Abstract
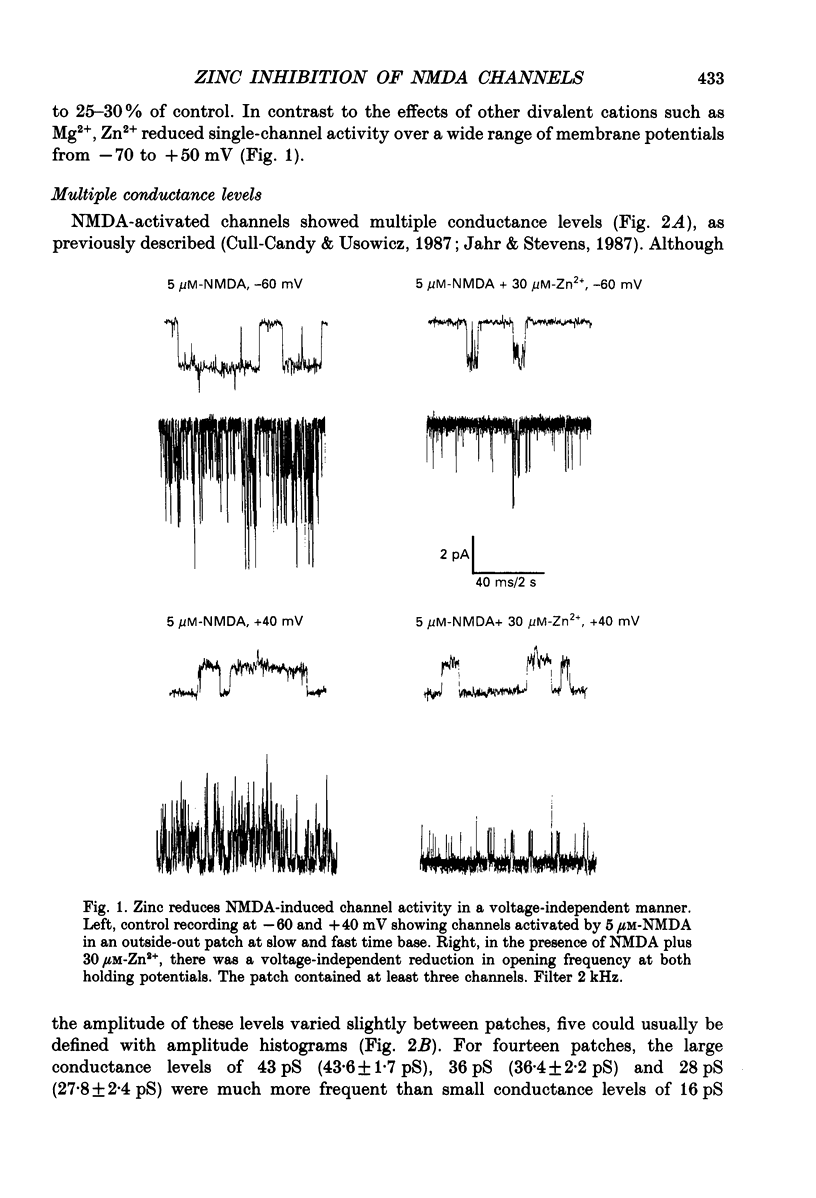
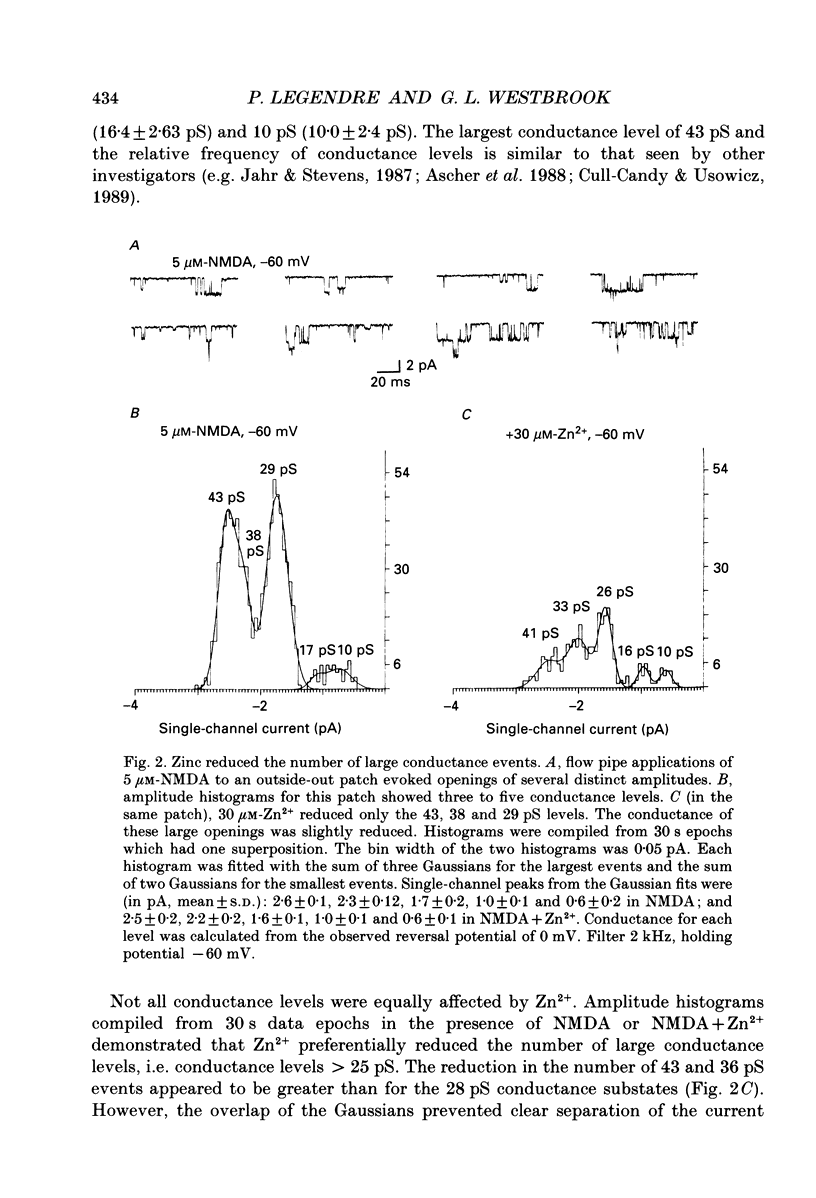
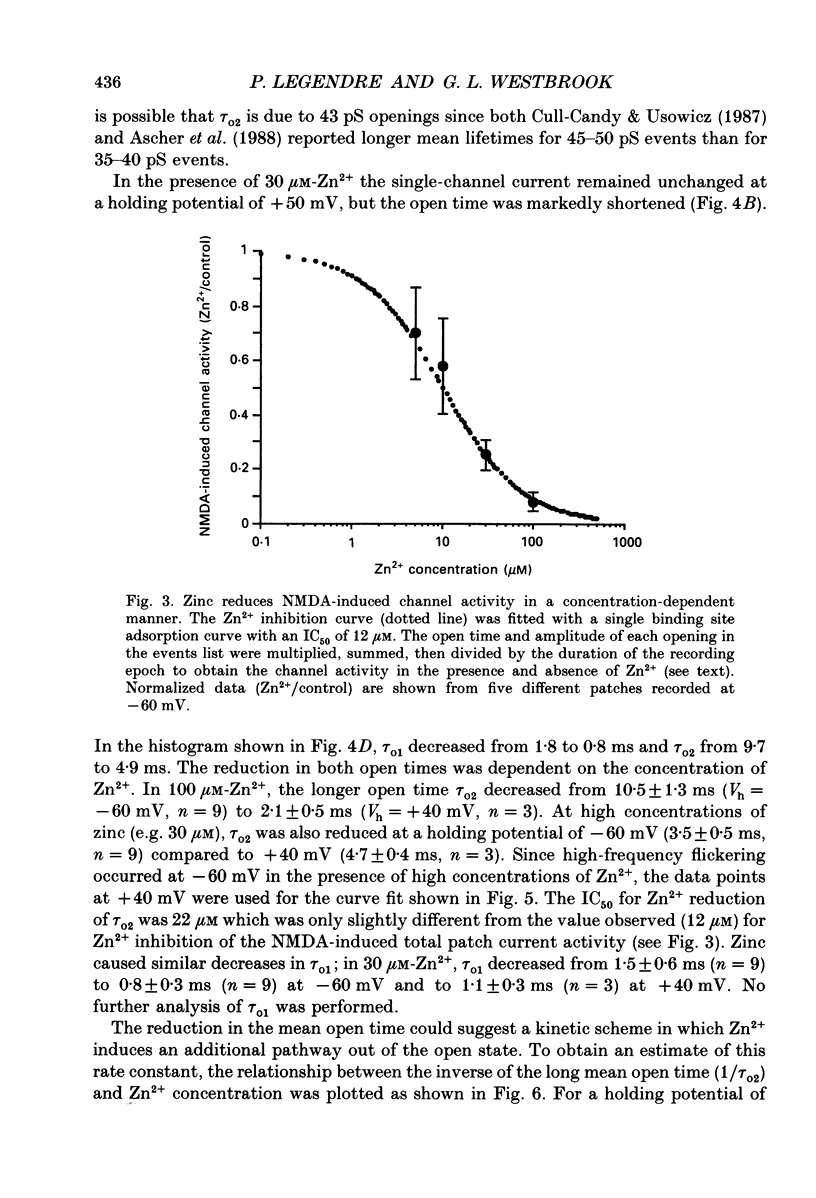
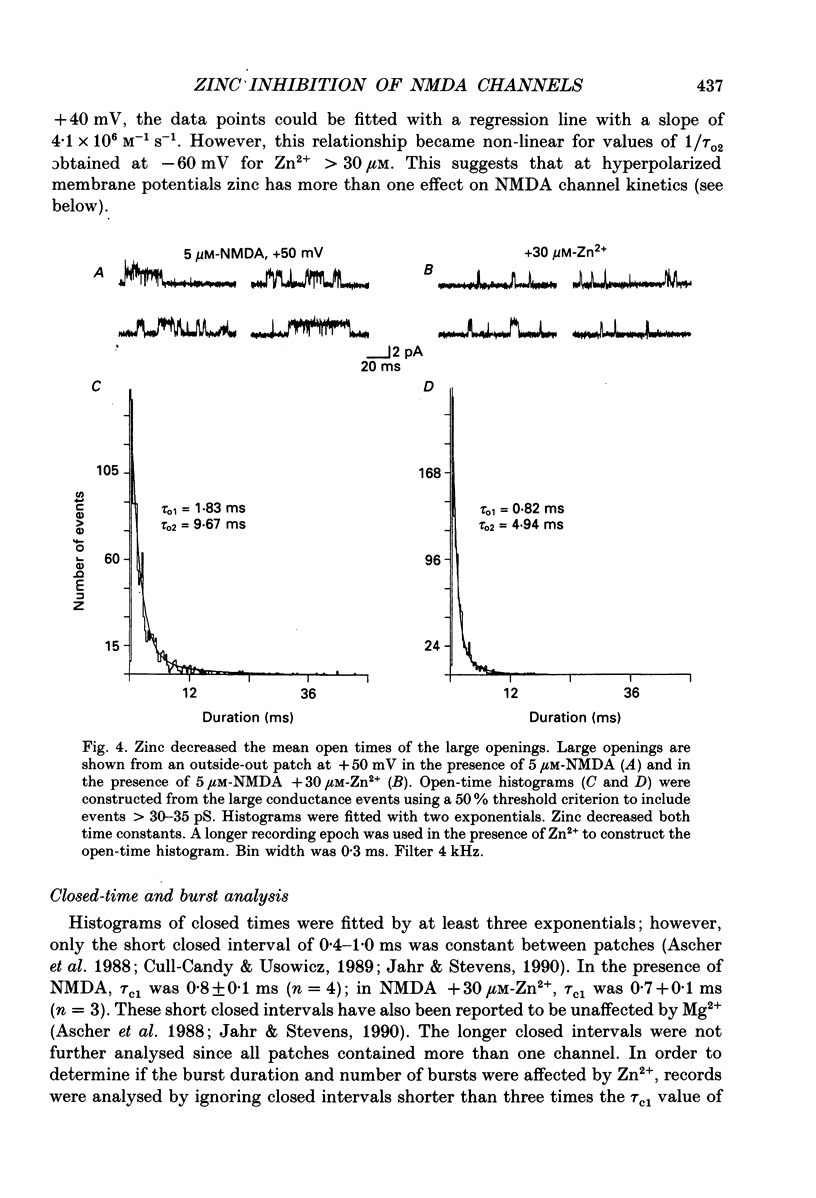
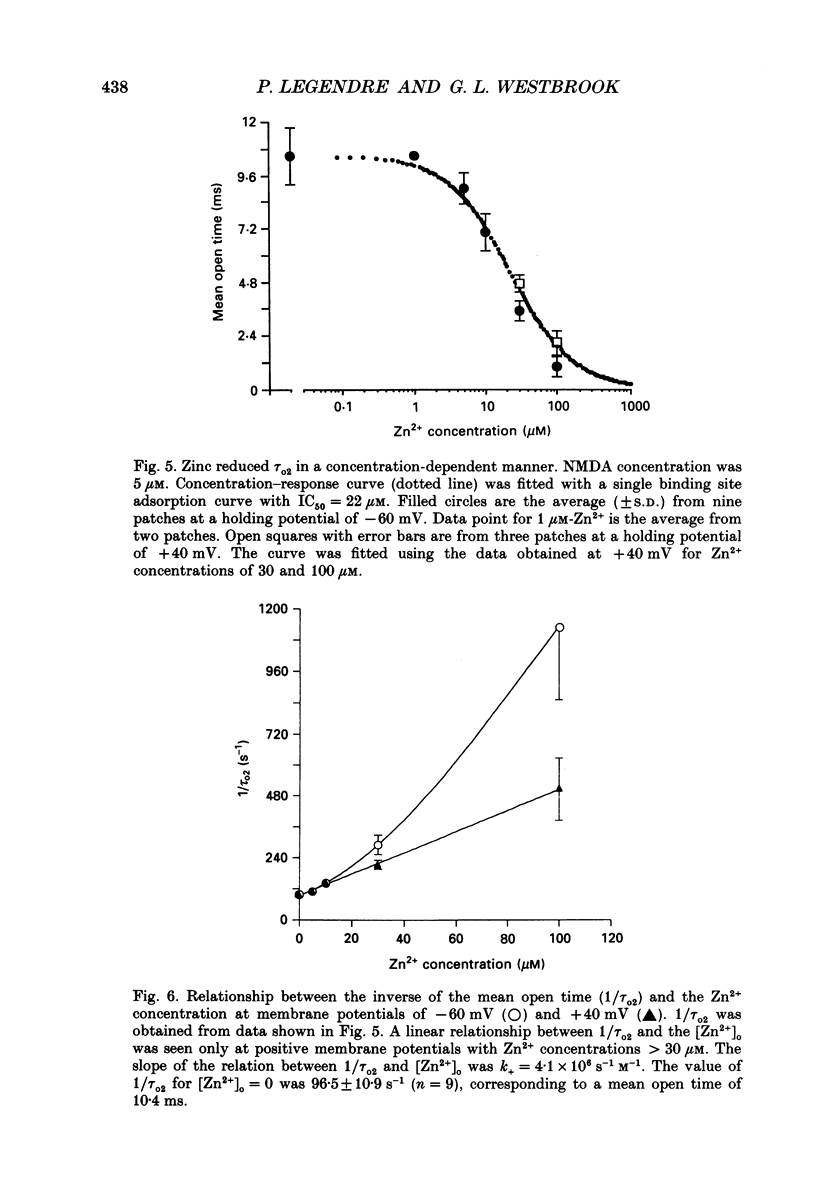
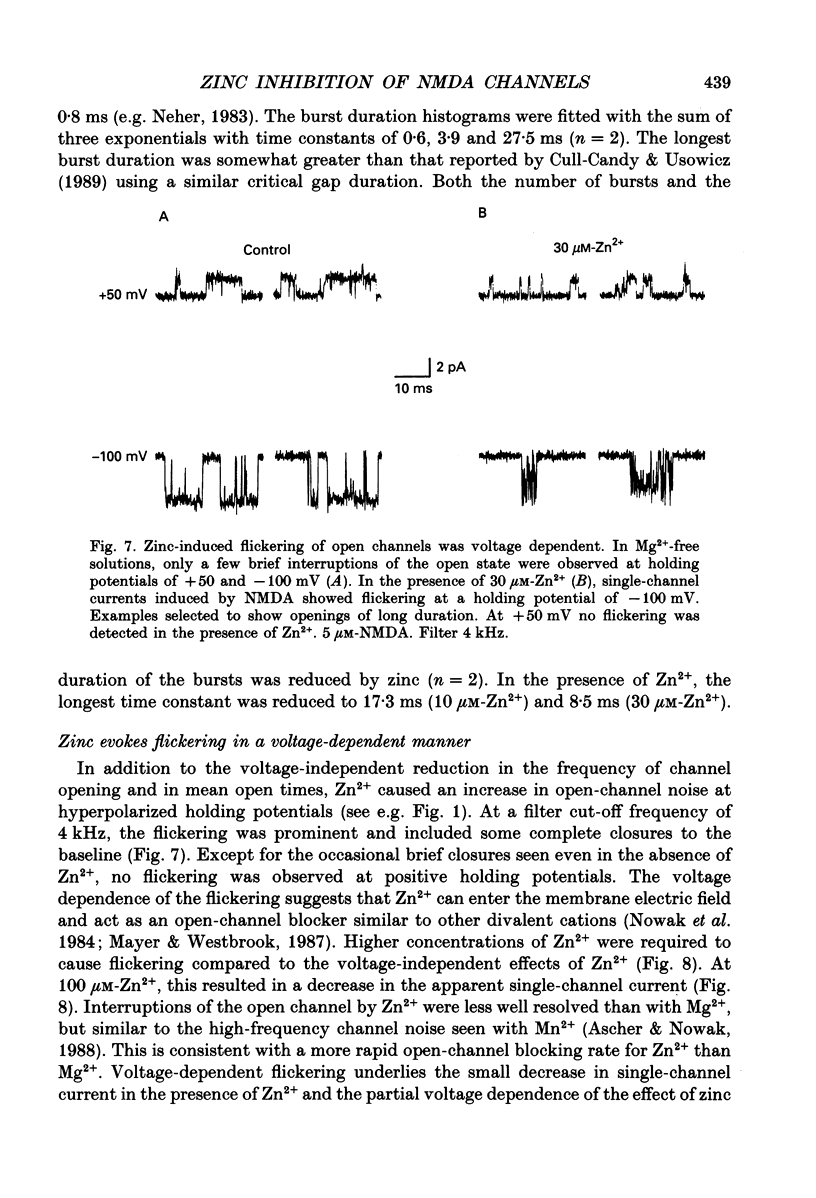
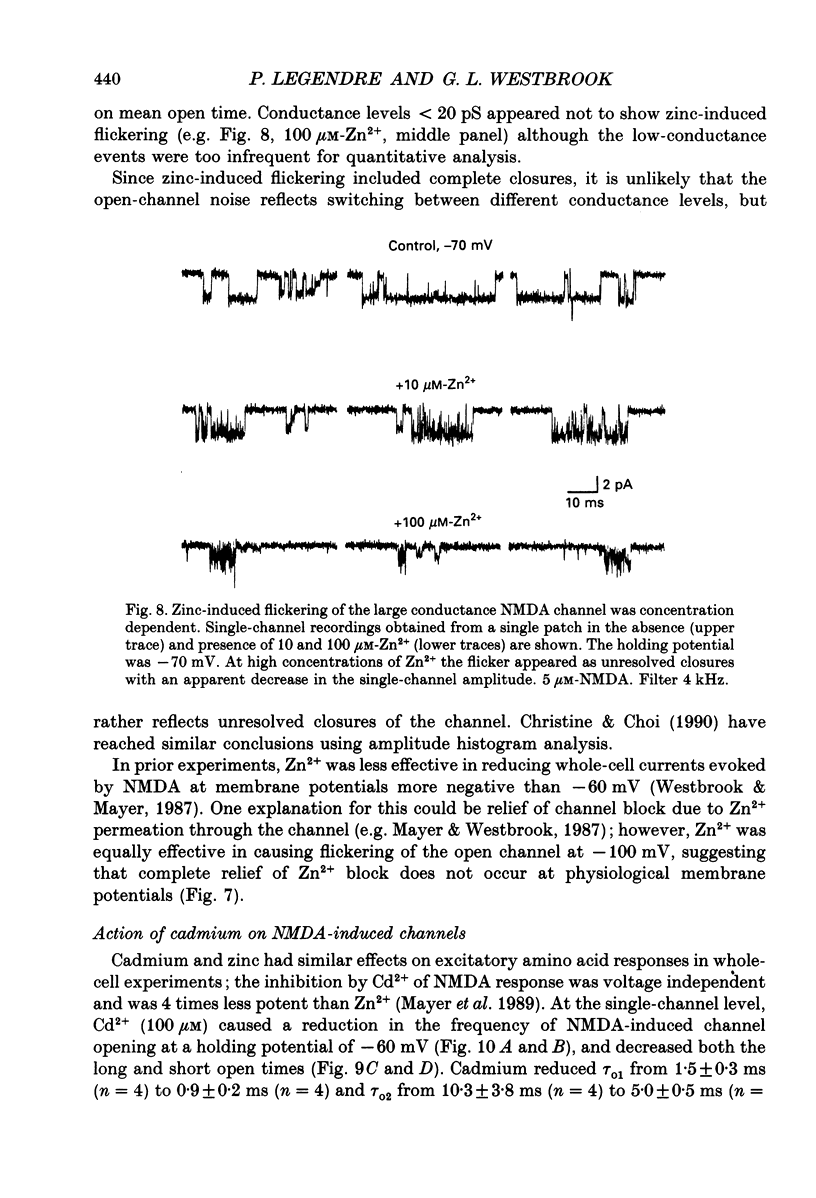
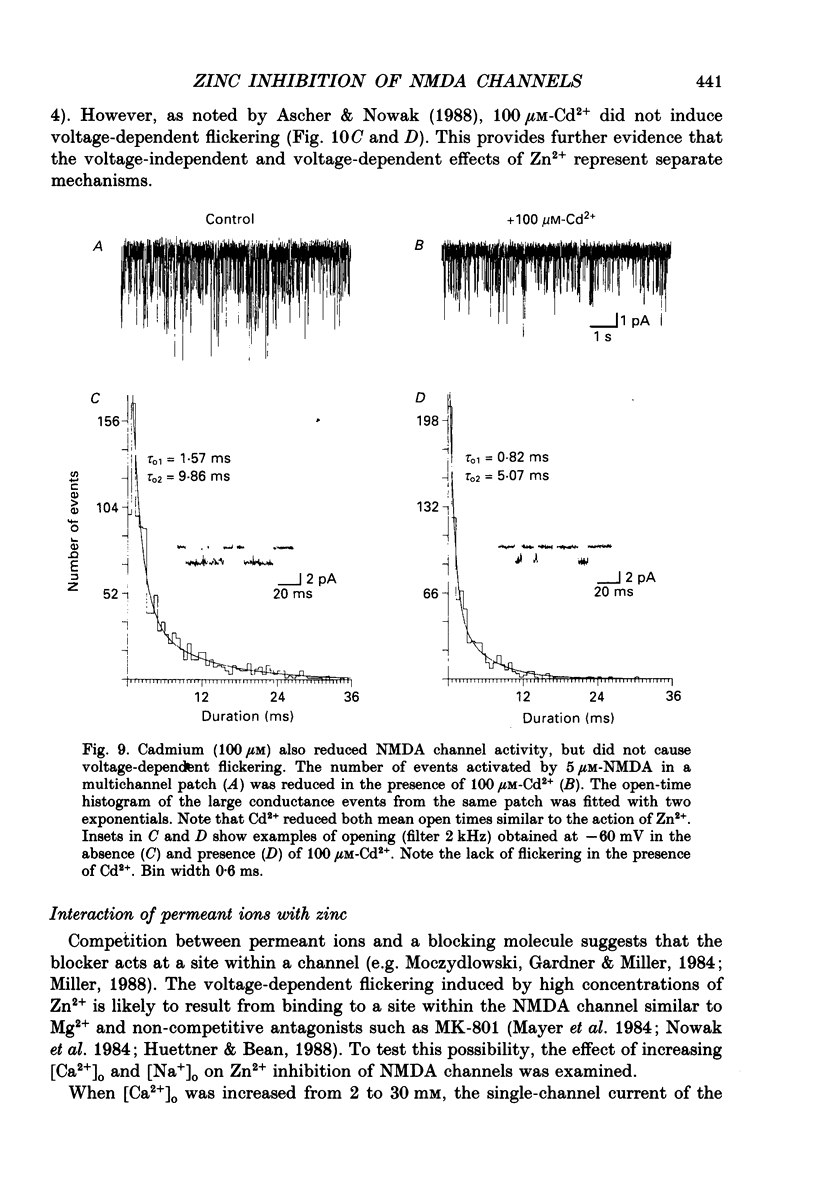
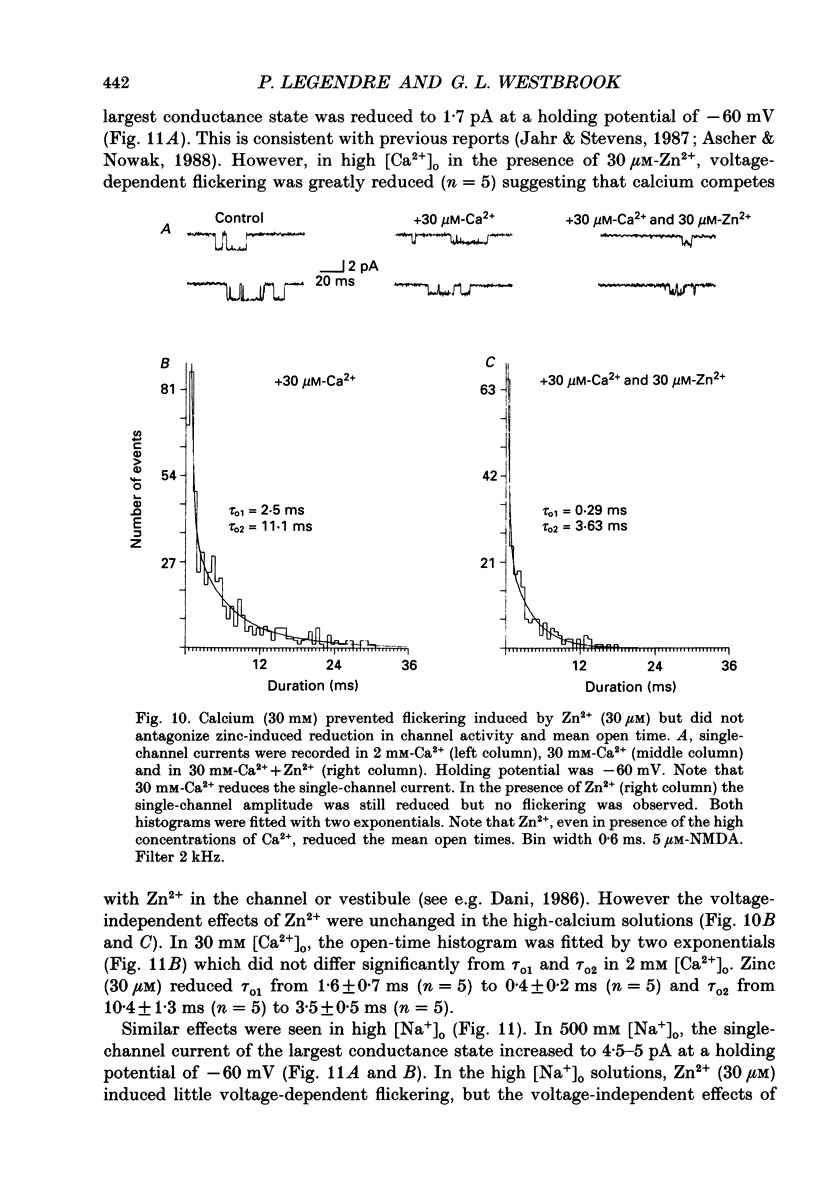
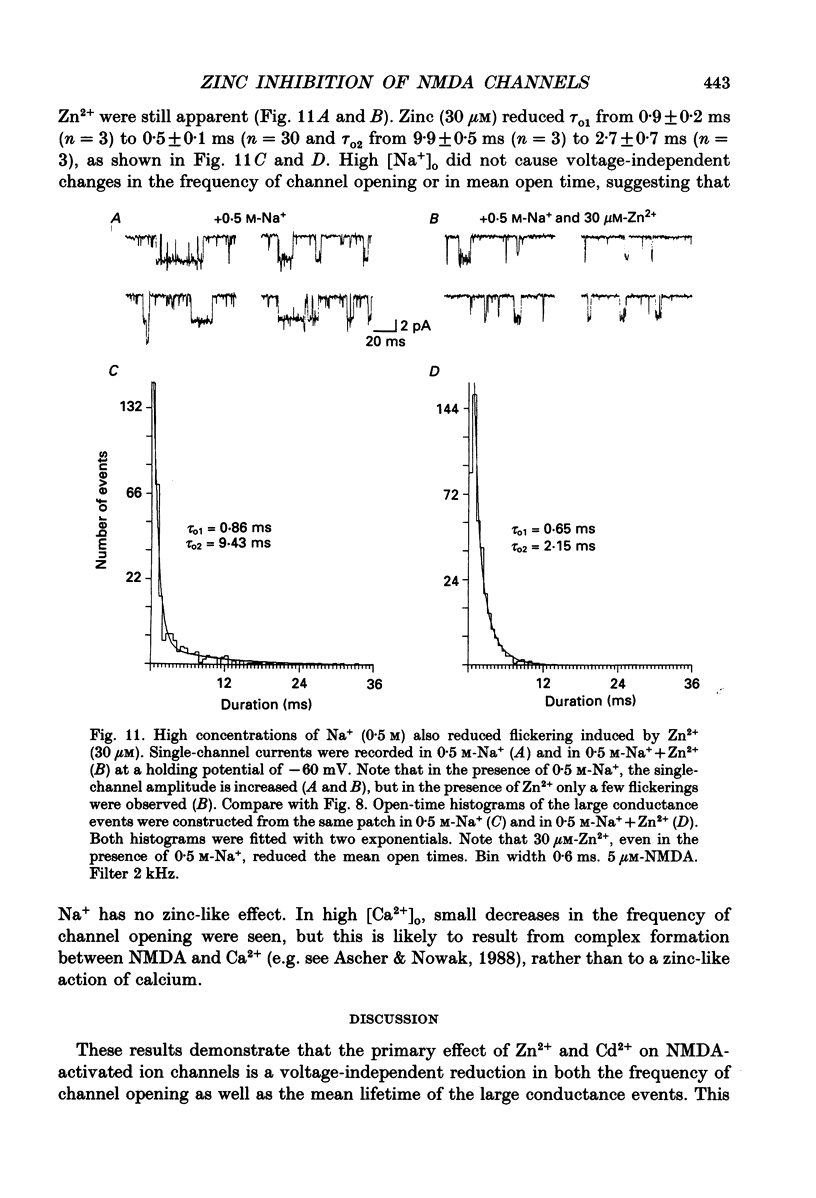
1. Single channels activated by N-methyl-D-aspartate (NMDA) were studied in outside-out patches