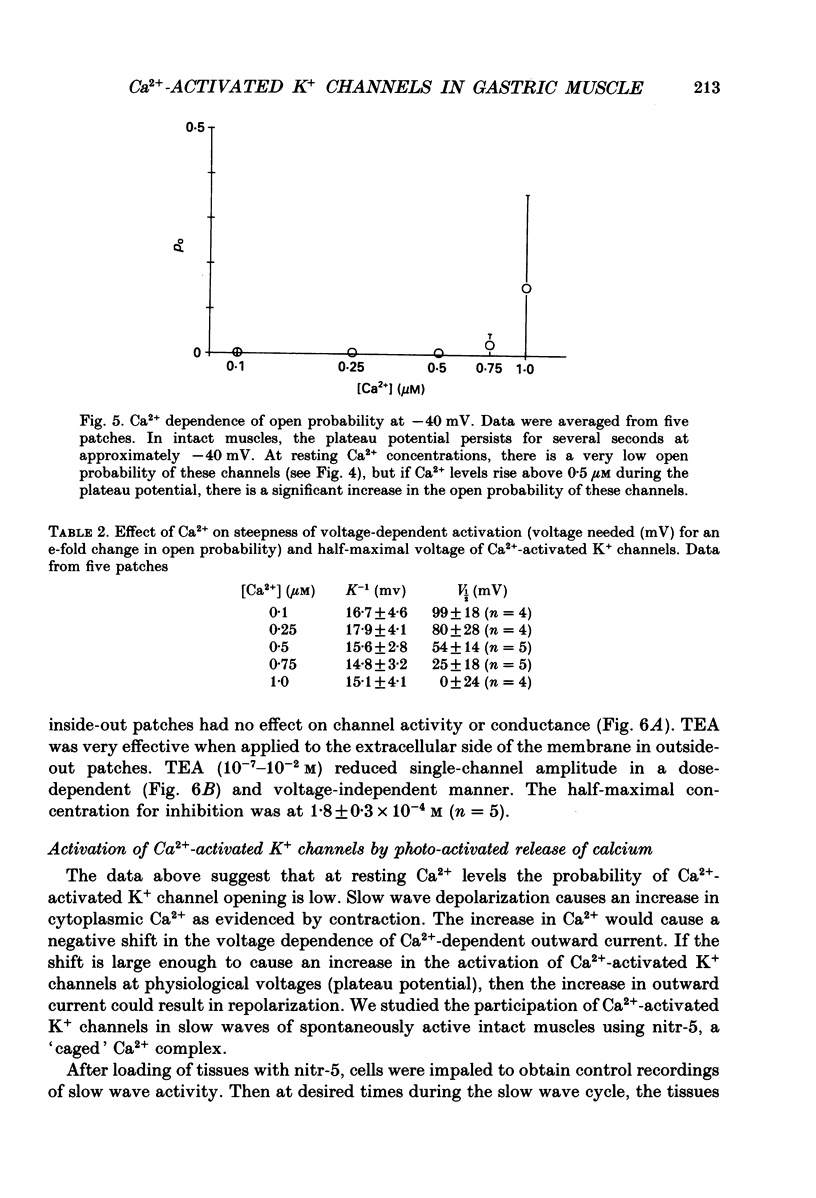
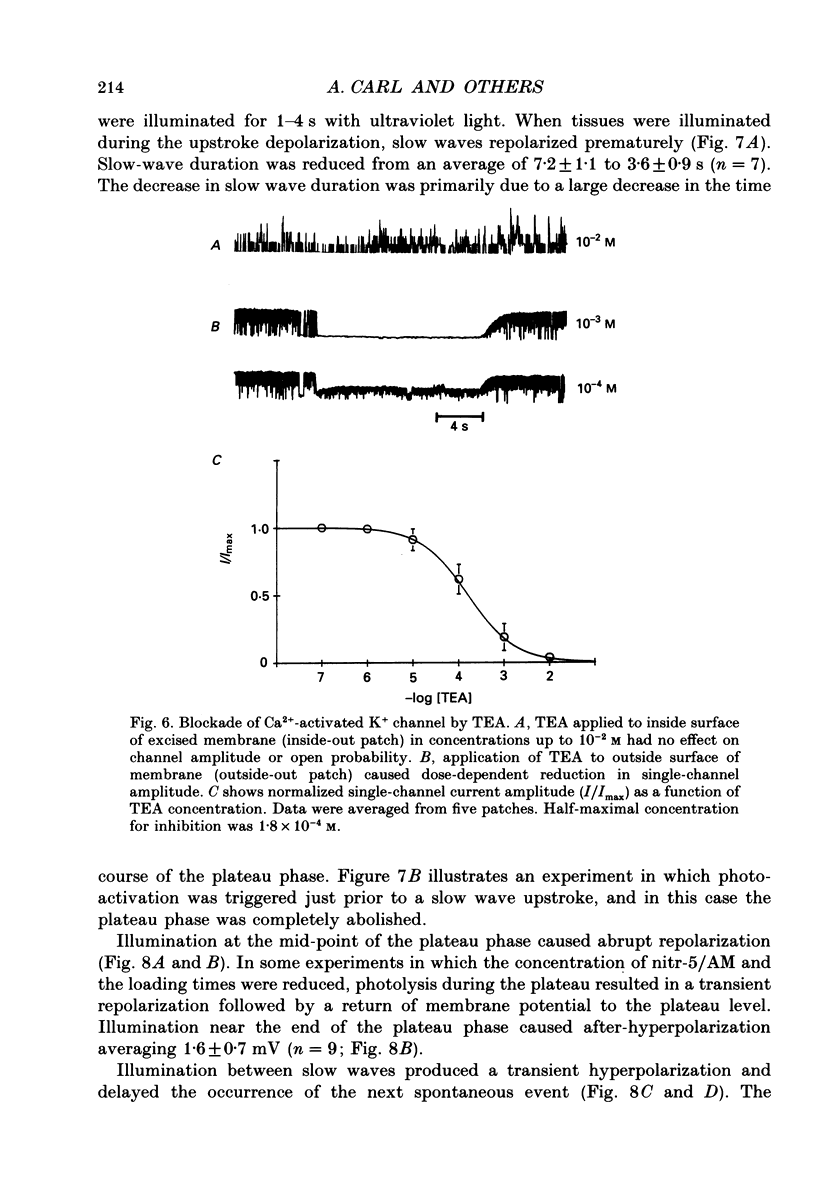
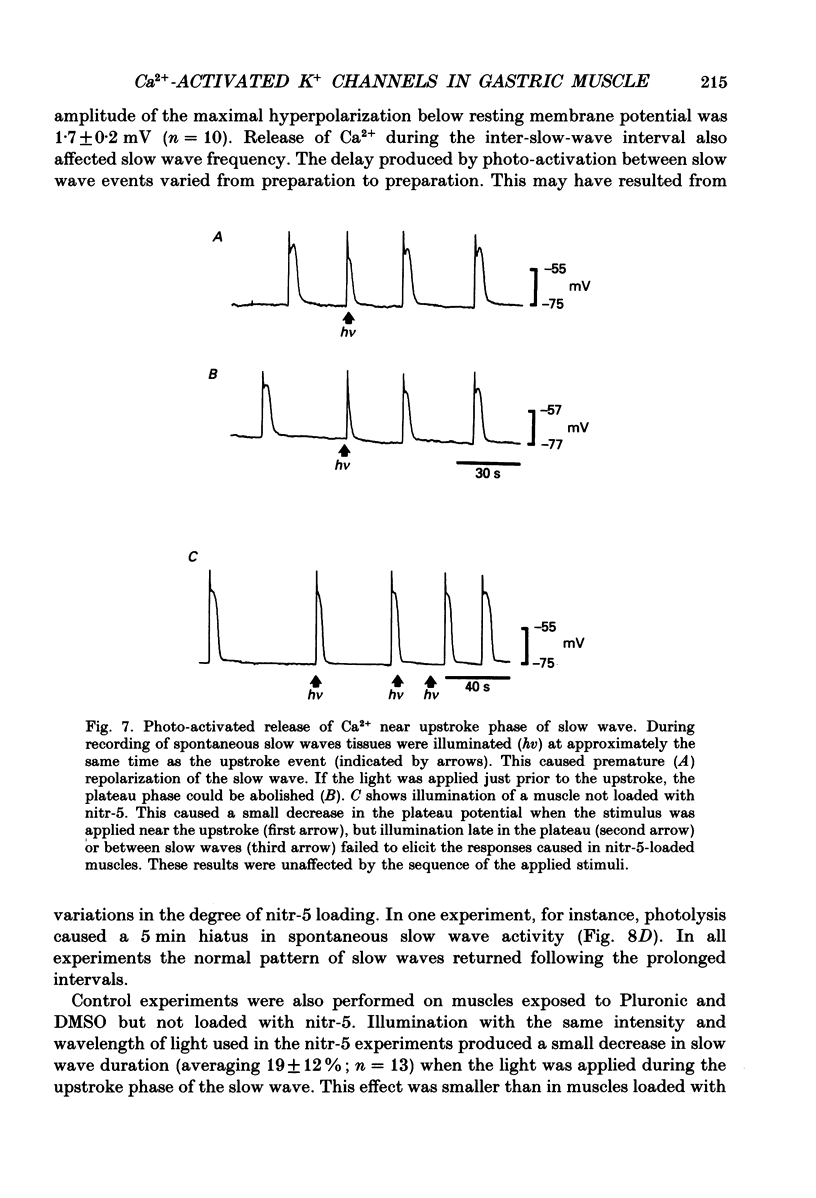
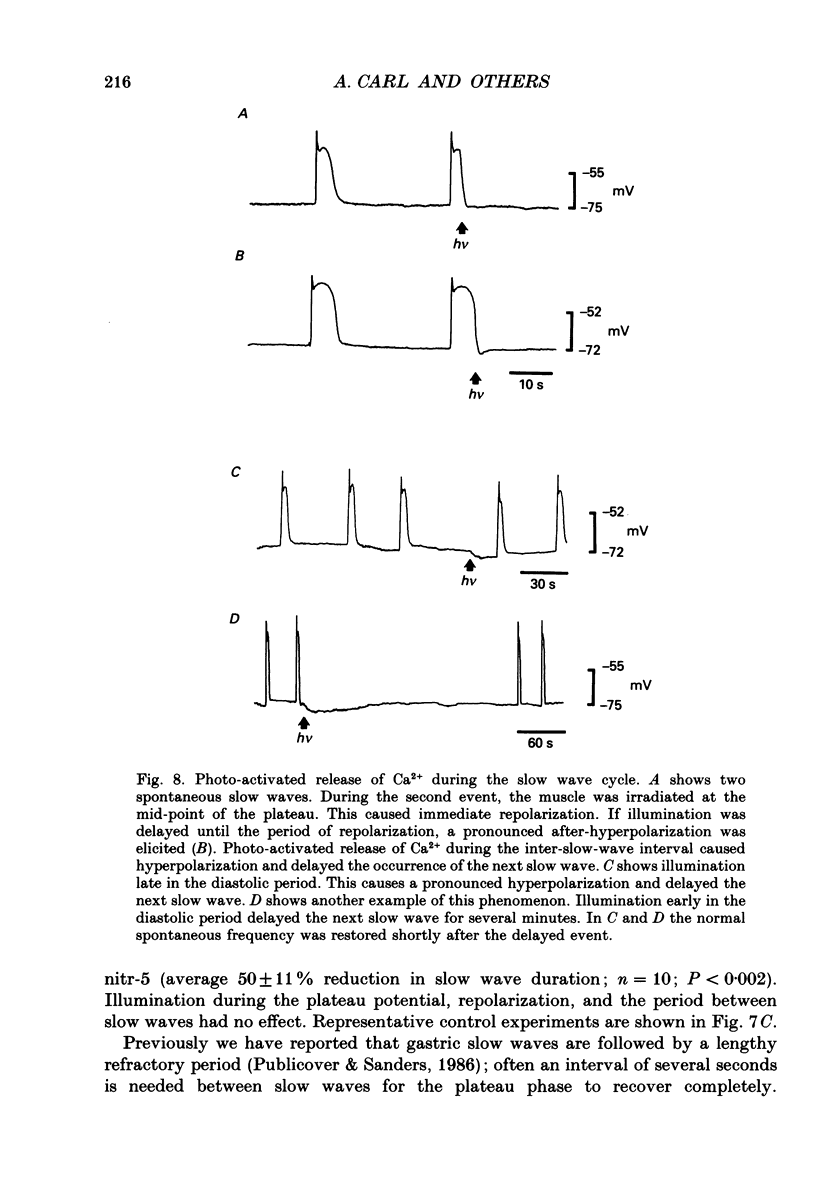
Abstract
1. The hypothesis that Ca2(+)-activated K+ channels participate in the repolarization of electrical slow waves was tested in isolated cells and intact muscles of the canine gastric antrum. 2. Freshly dispersed cells from the gastric antrum liberally express large conductance channels that were characterized as Ca2(+)-activated K+ channels by several criteria. 3. Mean slope conductance of these channels in symmetrical 140 mM-KCl solutions was 265 +/- 25 pS and reversal potential was 1.3 +/- 3.3 mV. The reversal potential was shifted when K+ was partially replaced with Na+ in a manner consistent with the Nernst equation for the K+ gradient. 4. Open probability was studied in excised patches in solutions containing 10(-7)-10(-6) M-Ca2+ with holding potentials ranging from -100 to +100 mV. Resulting activation curves were fitted by Boltzmann functions. 5. Increasing [Ca2+] from 10(-7) to 10(-6) M shifted the half-maximal activation from +99 to 0 mV. These data suggest that Ca2(+)-activated K+ channels may be activated in the voltage range and [Ca2+]i occurring during the plateau phase of the slow wave. 6. In intact muscles loaded with the photolabile Ca2+ chelator, nitr-5, photo-activated release of Ca2+ during the slow wave cycle produced changes consistent with activation of Ca2(+)-dependent outward currents. 7. The data are consistent with the idea that Ca2+ build-up during electrical slow waves shifts the activation voltage of Ca2(+)-activated K+ channels into the range of the plateau potential. Activation of these channels yields outward current and repolarization. 8. Since the force of contractions depends on slow wave amplitude and duration, regulation of these channels may be important in controlling gastric motility.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. S., MacKinnon R., Smith C., Miller C. Charybdotoxin block of single Ca2+-activated K+ channels. Effects of channel gating, voltage, and ionic strength. J Gen Physiol. 1988 Mar;91(3):317–333. doi: 10.1085/jgp.91.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A. J., Reed J. B., Sanders K. M. Slow wave heterogeneity within the circular muscle of the canine gastric antrum. J Physiol. 1985 Sep;366:221–232. doi: 10.1113/jphysiol.1985.sp015793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A. J., Sanders K. M. Gradient in excitation-contraction coupling in canine gastric antral circular muscle. J Physiol. 1985 Dec;369:283–294. doi: 10.1113/jphysiol.1985.sp015901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech D. J., Bolton T. B. Properties of the cromakalim-induced potassium conductance in smooth muscle cells isolated from the rabbit portal vein. Br J Pharmacol. 1989 Nov;98(3):851–864. doi: 10.1111/j.1476-5381.1989.tb14614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J., Takewaki T. Calcium-activated potassium channels in single smooth muscle cells of rabbit jejunum and guinea-pig mesenteric artery. J Physiol. 1986 Feb;371:45–67. doi: 10.1113/jphysiol.1986.sp015961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Lang R. J., Takewaki T., Benham C. D. Patch and whole-cell voltage clamp of single mammalian visceral and vascular smooth muscle cells. Experientia. 1985 Jul 15;41(7):887–894. doi: 10.1007/BF01970006. [DOI] [PubMed] [Google Scholar]
- Carl A., Sanders K. M. Ca2+-activated K channels of canine colonic myocytes. Am J Physiol. 1989 Sep;257(3 Pt 1):C470–C480. doi: 10.1152/ajpcell.1989.257.3.C470. [DOI] [PubMed] [Google Scholar]
- Cole W. C., Carl A., Sanders K. M. Muscarinic suppression of Ca2+-dependent K current in colonic smooth muscle. Am J Physiol. 1989 Sep;257(3 Pt 1):C481–C487. doi: 10.1152/ajpcell.1989.257.3.C481. [DOI] [PubMed] [Google Scholar]
- Cole W. C., Sanders K. M. Characterization of macroscopic outward currents of canine colonic myocytes. Am J Physiol. 1989 Sep;257(3 Pt 1):C461–C469. doi: 10.1152/ajpcell.1989.257.3.C461. [DOI] [PubMed] [Google Scholar]
- Cole W. C., Sanders K. M. G proteins mediate suppression of Ca2+-activated K current by acetylcholine in smooth muscle cells. Am J Physiol. 1989 Sep;257(3 Pt 1):C596–C600. doi: 10.1152/ajpcell.1989.257.3.C596. [DOI] [PubMed] [Google Scholar]
- Droogmans G., Callewaert G. Ca2+-channel current and its modification by the dihydropyridine agonist BAY k 8644 in isolated smooth muscle cells. Pflugers Arch. 1986 Mar;406(3):259–265. doi: 10.1007/BF00640911. [DOI] [PubMed] [Google Scholar]
- Ehrreich S. J., Furchgott R. F. Relaxation of mammalian smooth muscles by visible and ultraviolet radiation. Nature. 1968 May 18;218(5142):682–684. doi: 10.1038/218682a0. [DOI] [PubMed] [Google Scholar]
- Fujii K., Inoue R., Yamanaka K., Yoshitomi T. Effects of calcium antagonists on smooth muscle membranes of the canine stomach. Gen Pharmacol. 1985;16(3):217–221. doi: 10.1016/0306-3623(85)90072-2. [DOI] [PubMed] [Google Scholar]
- Gurney A. M., Tsien R. Y., Lester H. A. Activation of a potassium current by rapid photochemically generated step increases of intracellular calcium in rat sympathetic neurons. Proc Natl Acad Sci U S A. 1987 May;84(10):3496–3500. doi: 10.1073/pnas.84.10.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Langton P. D., Burke E. P., Sanders K. M. Participation of Ca currents in colonic electrical activity. Am J Physiol. 1989 Sep;257(3 Pt 1):C451–C460. doi: 10.1152/ajpcell.1989.257.3.C451. [DOI] [PubMed] [Google Scholar]
- Matsunaga K., Furchgott R. F. Interactions of light and sodium nitrite in producing relaxation of rabbit aorta. J Pharmacol Exp Ther. 1989 Feb;248(2):687–695. [PubMed] [Google Scholar]
- Morgan K. G., Muir T. C., Szurszewski J. H. The electrical basis for contraction and relaxation in canine fundal smooth muscle. J Physiol. 1981 Feb;311:475–488. doi: 10.1113/jphysiol.1981.sp013599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan K. G., Szurszewski J. H. Mechanisms of phasic and tonic actions of pentagastrin on canine gastric smooth muscle. J Physiol. 1980 Apr;301:229–242. doi: 10.1113/jphysiol.1980.sp013201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya Y., Kitamura K., Kuriyama H. Cellular calcium regulates outward currents in rabbit intestinal smooth muscle cell. Am J Physiol. 1987 Apr;252(4 Pt 1):C401–C410. doi: 10.1152/ajpcell.1987.252.4.C401. [DOI] [PubMed] [Google Scholar]
- Publicover N. G., Sanders K. M. Effects of frequency on the wave form of propagated slow waves in canine gastric antral muscle. J Physiol. 1986 Feb;371:179–189. doi: 10.1113/jphysiol.1986.sp015967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders K. M. Excitation-contraction coupling without Ca2+ action potentials in small intestine. Am J Physiol. 1983 May;244(5):C356–C361. doi: 10.1152/ajpcell.1983.244.5.C356. [DOI] [PubMed] [Google Scholar]
- Sato K., Ozaki H., Karaki H. Changes in cytosolic calcium level in vascular smooth muscle strip measured simultaneously with contraction using fluorescent calcium indicator fura 2. J Pharmacol Exp Ther. 1988 Jul;246(1):294–300. [PubMed] [Google Scholar]
- Singer J. J., Walsh J. V., Jr Characterization of calcium-activated potassium channels in single smooth muscle cells using the patch-clamp technique. Pflugers Arch. 1987 Feb;408(2):98–111. doi: 10.1007/BF00581337. [DOI] [PubMed] [Google Scholar]
