Abstract
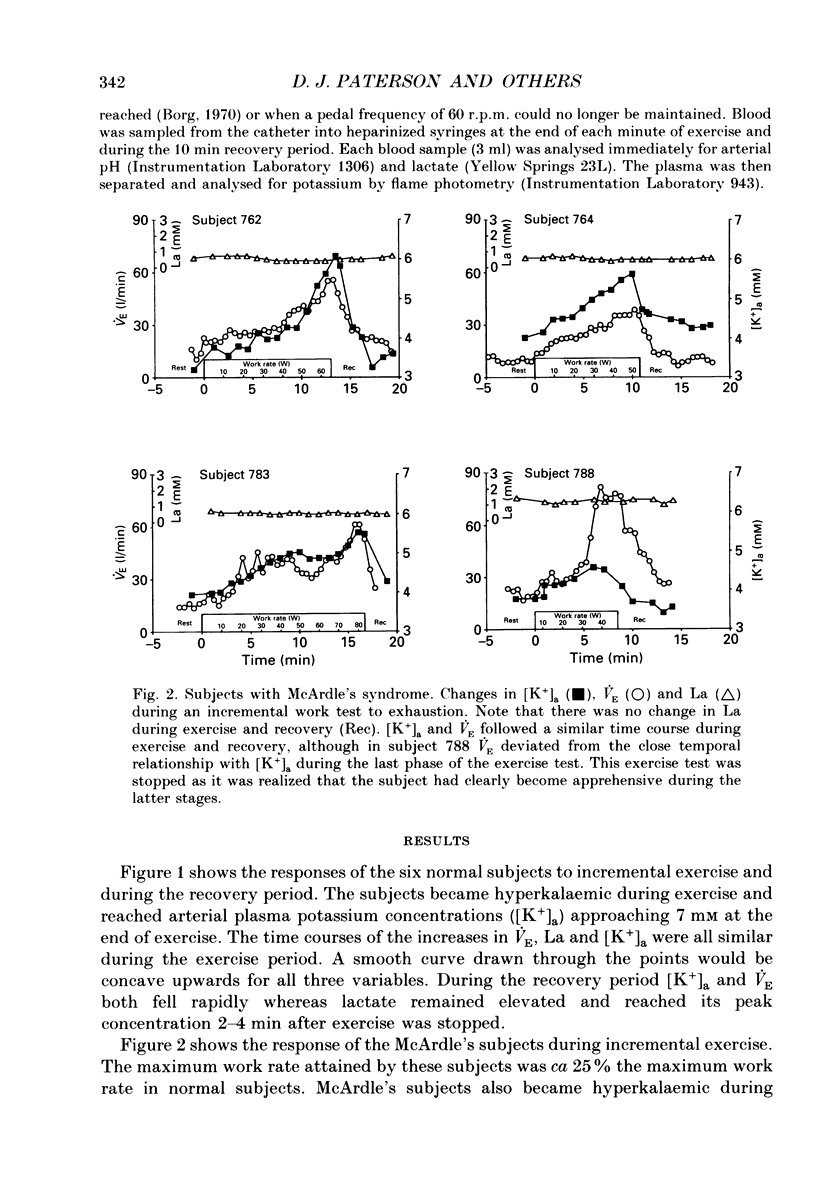
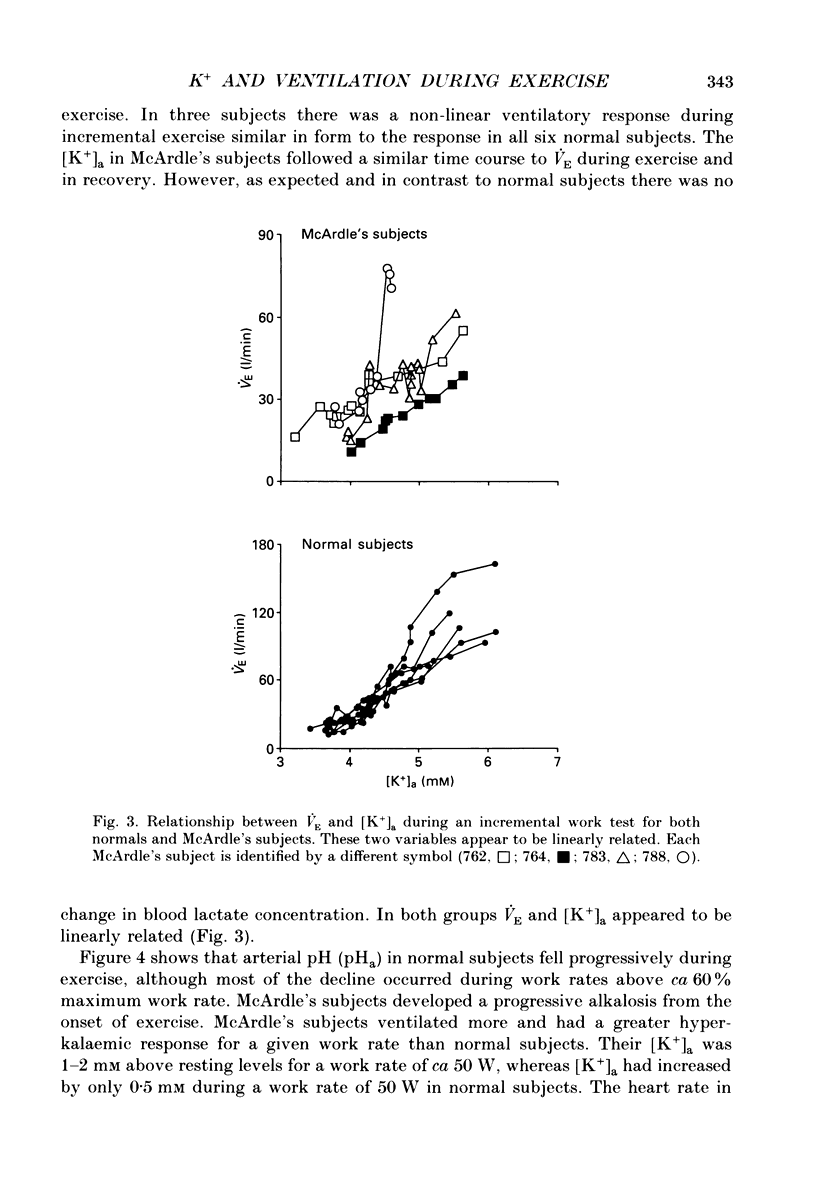
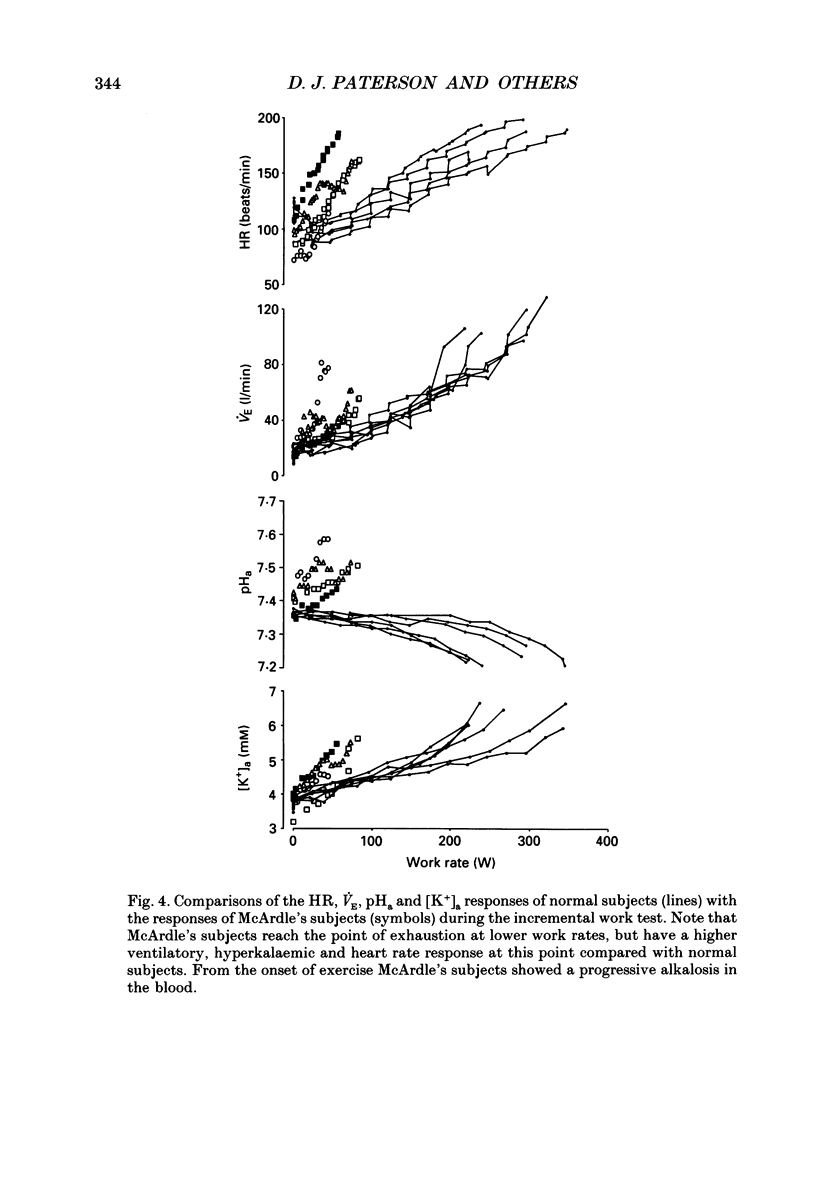
1. We have examined the relationship between ventilation (VE), lactate (La) and arterial plasma K+ concentrations [( K+]a) during incremental exercise in six normal subjects and in four subjects with McArdle's syndrome (myophosphorylase deficiency) who do not become acidotic during exercise. 2. In normal subjects, [K+]a rose to ca 7 mM at the point of exhaustion. The time courses of the increases in VE, La and [K+]a were all similar during the exercise period. La reached its peak concentration during the recovery from exercise when both VE and [K+]a were returning to resting levels. 3. McArdle's subjects, like normal subjects, had a non-linear ventilatory response during incremental exercise. Their [K+]a was closely related to VE throughout exercise and recovery. 4. The arterial pH of McArdle's subjects, rather than remaining constant, actually rose from the onset of exercise. 5. For a given level of exercise, the levels of VE and [K+]a were greater in the McArdle's subjects than in normal subjects. 6. These findings are consistent with the idea that hyperkalaemia may contribute significantly to the drive to breathe, especially during heavy exercise.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANNISTER R. G., CUNNINGHAM D. J. The effects on the respiration and performance during exercise of adding oxygen to the inspired air. J Physiol. 1954 Jul 28;125(1):118–137. doi: 10.1113/jphysiol.1954.sp005145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band D. M., Linton R. A., Kent R., Kurer F. L. The effect of peripheral chemodenervation on the ventilatory response to potassium. Respir Physiol. 1985 May;60(2):217–225. doi: 10.1016/0034-5687(85)90105-7. [DOI] [PubMed] [Google Scholar]
- Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2(2):92–98. [PubMed] [Google Scholar]
- Braakhekke J. P., de Bruin M. I., Stegeman D. F., Wevers R. A., Binkhorst R. A., Joosten E. M. The second wind phenomenon in McArdle's disease. Brain. 1986 Dec;109(Pt 6):1087–1101. doi: 10.1093/brain/109.6.1087. [DOI] [PubMed] [Google Scholar]
- Burger R. E., Estavillo J. A., Kumar P., Nye P. C., Paterson D. J. Effects of potassium, oxygen and carbon dioxide on the steady-state discharge of cat carotid body chemoreceptors. J Physiol. 1988 Jul;401:519–531. doi: 10.1113/jphysiol.1988.sp017176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle N. A., Haylett D. G. Effect of channel blockers on potassium efflux from metabolically exhausted frog skeletal muscle. J Physiol. 1987 Feb;383:31–43. doi: 10.1113/jphysiol.1987.sp016394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T. Regulation of active Na+-K+ transport in skeletal muscle. Physiol Rev. 1986 Jul;66(3):542–580. doi: 10.1152/physrev.1986.66.3.542. [DOI] [PubMed] [Google Scholar]
- Cunningham D. J. Studies on arterial chemoreceptors in man. J Physiol. 1987 Mar;384:1–26. doi: 10.1113/jphysiol.1987.sp016440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEJOURS P. Chemoreflexes in breathing. Physiol Rev. 1962 Jul;42:335–358. doi: 10.1152/physrev.1962.42.3.335. [DOI] [PubMed] [Google Scholar]
- Davies N. W. Modulation of ATP-sensitive K+ channels in skeletal muscle by intracellular protons. Nature. 1990 Jan 25;343(6256):375–377. doi: 10.1038/343375a0. [DOI] [PubMed] [Google Scholar]
- Friedland J., Paterson D. Potassium and fatigue. Lancet. 1988 Oct 22;2(8617):961–962. doi: 10.1016/s0140-6736(88)92627-x. [DOI] [PubMed] [Google Scholar]
- Hagberg J. M., Coyle E. F., Carroll J. E., Miller J. M., Martin W. H., Brooke M. H. Exercise hyperventilation in patients with McArdle's disease. J Appl Physiol Respir Environ Exerc Physiol. 1982 Apr;52(4):991–994. doi: 10.1152/jappl.1982.52.4.991. [DOI] [PubMed] [Google Scholar]
- Haller R. G., Lewis S. F. Abnormal ventilation during exercise in McArdle's syndrome: modulation by substrate availability. Neurology. 1986 May;36(5):716–719. doi: 10.1212/wnl.36.5.716. [DOI] [PubMed] [Google Scholar]
- Kono N., Mineo I., Sumi S., Shimizu T., Kang J., Nonaka K., Tarui S. Metabolic basis of improved exercise tolerance: muscle phosphorylase deficiency after glucagon administration. Neurology. 1984 Nov;34(11):1471–1476. doi: 10.1212/wnl.34.11.1471. [DOI] [PubMed] [Google Scholar]
- Lewis S. F., Haller R. G., Cook J. D., Blomqvist C. G. Metabolic control of cardiac output response to exercise in McArdle's disease. J Appl Physiol Respir Environ Exerc Physiol. 1984 Dec;57(6):1749–1753. doi: 10.1152/jappl.1984.57.6.1749. [DOI] [PubMed] [Google Scholar]
- Lewis S. F., Haller R. G., Cook J. D., Nunnally R. L. Muscle fatigue in McArdle's disease studied by 31P-NMR: effect of glucose infusion. J Appl Physiol (1985) 1985 Dec;59(6):1991–1994. doi: 10.1152/jappl.1985.59.6.1991. [DOI] [PubMed] [Google Scholar]
- Lewis S. F., Haller R. G. The pathophysiology of McArdle's disease: clues to regulation in exercise and fatigue. J Appl Physiol (1985) 1986 Aug;61(2):391–401. doi: 10.1152/jappl.1986.61.2.391. [DOI] [PubMed] [Google Scholar]
- Linton R. A., Band D. M. The effect of potassium on carotid chemoreceptor activity and ventilation in the cat. Respir Physiol. 1985 Jan;59(1):65–70. doi: 10.1016/0034-5687(85)90019-2. [DOI] [PubMed] [Google Scholar]
- McCloskey D. I., Mitchell J. H. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol. 1972 Jul;224(1):173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRET C. [Hyperoxia and regulation of ventilation during muscular exercise]. Helv Physiol Pharmacol Acta. 1960;18:72–97. [PubMed] [Google Scholar]
- Paterson D. J., Nye P. C. The effect of beta adrenergic blockade on the carotid body response to hyperkalaemia in the cat. Respir Physiol. 1988 Nov;74(2):229–237. doi: 10.1016/0034-5687(88)90107-7. [DOI] [PubMed] [Google Scholar]
- Paterson D. J., Robbins P. A., Conway J. Changes in arterial plasma potassium and ventilation during exercise in man. Respir Physiol. 1989 Dec;78(3):323–330. doi: 10.1016/0034-5687(89)90107-2. [DOI] [PubMed] [Google Scholar]
- SALMON S. E., TURNER C. E. MCARDLE'S DISEASE PRESENTING AS CONVULSION AND RHABDOMYOLYSIS. Am J Med. 1965 Jul;39:142–146. doi: 10.1016/0002-9343(65)90254-8. [DOI] [PubMed] [Google Scholar]
- Wasserman K., Whipp B. J., Koyal S. N., Cleary M. G. Effect of carotid body resection on ventilatory and acid-base control during exercise. J Appl Physiol. 1975 Sep;39(3):354–358. doi: 10.1152/jappl.1975.39.3.354. [DOI] [PubMed] [Google Scholar]
- Whipp B. J. Exercise hyperventilation in patients with McArdle's disease. J Appl Physiol Respir Environ Exerc Physiol. 1983 Nov;55(5):1638–1639. doi: 10.1152/jappl.1983.55.5.1638. [DOI] [PubMed] [Google Scholar]
- Woll K. H., Lönnendonker U., Neumcke B. ATP-sensitive potassium channels in adult mouse skeletal muscle: different modes of blockage by internal cations, ATP and tolbutamide. Pflugers Arch. 1989 Sep;414(6):622–628. doi: 10.1007/BF00582126. [DOI] [PubMed] [Google Scholar]