of cultured hippocampal neurones in the presence of glycine and absence of magnesium. The effects of the transition metal ions zinc and cadmium on NMDA channels were tested by placing the membrane patch at the mouth of one of an array of large barrelled flow pipes. 2. Amplitude histograms revealed several conductance levels between 5 and 45 pS with the majority of NMDA-activated openings greater than 25 pS. Zinc (5-100 microM) and cadmium (30-100 microM) reduced the number of large conductance events in a voltage-independent manner. Zinc (30 microM) reduced the large conductance openings by approximately 70-80%. The small number of events under 20 pS precluded quantitative assessment of the effects of zinc and cadmium on these conductance levels. Zinc inhibition of the calculated macroscopic current due to NMDA-activated channels could be fitted with a single binding site isotherm with an IC50 of 12 microM. 3. Zinc and cadmium also reduced the mean open time of the two largest conductance events of 38 and 43 pS; this reduction was voltage independent. Open-time histograms were fitted with the sum of two exponentials. In the presence of 5 microM-NMDA at -60 mV, tau o2 = 10.49 ms and tau o1 = 1.47 ms; in 30 microM-zinc, tau o2 = 3.49 and tau o1 = 0.8 ms. The 'blocking' rate constant calculated at a membrane potential of +40 mV from the slope of 1/tau o2 vs. [zinc]o was 4 x 10(6) M-1 S-1. 4. Closed-time analysis revealed brief (tau c = 0.4-1.0 ms) zinc-insensitive gaps; longer closed-time intervals were not