Abstract
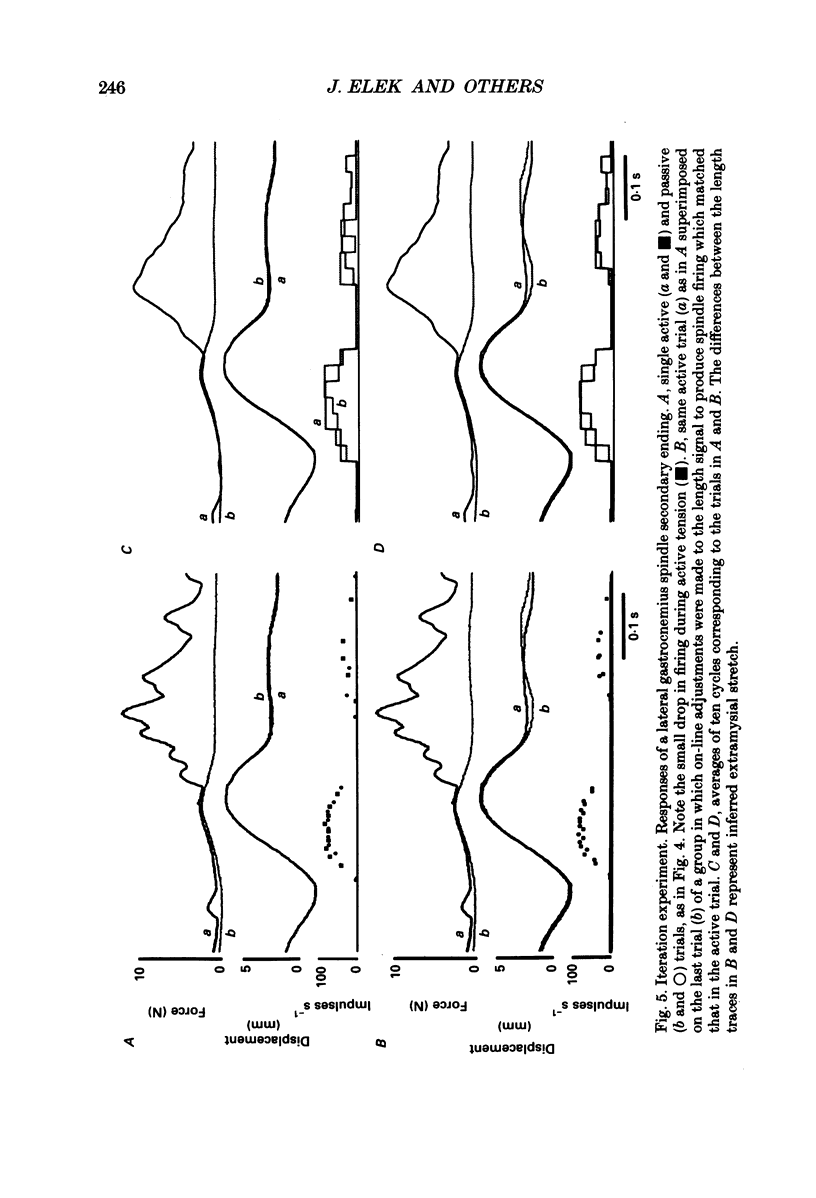
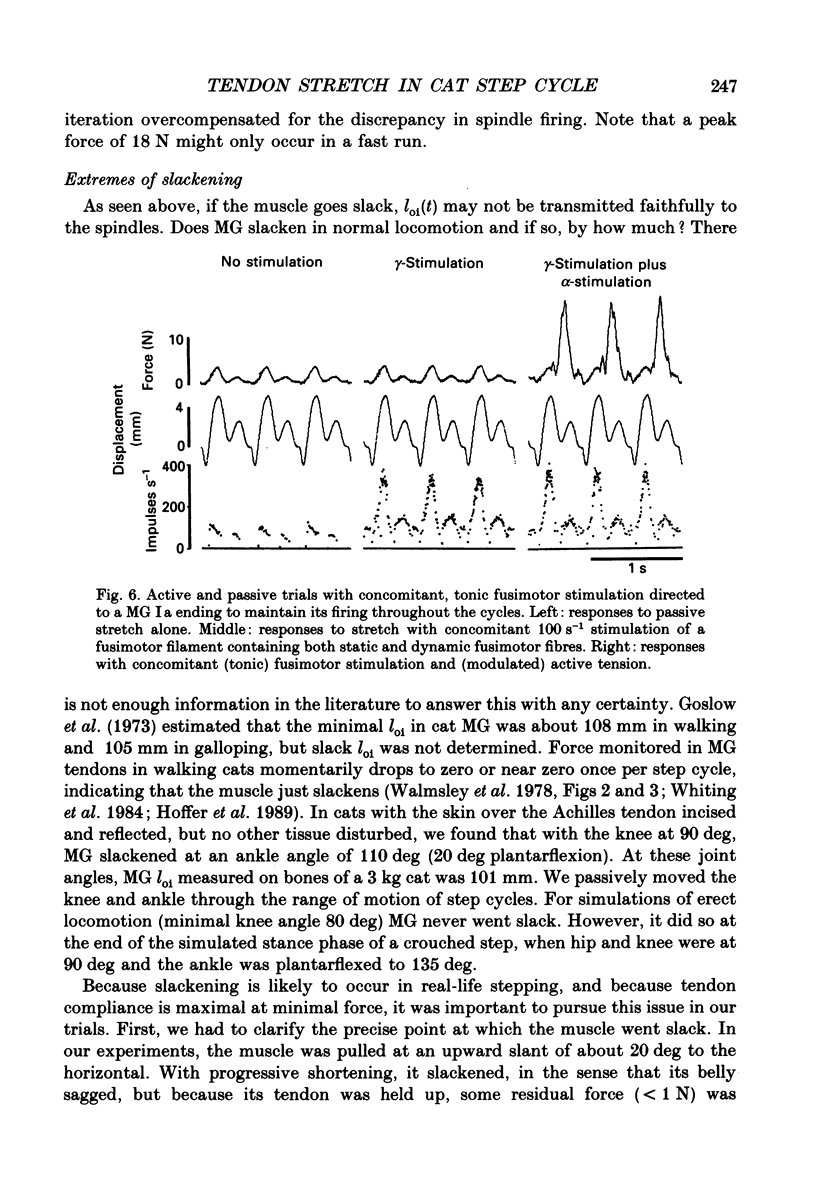
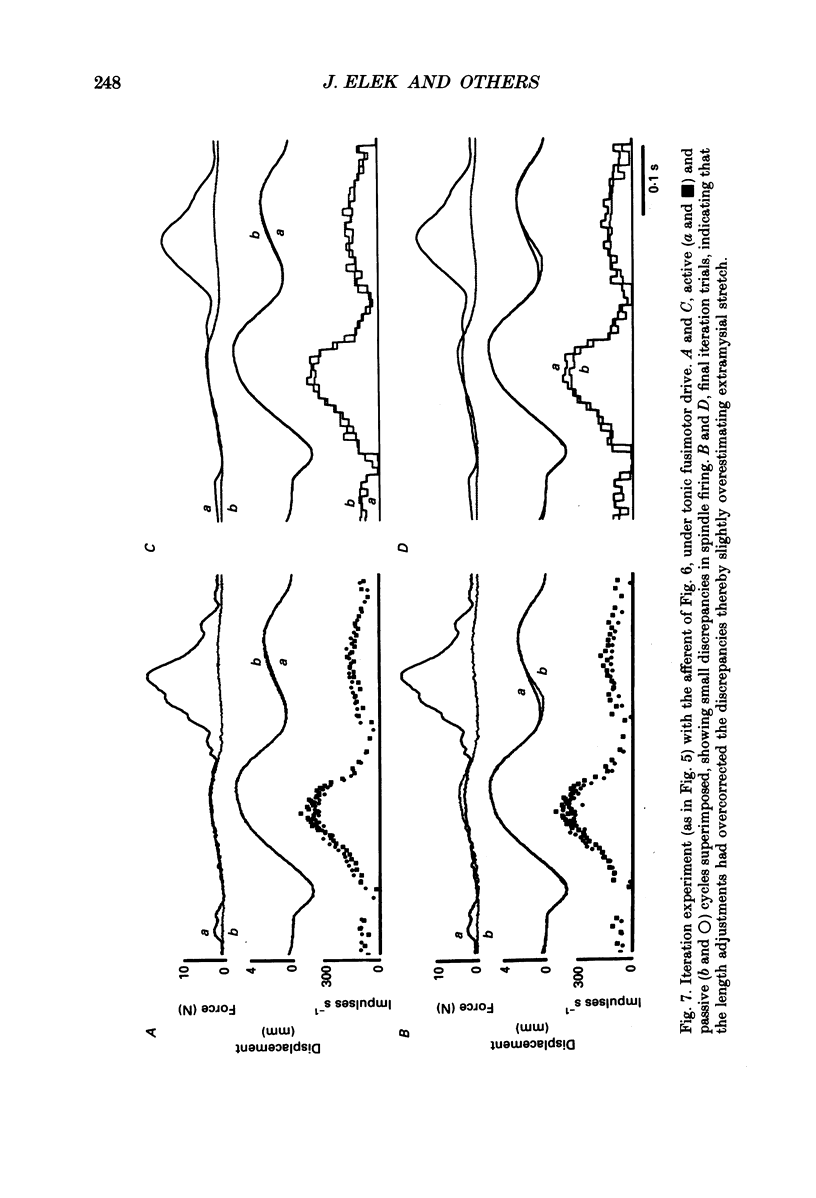
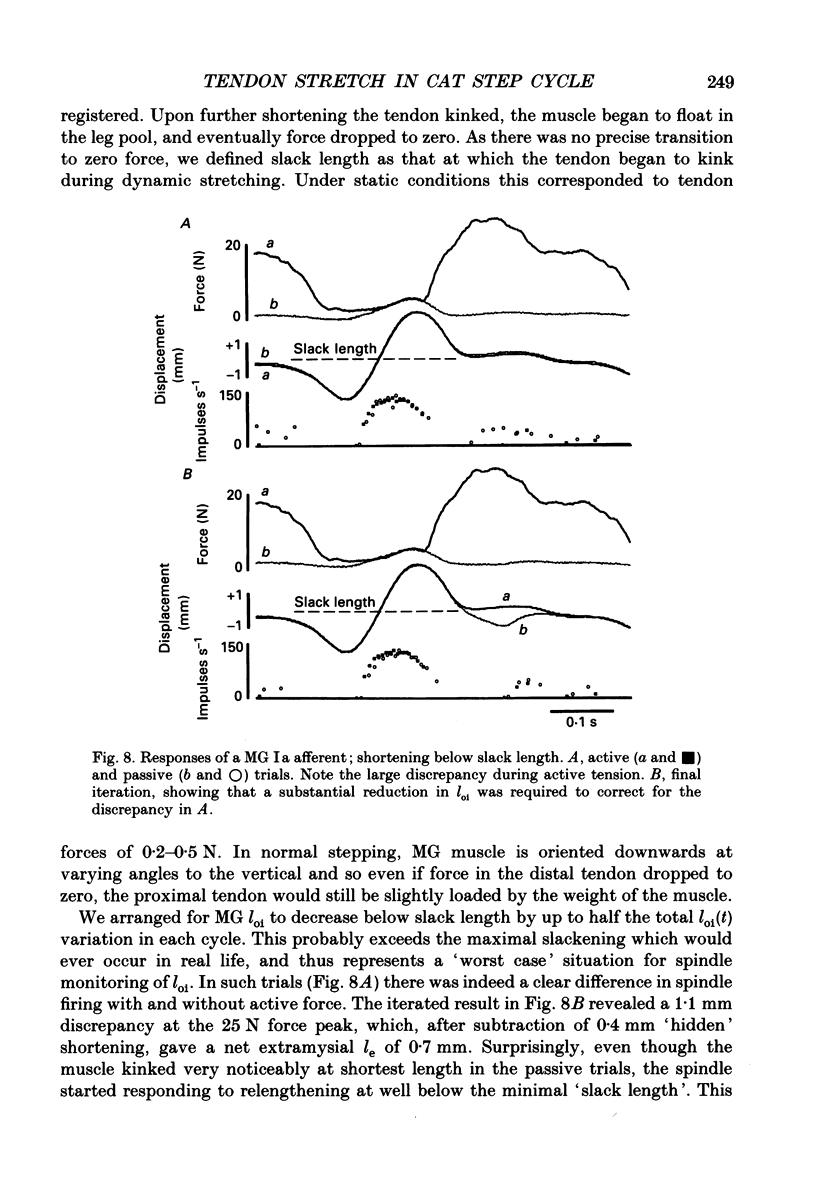
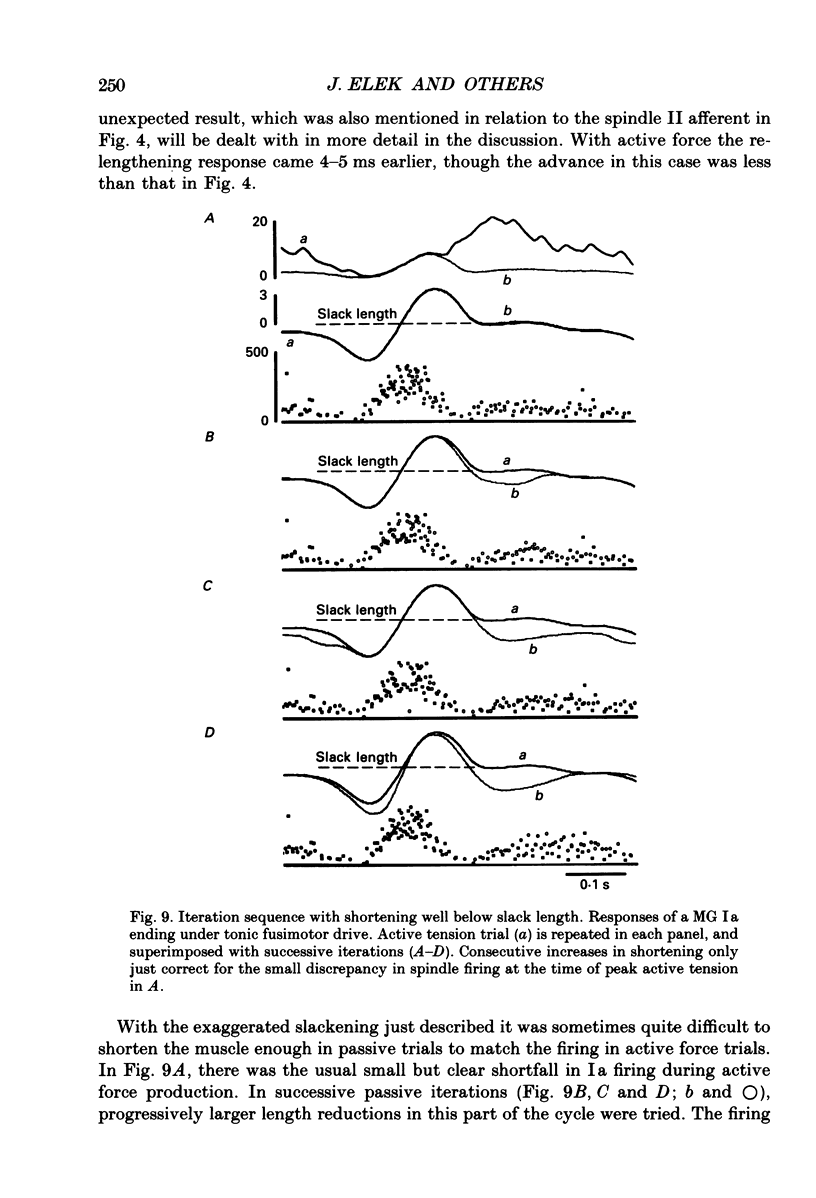
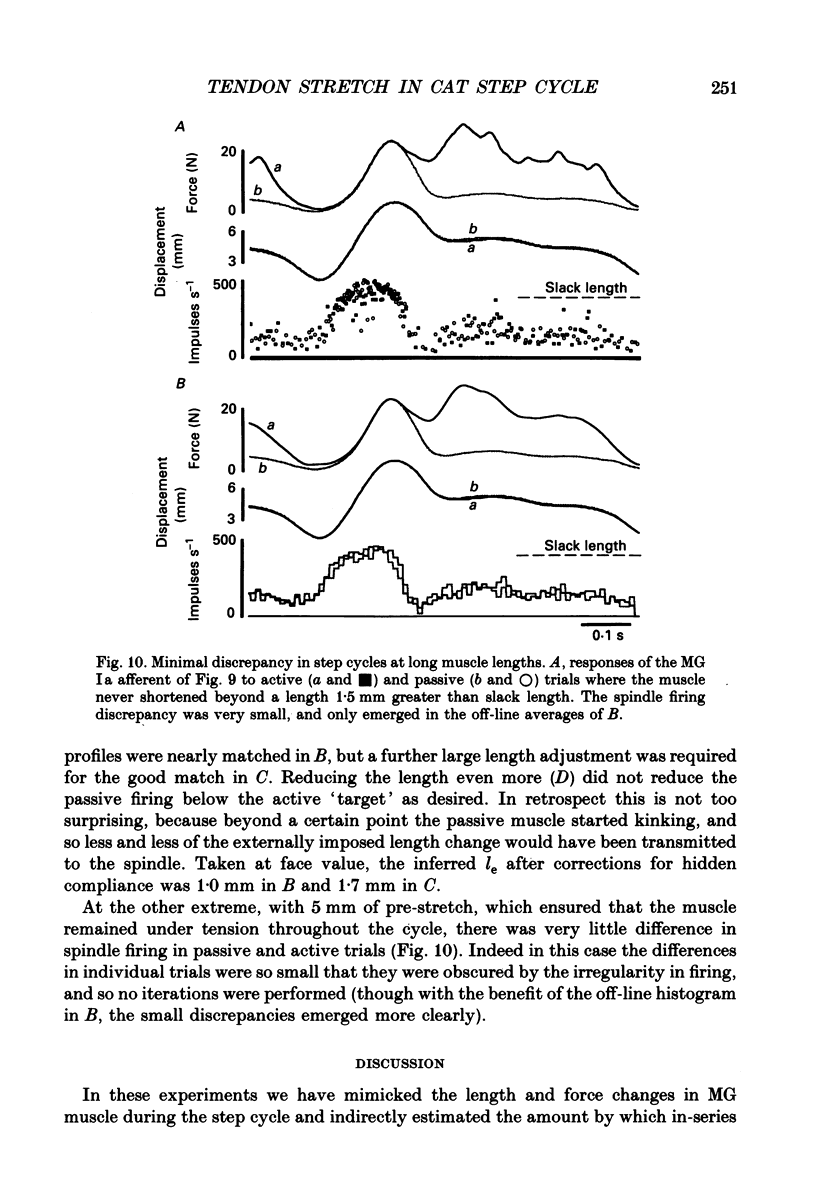
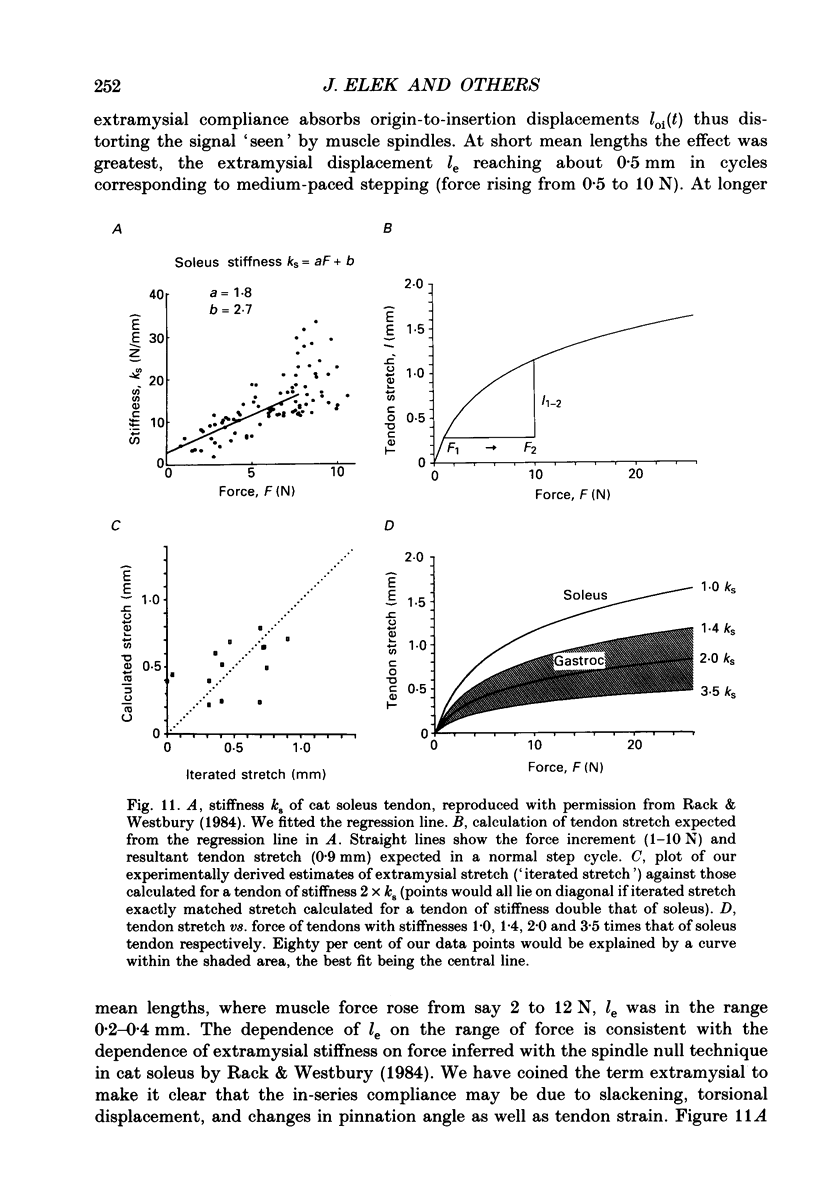
1. It has been claimed that stretch in the non-contractile (extramysial) portion of muscles is substantial, and may produce large discrepancies between the origin-to-insertion muscle length and the internal length variations 'seen' by muscle spindle endings. 2. In eight pentobarbitone-anaesthetized cats, we estimated stretch in the extramysial portion of medial gastrocnemius (MG) muscle with a method similar to the spindle null technique. 3. Length variations of MG previously monitored in a normal step cycle were reproduced with a computer-controlled length servo. The responses of test MG spindle endings were monitored in dorsal root filaments. Distributed stimulation of ventral root filaments, rate-modulated by the step-cycle EMG envelope, served to reproduce step-cycle forces. The filaments were selected so as to have no fusimotor action on the test spindle. 4. Spindle responses in active cycles were compared with those in passive cycles (stretch, but no distributed stimulation). In some cases concomitant tonic fusimotor stimulation was used to maintain spindle responsiveness throughout the cycle, both in active and passive trials. Generally, small discrepancies in spindle firing were seen. The passive trials were now repeated, with iterative adjustments of the length function, until the response matched the spindle firing profile in the active trial. The spindle 'saw' the same internal length change in the final passive trial as in the active trial. Any difference between the corresponding length profiles was attributed to extramysial displacement. 5. Extramysial displacement estimated in this was was maximal at short mean muscle lengths, reaching about 0.5 mm in a typical step cycle (force rising from 0 to 10 N). At longer mean muscle lengths where muscle force rose from say 2 to 12 N in the cycle, extramysial displacement was in the range 0.2-0.4 mm. 6. Except at very short lengths, the displacement was probably mainly tendinous. On this assumption, our results suggested that the stiffness of the MG tendinous compartment was force related, and about double that of cat soleus muscle at any given force. Calculations indicated that though the stretch was small, the MG tendon would store and release enough strain energy per cycle to contribute significantly to the E3 phase of the step cycle. The discrepancies in spindle firing were generally quite subtle, so we reject the claim that extramysial stretch poses a serious difficulty for inferences about fusimotion from chronic spindle afferent recordings.
Full text
PDF



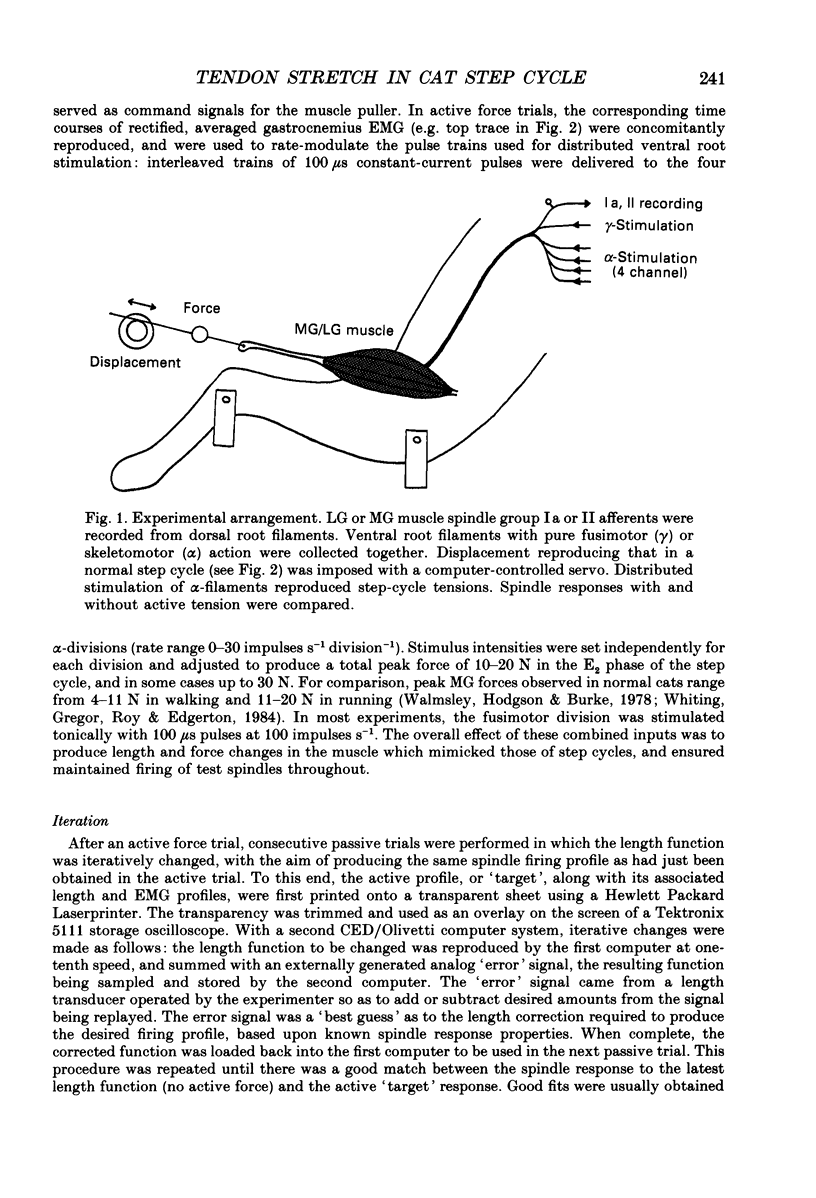
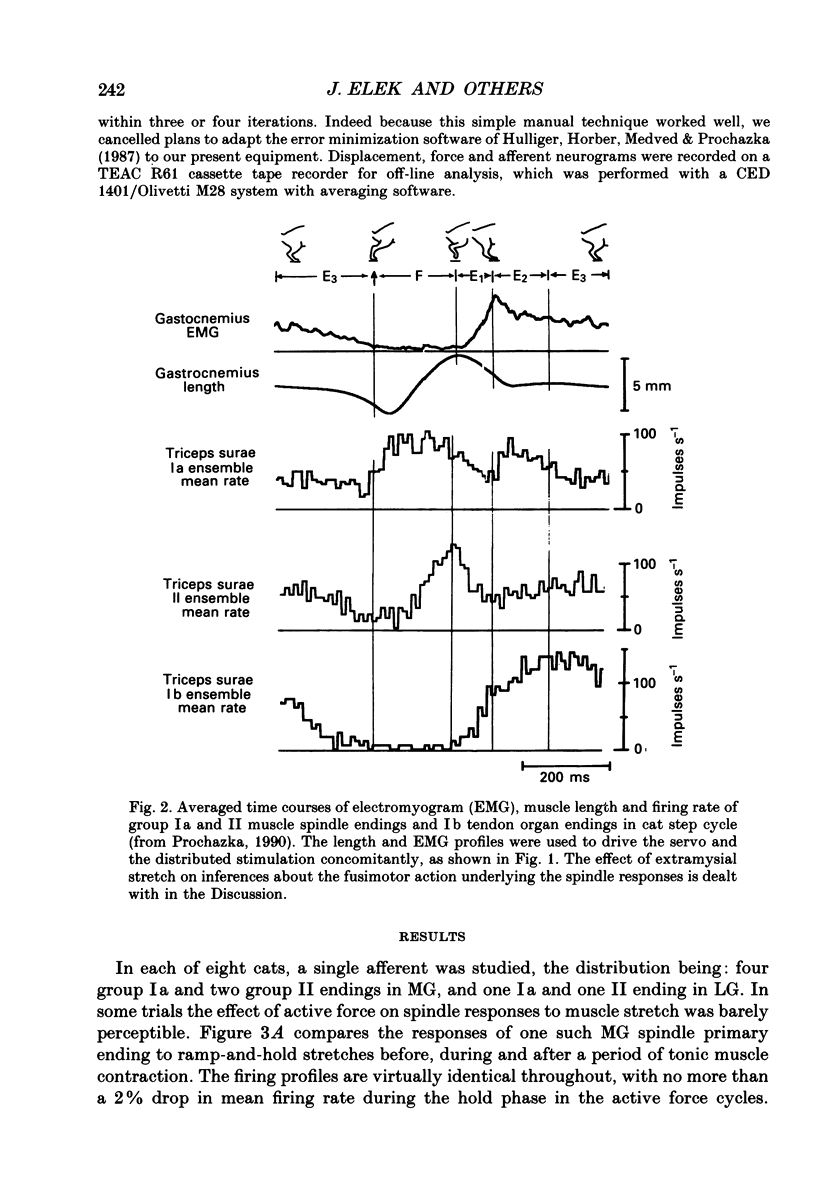
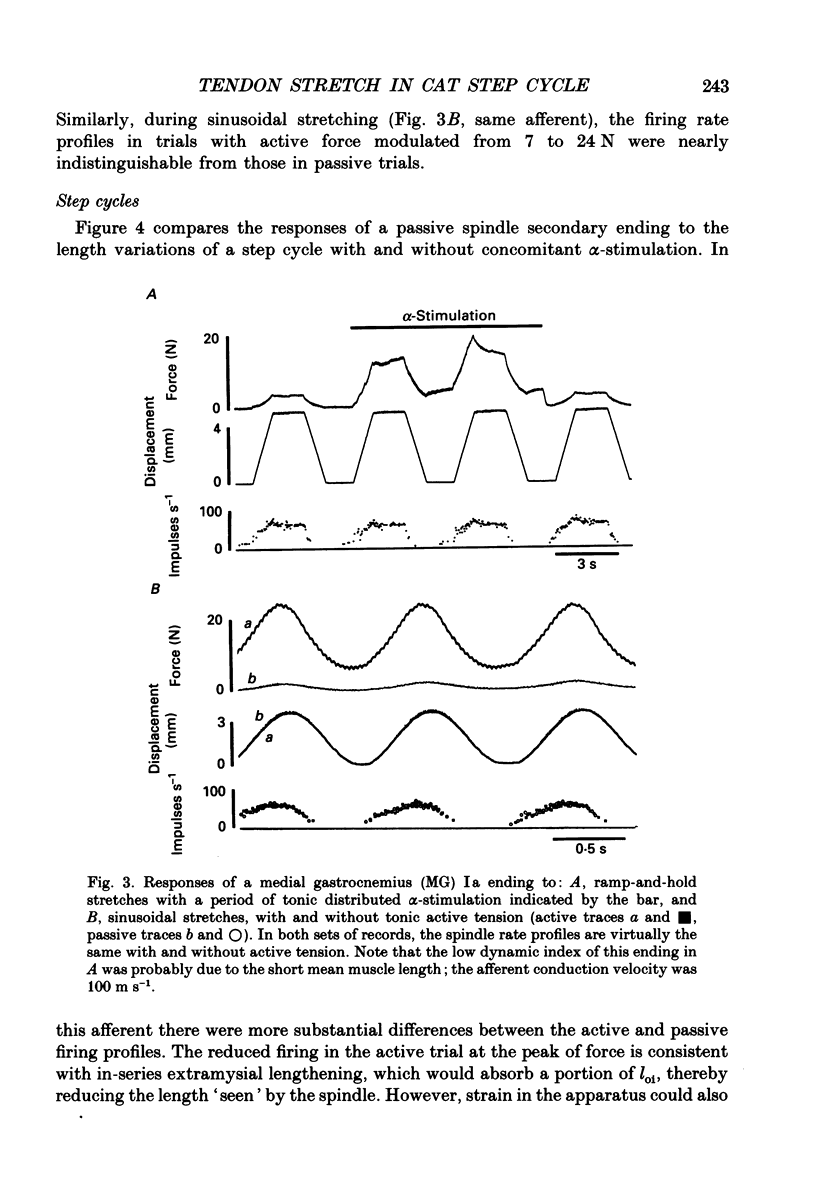
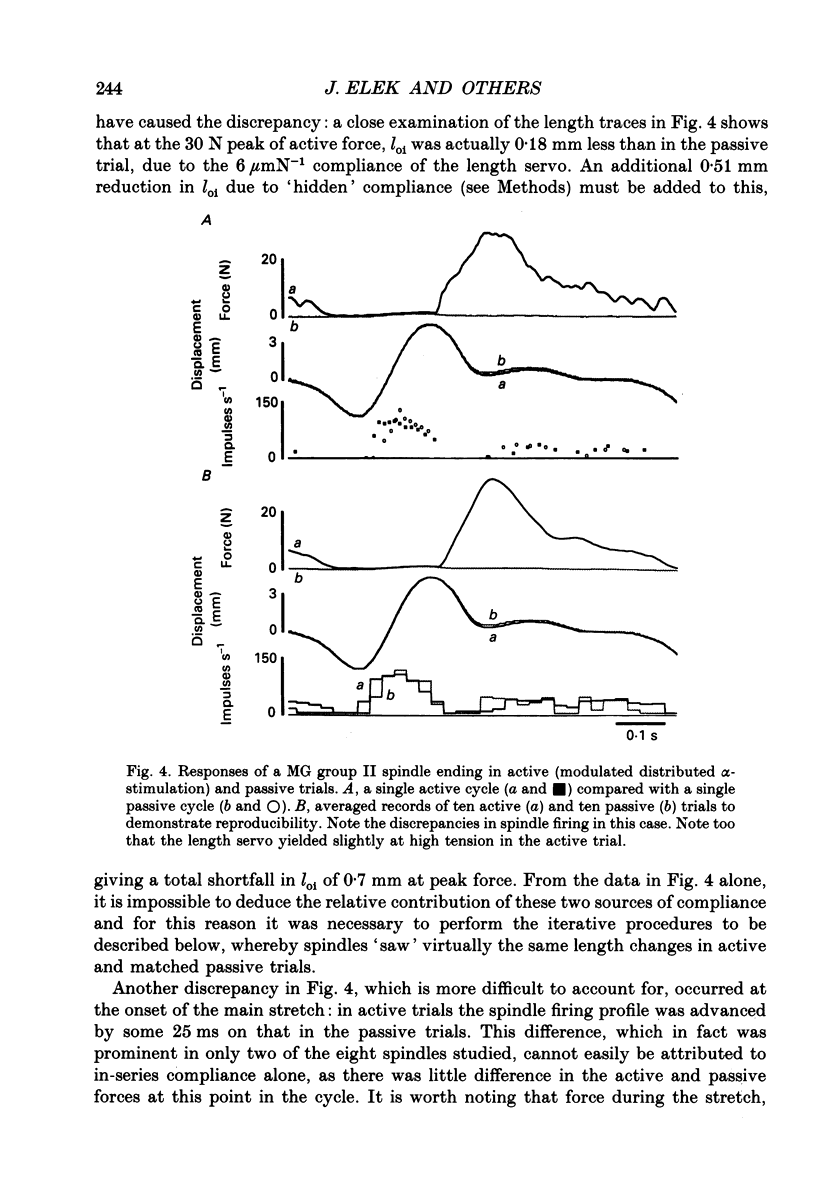







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander R. M., Bennet-Clark H. C. Storage of elastic strain energy in muscle and other tissues. Nature. 1977 Jan 13;265(5590):114–117. doi: 10.1038/265114a0. [DOI] [PubMed] [Google Scholar]
- Amis A., Prochazka A., Short D., Trend P. S., Ward A. Relative displacements in muscle and tendon during human arm movements. J Physiol. 1987 Aug;389:37–44. doi: 10.1113/jphysiol.1987.sp016645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler D. L., Grood E. S., Noyes F. R., Zernicke R. F. Biomechanics of ligaments and tendons. Exerc Sport Sci Rev. 1978;6:125–181. [PubMed] [Google Scholar]
- Cavagna G. A., Kaneko M. Mechanical work and efficiency in level walking and running. J Physiol. 1977 Jun;268(2):467–-81. doi: 10.1113/jphysiol.1977.sp011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows S. J., Rack P. M. Changes in the length of the human biceps brachii muscle during elbow movements. J Physiol. 1987 Feb;383:405–412. doi: 10.1113/jphysiol.1987.sp016416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslow G. E., Jr, Reinking R. M., Stuart D. G. The cat step cycle: hind limb joint angles and muscle lengths during unrestrained locomotion. J Morphol. 1973 Sep;141(1):1–41. doi: 10.1002/jmor.1051410102. [DOI] [PubMed] [Google Scholar]
- Gregor R. J., Roy R. R., Whiting W. C., Lovely R. G., Hodgson J. A., Edgerton V. R. Mechanical output of the cat soleus during treadmill locomotion: in vivo vs in situ characteristics. J Biomech. 1988;21(9):721–732. doi: 10.1016/0021-9290(88)90281-3. [DOI] [PubMed] [Google Scholar]
- Hoffer J. A., Caputi A. A., Pose I. E., Griffiths R. I. Roles of muscle activity and load on the relationship between muscle spindle length and whole muscle length in the freely walking cat. Prog Brain Res. 1989;80:75–60. doi: 10.1016/s0079-6123(08)62201-3. [DOI] [PubMed] [Google Scholar]
- Hulliger M., Horber F., Medved A., Prochazka A. An experimental simulation method for iterative and interactive reconstruction of unknown (fusimotor) inputs contributing to known (spindle afferent) responses. J Neurosci Methods. 1987 Oct;21(2-4):225–238. doi: 10.1016/0165-0270(87)90118-x. [DOI] [PubMed] [Google Scholar]
- Loeb G. E., Hoffer J. A., Pratt C. A. Activity of spindle afferents from cat anterior thigh muscles. I. Identification and patterns during normal locomotion. J Neurophysiol. 1985 Sep;54(3):549–564. doi: 10.1152/jn.1985.54.3.549. [DOI] [PubMed] [Google Scholar]
- Morgan D. L., Proske U., Warren D. Measurements of muscle stiffness and the mechanism of elastic storage of energy in hopping kangaroos. J Physiol. 1978 Sep;282:253–261. doi: 10.1113/jphysiol.1978.sp012461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. L. Separation of active and passive components of short-range stiffness of muscle. Am J Physiol. 1977 Jan;232(1):C45–C49. doi: 10.1152/ajpcell.1977.232.1.C45. [DOI] [PubMed] [Google Scholar]
- Prochazka A., Hulliger M., Zangger P., Appenteng K. 'Fusimotor set': new evidence for alpha-independent control of gamma-motoneurones during movement in the awake cat. Brain Res. 1985 Jul 22;339(1):136–140. doi: 10.1016/0006-8993(85)90632-8. [DOI] [PubMed] [Google Scholar]
- Prochazka A., Trend P., Hulliger M., Vincent S. Ensemble proprioceptive activity in the cat step cycle: towards a representative look-up chart. Prog Brain Res. 1989;80:61–60. doi: 10.1016/s0079-6123(08)62200-1. [DOI] [PubMed] [Google Scholar]
- Proske U., Morgan D. L. Tendon stiffness: methods of measurement and significance for the control of movement. A review. J Biomech. 1987;20(1):75–82. doi: 10.1016/0021-9290(87)90269-7. [DOI] [PubMed] [Google Scholar]
- Rack P. M., Westbury D. R. Elastic properties of the cat soleus tendon and their functional importance. J Physiol. 1984 Feb;347:479–495. doi: 10.1113/jphysiol.1984.sp015077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rack P. M., Westbury D. R. The effects of length and stimulus rate on tension in the isometric cat soleus muscle. J Physiol. 1969 Oct;204(2):443–460. doi: 10.1113/jphysiol.1969.sp008923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rack P. M., Westbury D. R. The short range stiffness of active mammalian muscle and its effect on mechanical properties. J Physiol. 1974 Jul;240(2):331–350. doi: 10.1113/jphysiol.1974.sp010613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell J. C., Traub M. M., Day B. L., Obeso J. A., Thomas P. K., Marsden C. D. Manual motor performance in a deafferented man. Brain. 1982 Sep;105(Pt 3):515–542. doi: 10.1093/brain/105.3.515. [DOI] [PubMed] [Google Scholar]
