantitative injections of cyclic AMP (internal concentrations up to 50 microM) directly into the cells from a second pressure injection pipette. 5. Similar results were obtained with quantitative injections of the catalytic subunit (C-subunit) of the cyclic AMP-dependent protein kinase (internal concentrations 10(-4) units of enzyme) directly into the cells from a second pressure injection pipette. 6. Injection of the non-hydrolysable GTP analogue, GTP-gamma-S (internal concentrations 100 microM), which stimulates G-proteins, produced a prolonged increase in CAN current amplitude by as much as 300%. 7. External application of serotonin (100-200 microM) caused a transition from bursting to beating activity of the neurones and mimicked cyclic AMP's effects on CAN currents. Two other neurotransmitters, dopamine and acetylcholine, were not significantly effective in reducing CAN currents. 8. Injection of a peptide inhibitor of cyclic AMP-dependent protein kinase suppressed serotonin's action on bursting and on CAN current. 9. Our results indicate that CAN currents in Helix burster neurones are modulated by cyclic AMP-dependent membrane phosphorylation. They suggest that the physiological transmitter that induces this second messenger action is serotonin. The dual control of CAN channels by two second messengers, namely Ca2+ and cyclic AMP, has important functional implications. While Ca2+ activates these channels which generate the pacemaker current in these neurones, cyclic AMP-dependent phosphorylation down-regulates them, thereby resulting in modulation of neuronal bursting activity.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldenhoff J. B., Hofmeier G., Lux H. D., Swandulla D. Stimulation of a sodium influx by cAMP in Helix neurons. Brain Res. 1983 Oct 16;276(2):289–296. doi: 10.1016/0006-8993(83)90736-9. [DOI] [PubMed] [Google Scholar]
- Ascher P. Inhibitory and excitatory effects of dopamine on Aplysia neurones. J Physiol. 1972 Aug;225(1):173–209. doi: 10.1113/jphysiol.1972.sp009933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson J. A., Levitan I. B. Serotonin increases an anomalously rectifying K+ current in the Aplysia neuron R15. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3522–3525. doi: 10.1073/pnas.80.11.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson M., Gola M. Current-voltage relations in ILD- or dopamine-stabilized bursting neurone in APLysia. Comp Biochem Physiol C. 1976;54(2):109–113. doi: 10.1016/0306-4492(76)90073-3. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Hockberger P. A novel membrane sodium current induced by injection of cyclic nucleotides into gastropod neurones. J Physiol. 1984 Sep;354:139–162. doi: 10.1113/jphysiol.1984.sp015368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs J., Thompson S. Forskolin's effect on transient K current in nudibranch neurons is not reproduced by cAMP. J Neurosci. 1987 Feb;7(2):443–452. doi: 10.1523/JNEUROSCI.07-02-00443.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell G. A., Osborne N. N. Subcellular localization of serotonin in an identified serotonin-containing neurone. Nature. 1970 Jan 31;225(5231):470–472. doi: 10.1038/225470a0. [DOI] [PubMed] [Google Scholar]
- Drummond A. H., Benson J. A., Levitan I. B. Serotonin-induced hyperpolarization of an indentified Aplysia neuron is mediated by cyclic AMP. Proc Natl Acad Sci U S A. 1980 Aug;77(8):5013–5017. doi: 10.1073/pnas.77.8.5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton A., Dyball R. E. Phasic firing enhances vasopressin release from the rat neurohypophysis. J Physiol. 1979 May;290(2):433–440. doi: 10.1113/jphysiol.1979.sp012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerschenfeld H. M. Chemical transmission in invertebrate central nervous systems and neuromuscular junctions. Physiol Rev. 1973 Jan;53(1):1–119. doi: 10.1152/physrev.1973.53.1.1. [DOI] [PubMed] [Google Scholar]
