Abstract
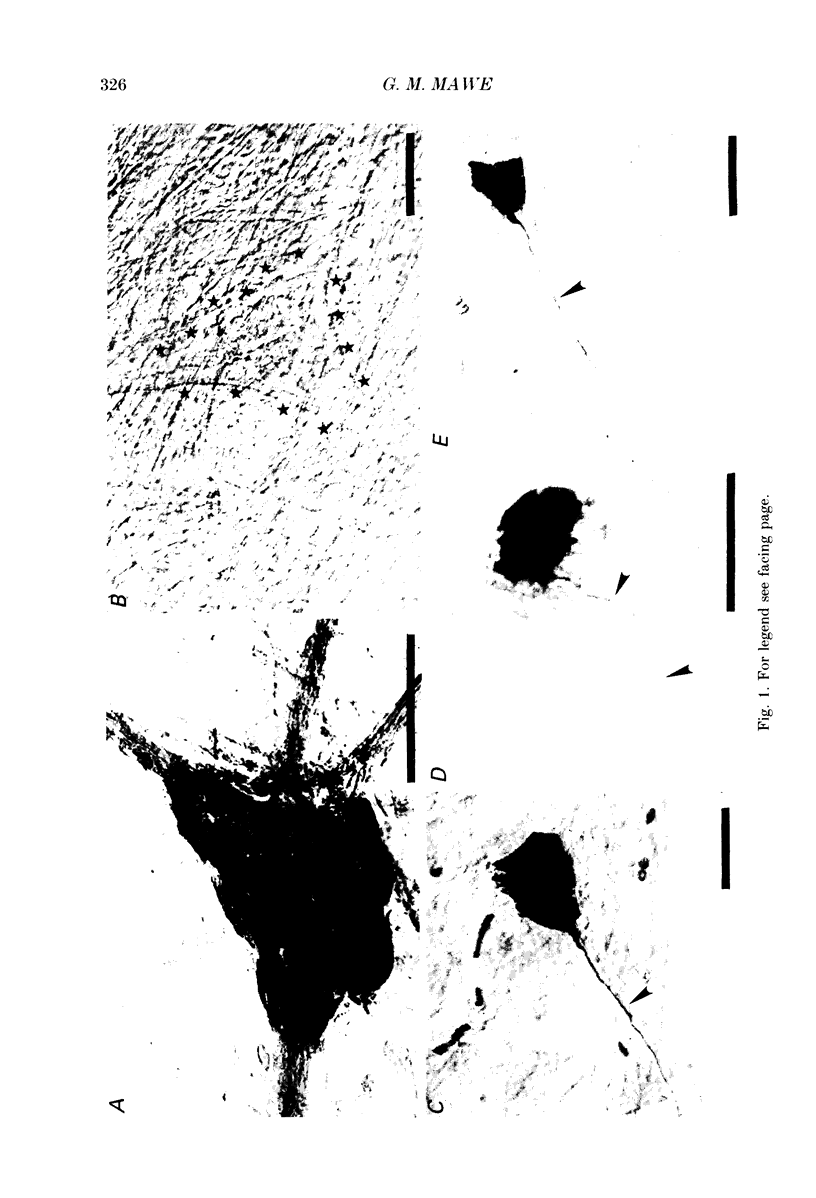
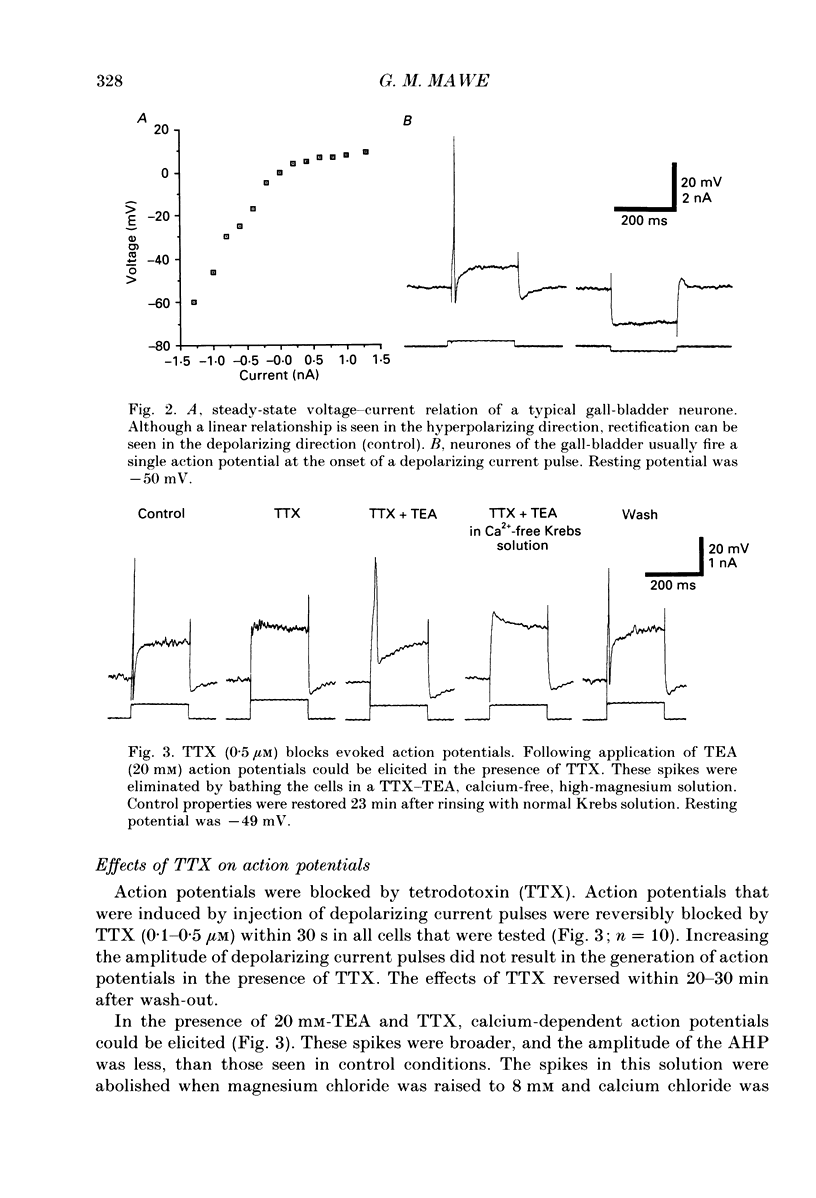
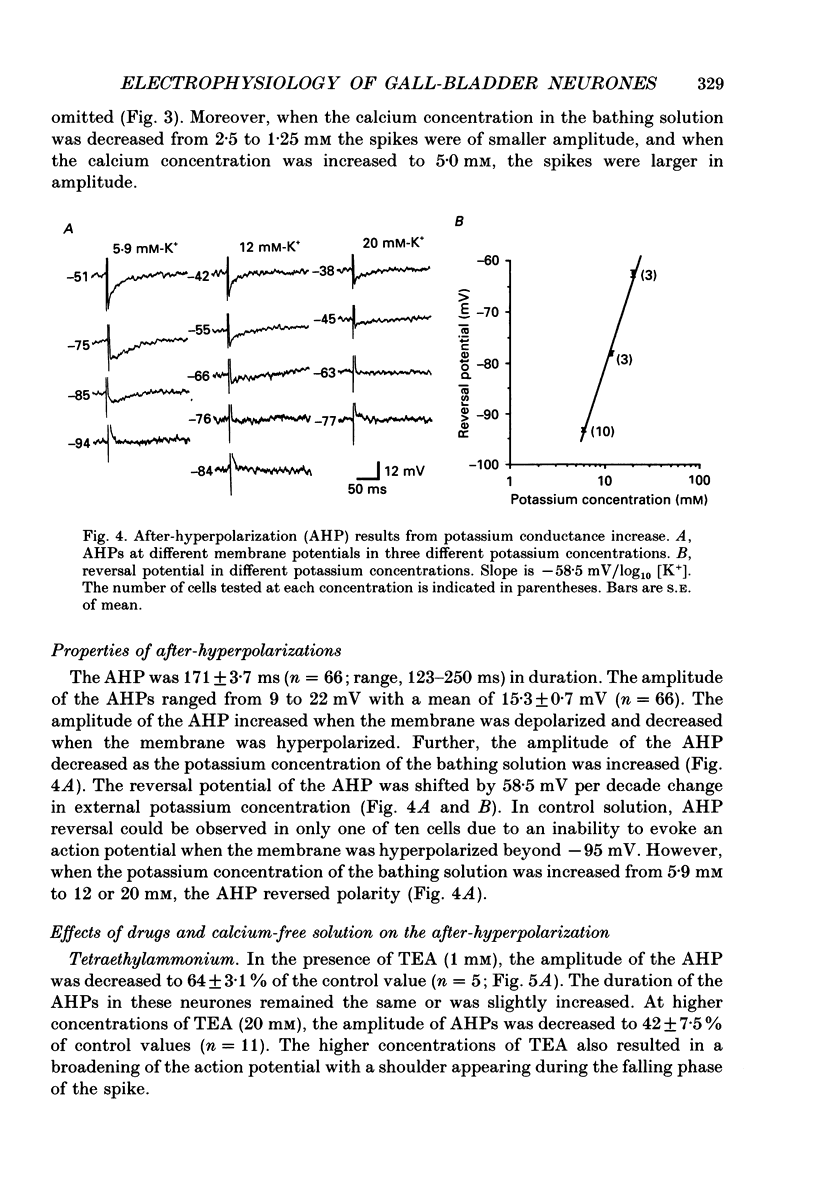
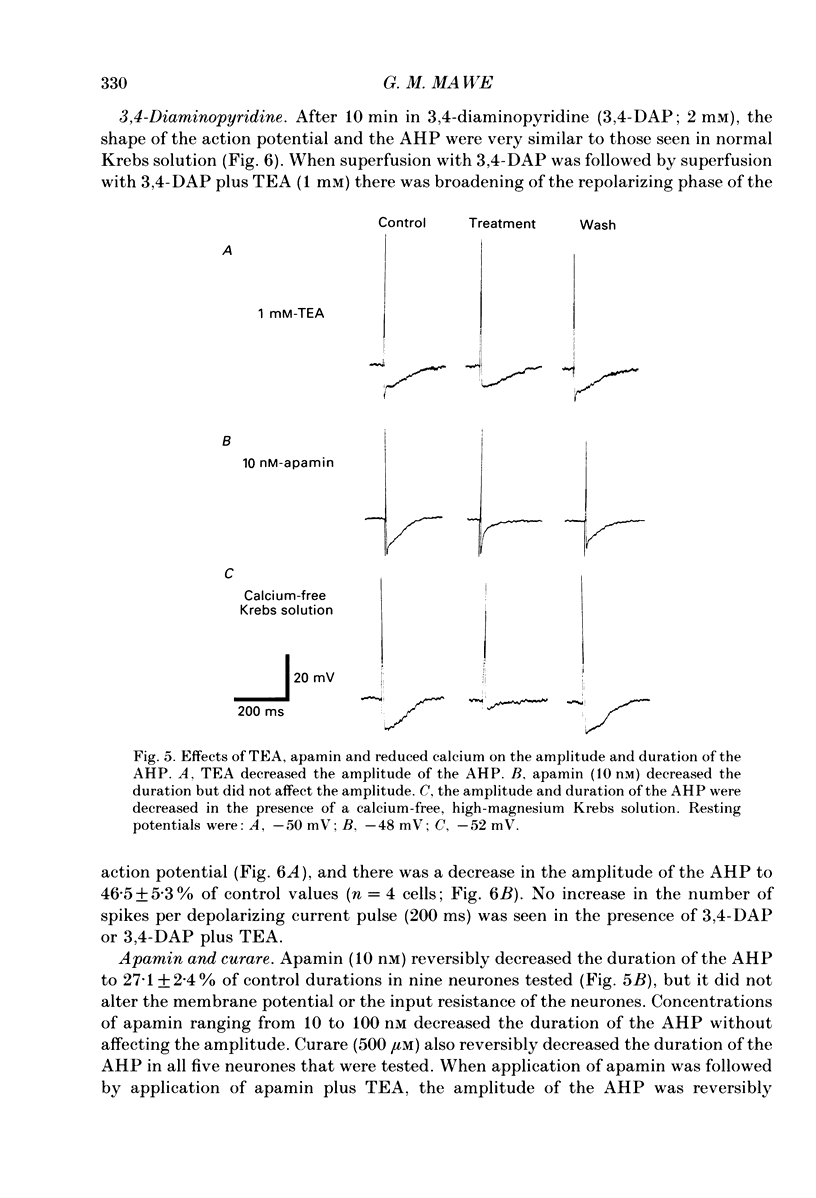
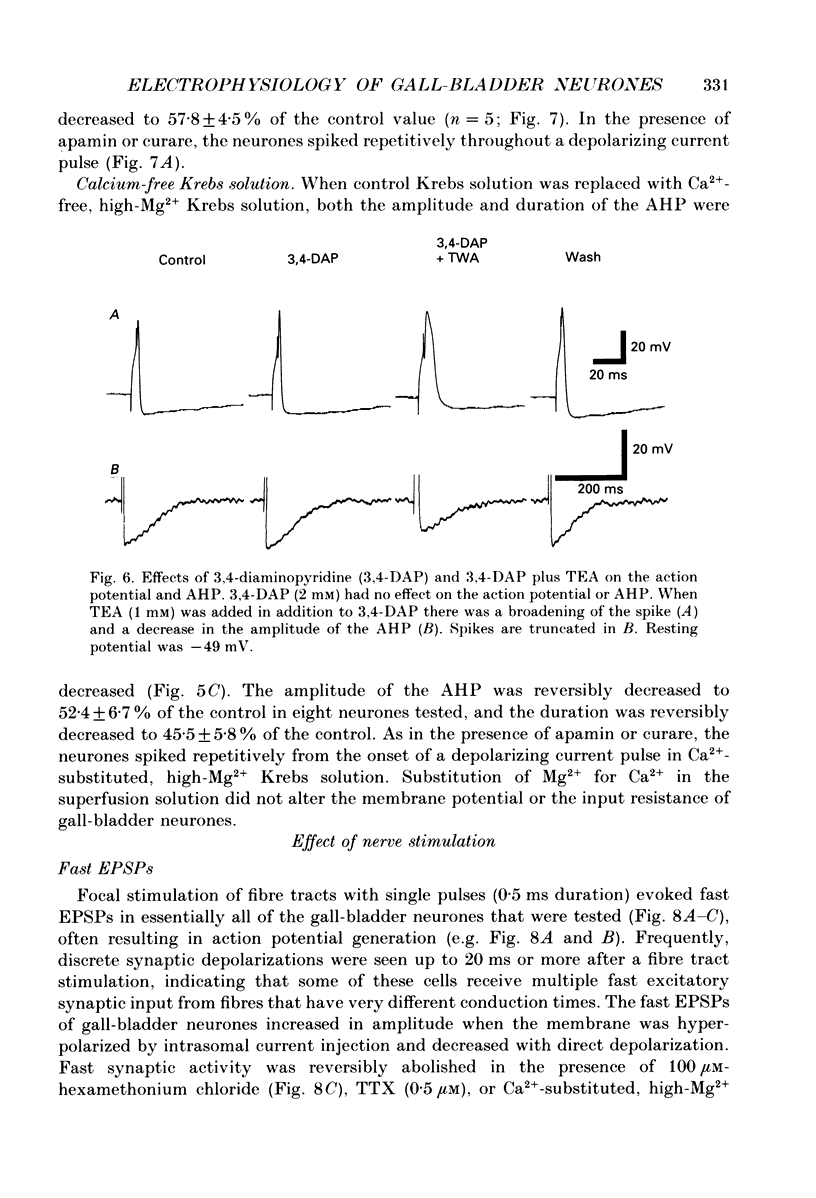
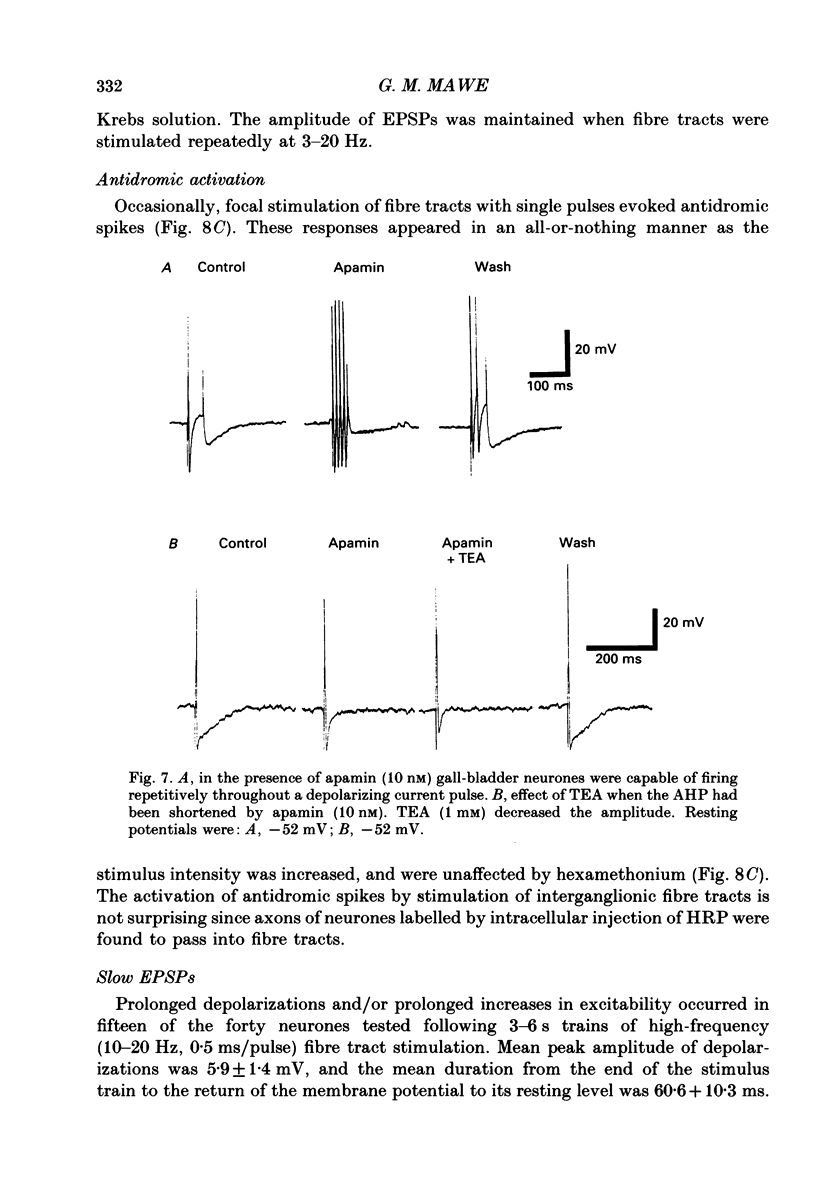
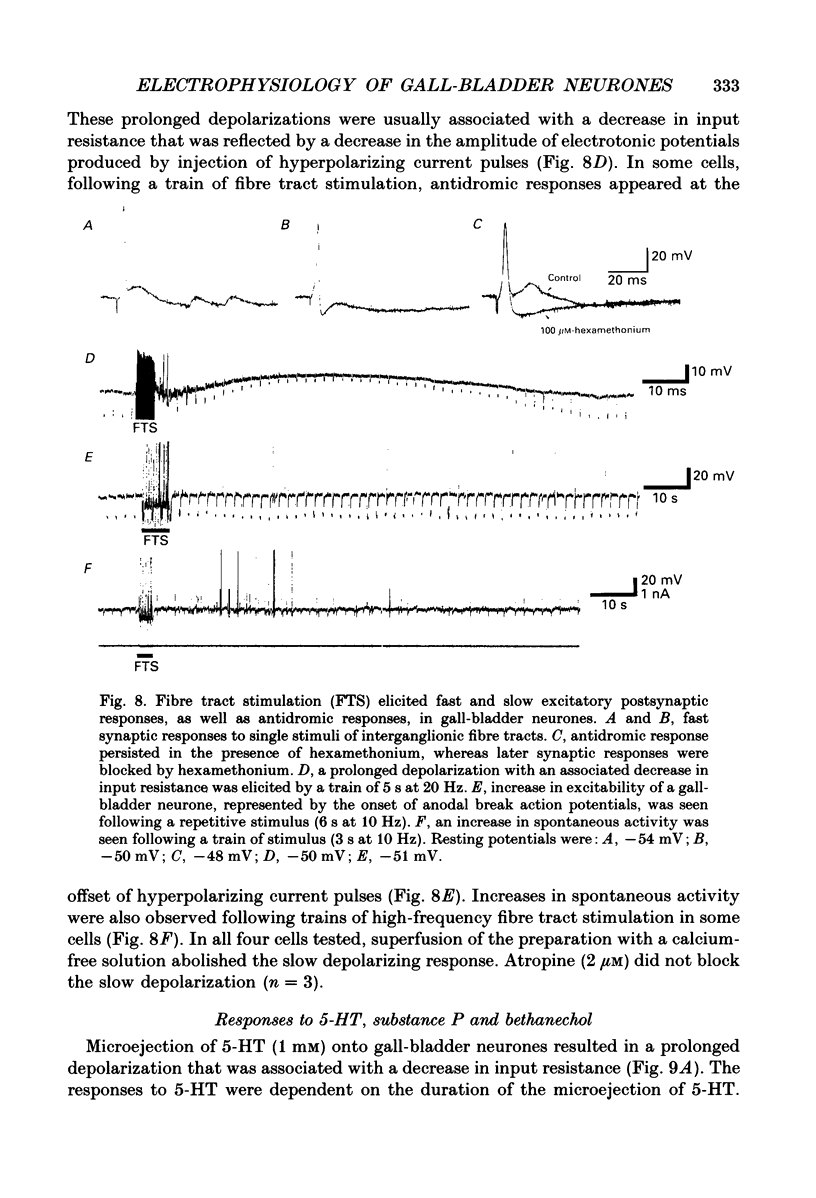
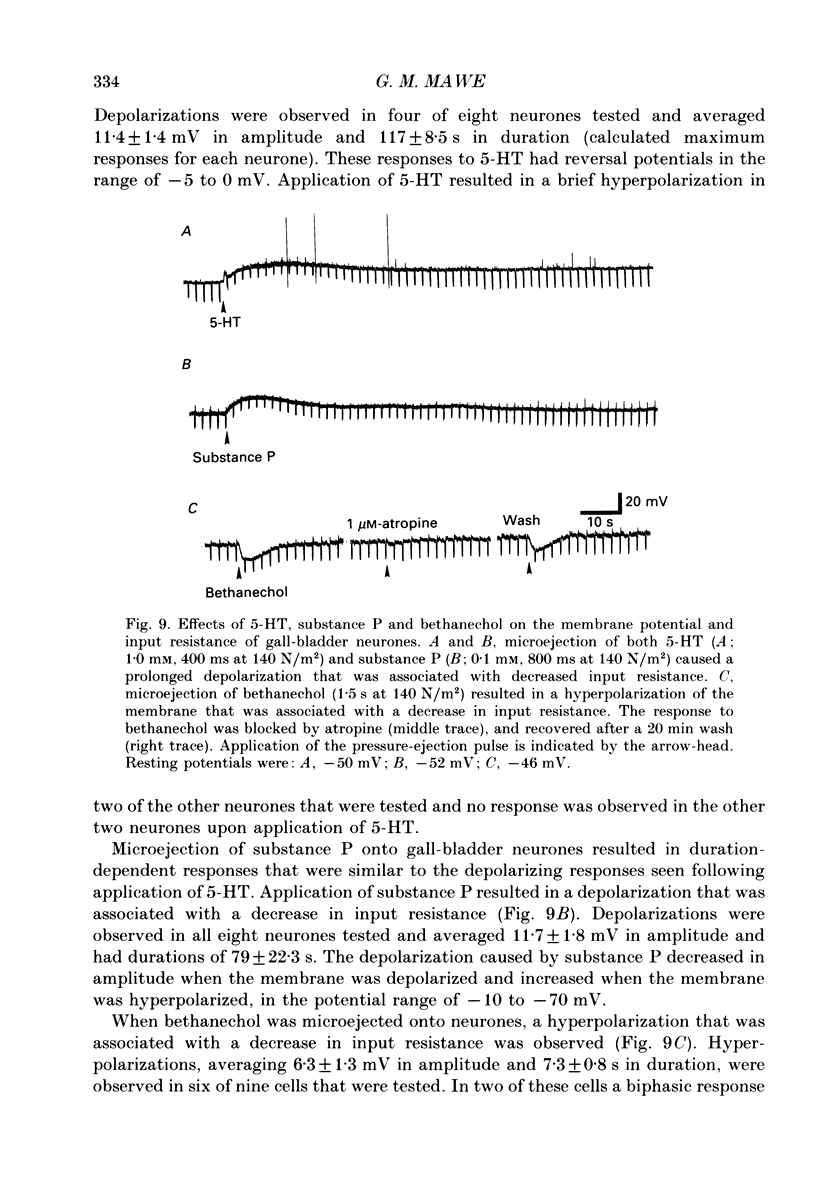
1. Intracellular recordings were made from neurones of the guinea-pig gall-bladder in vitro. Intracellular injection of horseradish peroxidase revealed a simple structure, consisting of a soma and a single process, but no discernible dendritic arborization. 2. The resting membrane potential was -50.5 +/- 0.4 mV and the input resistance was 80 M omega. 