- Sanes J. N., Mauritz K. H., Dalakas M. C., Evarts E. V. Motor control in humans with large-fiber sensory neuropathy. Hum Neurobiol. 1985;4(2):101–114. [PubMed] [Google Scholar]
- Walmsley B., Hodgson J. A., Burke R. E. Forces produced by medial gastrocnemius and soleus muscles during locomotion in freely moving cats. J Neurophysiol. 1978 Sep;41(5):1203–1216. doi: 10.1152/jn.1978.41.5.1203. [DOI] [PubMed] [Google Scholar]
- Walmsley B., Proske U. Comparison of stiffness of soleus and medial gastrocnemius muscles in cats. J Neurophysiol. 1981 Aug;46(2):250–259. doi: 10.1152/jn.1981.46.2.250. [DOI] [PubMed] [Google Scholar]
- Whiting W. C., Gregor R. J., Roy R. R., Edgerton V. R. A technique for estimating mechanical work of individual muscles in the cat during treadmill locomotion. J Biomech. 1984;17(9):685–694. doi: 10.1016/0021-9290(84)90122-2. [DOI] [PubMed] [Google Scholar]
- Zajac F. E. Muscle and tendon: properties, models, scaling, and application to biomechanics and motor control. Crit Rev Biomed Eng. 1989;17(4):359–411. [PubMed] [Google Scholar]