- Heyer C. B., Lux H. D. Control of the delayed outward potassium currents in bursting pace-maker neurones of the snail, Helix pomatia. J Physiol. 1976 Nov;262(2):349–382. doi: 10.1113/jphysiol.1976.sp011599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockberger P. E., Swandulla D. Direct ion channel gating: a new function for intracellular messengers. Cell Mol Neurobiol. 1987 Sep;7(3):229–236. doi: 10.1007/BF00711301. [DOI] [PubMed] [Google Scholar]
- Hockberger P., Yamane T. Compartmentalization of cyclic AMP elevation in neurons of Aplysia californica. Cell Mol Neurobiol. 1987 Mar;7(1):19–33. doi: 10.1007/BF00734987. [DOI] [PubMed] [Google Scholar]
- Hofmeier G., Lux H. D. The time courses of intracellular free calcium and related electrical effects after injection of CaCl2 into neurons of the snail, Helix pomatia. Pflugers Arch. 1981 Sep;391(3):242–251. doi: 10.1007/BF00596178. [DOI] [PubMed] [Google Scholar]
- Hoshi T., Garber S. S., Aldrich R. W. Effect of forskolin on voltage-gated K+ channels is independent of adenylate cyclase activation. Science. 1988 Jun 17;240(4859):1652–1655. doi: 10.1126/science.2454506. [DOI] [PubMed] [Google Scholar]
- Joost H. G., Habberfield A. D., Simpson I. A., Laurenza A., Seamon K. B. Activation of adenylate cyclase and inhibition of glucose transport in rat adipocytes by forskolin analogues: structural determinants for distinct sites of action. Mol Pharmacol. 1988 Apr;33(4):449–453. [PubMed] [Google Scholar]
- Kaczmarek L. K., Jennings K., Strumwasser F. Neurotransmitter modulation, phosphodiesterase inhibitor effects, and cyclic AMP correlates of afterdischarge in peptidergic neurites. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5200–5204. doi: 10.1073/pnas.75.10.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkut G. A., Lambert J. D., Gayton R. J., Loker J. E., Walker R. J. Mapping of nerve cells in the suboesophageal ganglia of Helix aspersa. Comp Biochem Physiol A Comp Physiol. 1975 Jan 1;50(1A):1–25. doi: 10.1016/s0010-406x(75)80194-0. [DOI] [PubMed] [Google Scholar]
- Kononenko N. I., Kostyuk P. G., Shcherbatko A. D. The effect of intracellular cAMP injections on stationary membrane conductance and voltage- and time-dependent ionic currents in identified snail neurons. Brain Res. 1983 Jun 6;268(2):321–338. doi: 10.1016/0006-8993(83)90499-7. [DOI] [PubMed] [Google Scholar]
- Kramer R. H., Levitan E. S., Wilson M. P., Levitan I. B. Mechanism of calcium-dependent inactivation of a potassium current in Aplysia neuron R15: interaction between calcium and cyclic AMP. J Neurosci. 1988 May;8(5):1804–1813. doi: 10.1523/JNEUROSCI.08-05-01804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause D., Lee S. C., Deutsch C. Forskolin effects on the voltage-gated K+ conductance of human T cells. Pflugers Arch. 1988 Jul;412(1-2):133–140. doi: 10.1007/BF00583742. [DOI] [PubMed] [Google Scholar]
- Levitan E. S., Levitan I. B. Serotonin acting via cyclic AMP enhances both the hyperpolarizing and depolarizing phases of bursting pacemaker activity in the Aplysia neuron R15. J Neurosci. 1988 Apr;8(4):1152–1161. doi: 10.1523/JNEUROSCI.08-04-01152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner E., Metzger H. The action of forskolin on muscle cells is modified by hormones, calcium ions and calcium antagonists. Arzneimittelforschung. 1983;33(10):1436–1441. [PubMed] [Google Scholar]
- Lotshaw D. P., Levitan E. S., Levitan I. B. Fine tuning of neuronal electrical activity: modulation of several ion channels by intracellular messengers in a single identified nerve cell. J Exp Biol. 1986 Sep;124:307–322. doi: 10.1242/jeb.124.1.307. [DOI] [PubMed] [Google Scholar]
- Müller T. H., Swandulla D., Lux H. D. Activation of three types of membrane currents by various divalent cations in identified molluscan pacemaker neurons. J Gen Physiol. 1989 Dec;94(6):997–1014. doi: 10.1085/jgp.94.6.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neer E. J., Clapham D. E. Roles of G protein subunits in transmembrane signalling. Nature. 1988 May 12;333(6169):129–134. doi: 10.1038/333129a0. [DOI] [PubMed] [Google Scholar]
- Osterrieder W., Brum G., Hescheler J., Trautwein W., Flockerzi V., Hofmann F. Injection of subunits of cyclic AMP-dependent protein kinase into cardiac myocytes modulates Ca2+ current. Nature. 1982 Aug 5;298(5874):576–578. doi: 10.1038/298576a0. [DOI] [PubMed] [Google Scholar]
- Parnas I., Strumwasser F. Mechanisms of long-lasting inhibition of a bursting pacemaker neuron. J Neurophysiol. 1974 Jul;37(4):609–620. doi: 10.1152/jn.1974.37.4.609. [DOI] [PubMed] [Google Scholar]
- Partridge L. D., Swandulla D. Calcium-activated non-specific cation channels. Trends Neurosci. 1988 Feb;11(2):69–72. doi: 10.1016/0166-2236(88)90167-1. [DOI] [PubMed] [Google Scholar]
- Partridge L. D., Swandulla D. Single Ca-activated cation channels in bursting neurons of Helix. Pflugers Arch. 1987 Dec;410(6):627–631. doi: 10.1007/BF00581323. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: its biological and chemical properties. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:1–150. [PubMed] [Google Scholar]
- Seamon K., Daly J. W. Activation of adenylate cyclase by the diterpene forskolin does not require the guanine nucleotide regulatory protein. J Biol Chem. 1981 Oct 10;256(19):9799–9801. [PubMed] [Google Scholar]
- Swandulla D. Cationic membrane conductances induced by intracellularly elevated cAMP and Ca2+: measurements with ion-selective microelectrodes. Can J Physiol Pharmacol. 1987 May;65(5):898–903. doi: 10.1139/y87-145. [DOI] [PubMed] [Google Scholar]
- Swandulla D., Lux H. D. Activation of a nonspecific cation conductance by intracellular Ca2+ elevation in bursting pacemaker neurons of Helix pomatia. J Neurophysiol. 1985 Dec;54(6):1430–1443. doi: 10.1152/jn.1985.54.6.1430. [DOI] [PubMed] [Google Scholar]
- Swandulla D., Lux H. D. Changes in ionic conductances induced by cAMP in Helix neurons. Brain Res. 1984 Jul 2;305(1):115–122. doi: 10.1016/0006-8993(84)91126-0. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Intracellular pH of snail neurones measured with a new pH-sensitive glass mirco-electrode. J Physiol. 1974 Apr;238(1):159–180. doi: 10.1113/jphysiol.1974.sp010516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volterra A., Siegelbaum S. A. Role of two different guanine nucleotide-binding proteins in the antagonistic modulation of the S-type K+ channel by cAMP and arachidonic acid metabolites in Aplysia sensory neurons. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7810–7814. doi: 10.1073/pnas.85.20.7810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagoner P. K., Pallotta B. S. Modulation of acetylcholine receptor desensitization by forskolin is independent of cAMP. Science. 1988 Jun 17;240(4859):1655–1657. doi: 10.1126/science.2454507. [DOI] [PubMed] [Google Scholar]
- Zünkler B. J., Trube G., Ohno-Shosaku T. Forskolin-induced block of delayed rectifying K+ channels in pancreatic beta-cells is not mediated by cAMP. Pflugers Arch. 1988 Jun;411(6):613–619. doi: 10.1007/BF00580856. [DOI] [PubMed] [Google Scholar]
- de Peyer J. E., Cachelin A. B., Levitan I. B., Reuter H. Ca2+ -activated K+ conductance in internally perfused snail neurons is enhanced by protein phosphorylation. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4207–4211. doi: 10.1073/pnas.79.13.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]