- Szurszewski J. H. A study of the canine gastric action potential in the presence of tetraethylammonium chloride. J Physiol. 1978 Apr;277:91–102. doi: 10.1113/jphysiol.1978.sp012262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szurszewski J. H. Mechanism of action of pentagastrin and acetylcholine on the longitudinal muscle of the canine antrum. J Physiol. 1975 Nov;252(2):335–361. doi: 10.1113/jphysiol.1975.sp011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillotson D. Inactivation of Ca conductance dependent on entry of Ca ions in molluscan neurons. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1497–1500. doi: 10.1073/pnas.76.3.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Zucker R. S. Control of cytoplasmic calcium with photolabile tetracarboxylate 2-nitrobenzhydrol chelators. Biophys J. 1986 Nov;50(5):843–853. doi: 10.1016/S0006-3495(86)83525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J. V., Jr, Singer J. J. Identification and characterization of major ionic currents in isolated smooth muscle cells using the voltage-clamp technique. Pflugers Arch. 1987 Feb;408(2):83–97. doi: 10.1007/BF00581336. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Fay F. S. Calcium transients and resting levels in isolated smooth muscle cells as monitored with quin 2. Am J Physiol. 1986 May;250(5 Pt 1):C779–C791. doi: 10.1152/ajpcell.1986.250.5.C779. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Honeyman T. W., Fay F. S. Beta-adrenergic actions on membrane electrical properties of dissociated smooth muscle cells. Am J Physiol. 1988 Mar;254(3 Pt 1):C423–C431. doi: 10.1152/ajpcell.1988.254.3.C423. [DOI] [PubMed] [Google Scholar]
- el-Sharkawy T. Y., Morgan K. G., Szurszewski J. H. Intracellular electrical activity of canine and human gastric smooth muscle. J Physiol. 1978 Jun;279:291–307. doi: 10.1113/jphysiol.1978.sp012345. [DOI] [PMC free article] [PubMed] [Google Scholar]