3. Gall-bladder neurones spiked only once at the onset of depolarizing current pulses. Action potentials were blocked by tetrodotoxin, but a Ca2(+)-dependent spike could be elicited in the presence of tetrodotoxin and tetraethylammonium. 4. Action potential after-hyperpolarizations had a duration of 172 +/- 3.7 ms and reversed at a membrane potential of -93 mV; this reversal potential was linearly related to the logarithm of the external potassium concentration. The initial phase of the after-hyperpolarization was inhibited by tetraethylammonium (1-10 mM) and was not affected by 3,4-diaminopyridine. The late phase of the after-hyperpolarization was blocked by apamin (10 nM) or curare (500 microM). Both the early and late phases of the after-hyperpolarization were inhibited when the preparation was perfused with a calcium-free, high-magnesium solution. The calcium-free, high-magnesium solution had no effect on the membrane potential or input resistance of these cells. 5. Fast excitatory synaptic responses and antidromic responses were elicited in gall-bladder neurones by focal stimulation of fibre tracts. High-frequency fibre tract stimulation often resulted in prolonged, calcium-dependent, depolarizations that were associated with a decrease in input resistance. 6. 5-Hydroxytryptamine and substance P caused depolarizations that were associated with a decrease in input resistance. Bethanechol caused hyperpolarizations that were associated with a decrease in input resistance and which were blocked by atropine.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cai W. Q., Gabella G. Innervation of the gall bladder and biliary pathways in the guinea-pig. J Anat. 1983 Jan;136(Pt 1):97–109. [PMC free article] [PubMed] [Google Scholar]
- Castle N. A., Haylett D. G., Jenkinson D. H. Toxins in the characterization of potassium channels. Trends Neurosci. 1989 Feb;12(2):59–65. doi: 10.1016/0166-2236(89)90137-9. [DOI] [PubMed] [Google Scholar]