analysed since all patches contained more than one channel. Both burst duration and the number of bursts were reduced in the presence of zinc. 5. At holding potentials negative to -40 mV in magnesium-free solutions, zinc also induced high-frequency flickering of the open channel which included complete channel closures at 4 kHz filtering. No zinc-induced flickering was seen at positive membrane potentials. The flickering was dose dependent, becoming prominent at zinc concentrations above 30 microM. Cadmium did not induce flickering at concentrations up to 100 microM.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aizenman E., Lipton S. A., Loring R. H. Selective modulation of NMDA responses by reduction and oxidation. Neuron. 1989 Mar;2(3):1257–1263. doi: 10.1016/0896-6273(89)90310-3. [DOI] [PubMed] [Google Scholar]
- Ascher P., Bregestovski P., Nowak L. N-methyl-D-aspartate-activated channels of mouse central neurones in magnesium-free solutions. J Physiol. 1988 May;399:207–226. doi: 10.1113/jphysiol.1988.sp017076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988 May;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf S. Y., Chung S. H. Release of endogenous Zn2+ from brain tissue during activity. Nature. 1984 Apr 19;308(5961):734–736. doi: 10.1038/308734a0. [DOI] [PubMed] [Google Scholar]
- Choi D. W., Yokoyama M., Koh J. Zinc neurotoxicity in cortical cell culture. Neuroscience. 1988 Jan;24(1):67–79. doi: 10.1016/0306-4522(88)90312-0. [DOI] [PubMed] [Google Scholar]
- Christine C. W., Choi D. W. Effect of zinc on NMDA receptor-mediated channel currents in cortical neurons. J Neurosci. 1990 Jan;10(1):108–116. doi: 10.1523/JNEUROSCI.10-01-00108.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D. E., Neher E. Substance P reduces acetylcholine-induced currents in isolated bovine chromaffin cells. J Physiol. 1984 Feb;347:255–277. doi: 10.1113/jphysiol.1984.sp015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. Multiple-conductance channels activated by excitatory amino acids in cerebellar neurons. