Abstract
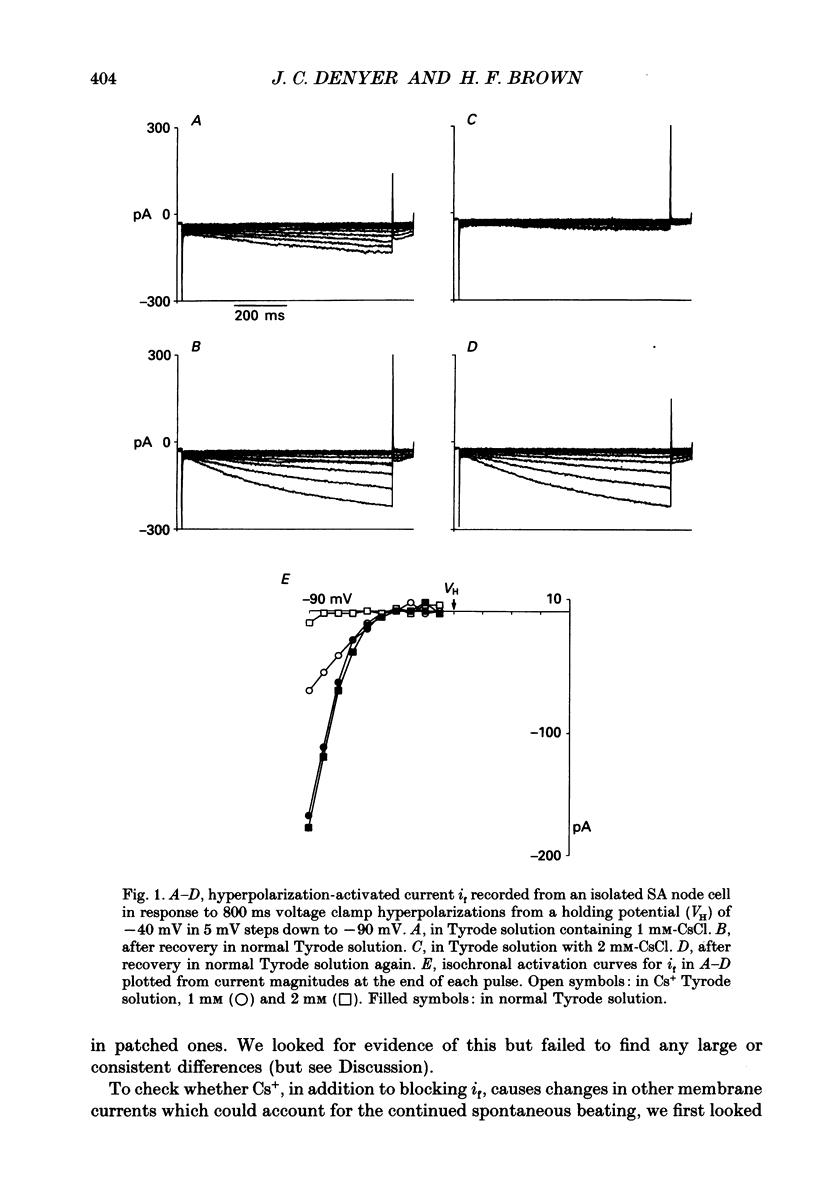
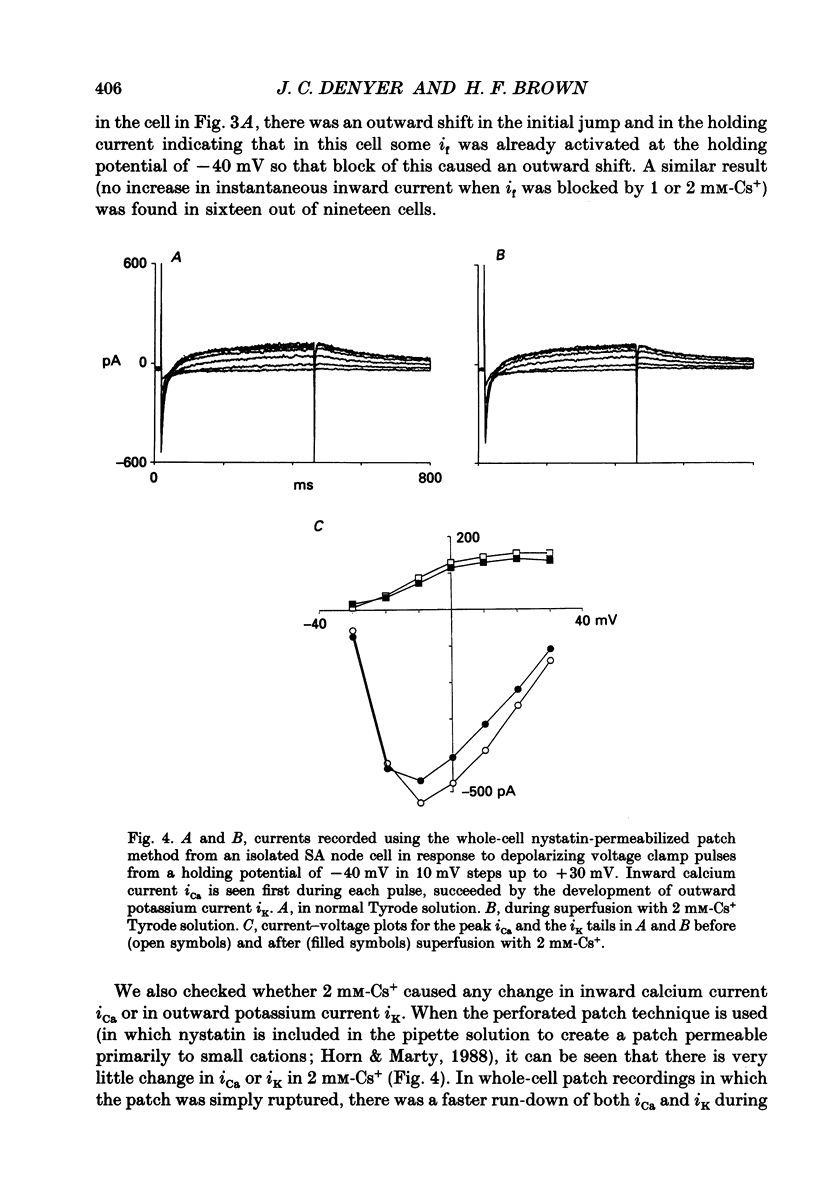
1. Experiments have been carried out using the whole-cell patch clamp technique to investigate how the spontaneous pacemaker activity of rabbit isolated sino-atrial (SA) node cells is affected by the block of the hyperpolarization-activated current if by 1 and 2 mM-caesium. 2. Two millimolar caesium reduced the amount of if activated at -90 mV to less than 10% of the control value. In the pacemaking range of SA node cells, if was often completely blocked by this concentration of Cs+. 3. Two millimolar caesium slowed but did not arrest spontaneous pacemaking in isolated SA node cells. In freely beating non-patched cells, 2 mM-CsCl caused a 30% reduction in rate of beating, indicating that in all cells observed if was normally contributing to pacemaking. 4. No increase in instantaneous inward current was seen in response to hyperpolarizing voltage clamp pulses from a holding potential of -40 mV when 1 or 2 mM-CsCl was applied to SA node cells. These concentrations of Cs+ do not therefore induce an 'extra' inward current. 5. Neither the inward calcium current (iCa) nor the outward potassium current (iK) showed changes which could be attributed to Cs+ application. 6. Since spontaneous pacemaking continues during Cs+ block of if while other membrane currents show no Cs(+)-induced changes which could account for this, these experiments provide strong, though indirect, evidence for the presence in SA node of a time-independent background current, ib, which will contribute to a mode of pacemaking controlled by the decay of potassium conductance (gK). The nature of this inward current has yet to be clarified. 7. The results strongly suggest that the hyperpolarization-activated current if normally makes an important contribution to the pacemaker depolarization of all SA node cells.
Full text
PDF


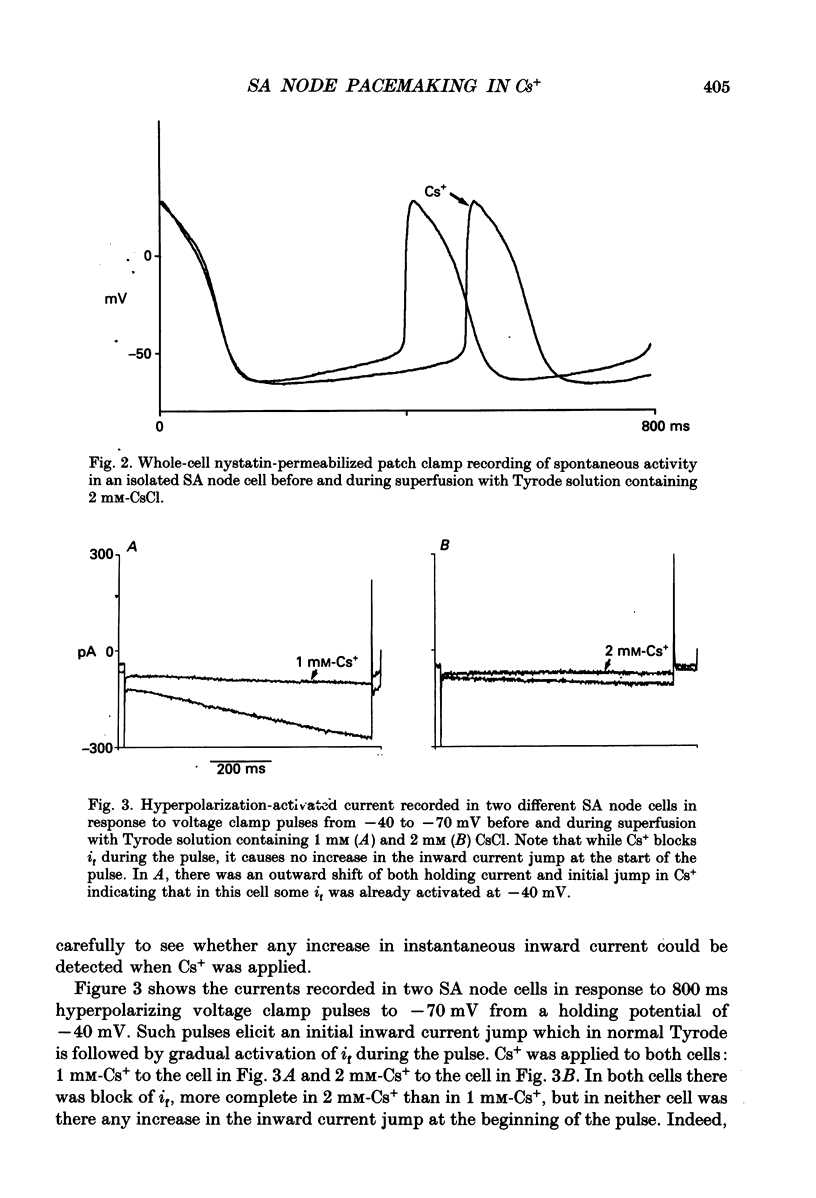



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown H., Difrancesco D., Noble S. Cardiac pacemaker oscillation and its modulation by autonomic transmitters. J Exp Biol. 1979 Aug;81:175–204. doi: 10.1242/jeb.81.1.175. [DOI] [PubMed] [Google Scholar]
- Denyer J. C., Brown H. F. Rabbit sino-atrial node cells: isolation and electrophysiological properties. J Physiol. 1990 Sep;428:405–424. doi: 10.1113/jphysiol.1990.sp018219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Ducouret P., Robinson R. B. Muscarinic modulation of cardiac rate at low acetylcholine concentrations. Science. 1989 Feb 3;243(4891):669–671. doi: 10.1126/science.2916119. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Ferroni A., Mazzanti M., Tromba C. Properties of the hyperpolarizing-activated current (if) in cells isolated from the rabbit sino-atrial node. J Physiol. 1986 Aug;377:61–88. doi: 10.1113/jphysiol.1986.sp016177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerr T., Denger R., Trautwein W. Calcium currents in single SA nodal cells of the rabbit heart studied with action potential clamp. Pflugers Arch. 1989 Apr;413(6):599–603. doi: 10.1007/BF00581808. [DOI] [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H. Modulation by intracellular Ca2+ of the hyperpolarization-activated inward current in rabbit single sino-atrial node cells. J Physiol. 1989 Feb;409:121–141. doi: 10.1113/jphysiol.1989.sp017488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C. Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog Biophys Mol Biol. 1988;52(3):165–247. doi: 10.1016/0079-6107(88)90014-4. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irisawa H. Comparative physiology of the cardiac pacemaker mechanism. Physiol Rev. 1978 Apr;58(2):461–498. doi: 10.1152/physrev.1978.58.2.461. [DOI] [PubMed] [Google Scholar]
- Noma A., Morad M., Irisawa H. Does the "pacemaker current" generate the diastolic depolarization in the rabbit SA node cells? Pflugers Arch. 1983 May;397(3):190–194. doi: 10.1007/BF00584356. [DOI] [PubMed] [Google Scholar]