- Erde S. M., Sherman D., Gershon M. D. Morphology and serotonergic innervation of physiologically identified cells of the guinea pig's myenteric plexus. J Neurosci. 1985 Mar;5(3):617–633. doi: 10.1523/JNEUROSCI.05-03-00617.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forehand C. J., Konopka L. M. Frog sympathetic ganglion cells have local axon collaterals. J Comp Neurol. 1989 Nov 8;289(2):294–303. doi: 10.1002/cne.902890209. [DOI] [PubMed] [Google Scholar]
- Goehler L. E., Sternini C., Brecha N. C. Calcitonin gene-related peptide immunoreactivity in the biliary pathway and liver of the guinea-pig: distribution and colocalization with substance P. Cell Tissue Res. 1988 Jul;253(1):145–150. doi: 10.1007/BF00221749. [DOI] [PubMed] [Google Scholar]
- Goh J. W., Kelly M. E., Pennefather P. S. Electrophysiological function of the delayed rectifier (IK) in bullfrog sympathetic ganglion neurones. Pflugers Arch. 1989 Mar;413(5):482–486. doi: 10.1007/BF00594177. [DOI] [PubMed] [Google Scholar]
- Grafe P., Mayer C. J., Wood J. D. Synaptic modulation of calcium-dependent potassium conductance in myenteric neurones in the guinea-pig. J Physiol. 1980 Aug;305:235–248. doi: 10.1113/jphysiol.1980.sp013360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawe G. M., Gershon M. D. Structure, afferent innervation, and transmitter content of ganglia of the guinea pig gallbladder: relationship to the enteric nervous system. J Comp Neurol. 1989 May 15;283(3):374–390. doi: 10.1002/cne.902830306. [DOI] [PubMed] [Google Scholar]
- Schemann M., Wood J. D. Electrical behaviour of myenteric neurones in the gastric corpus of the guinea-pig. J Physiol. 1989 Oct;417:501–518. doi: 10.1113/jphysiol.1989.sp017815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider W. D. The dendritic complexity and innervation of submandibular neurons in five species of mammals. J Neurosci. 1987 Jun;7(6):1760–1768. doi: 10.1523/JNEUROSCI.07-06-01760.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]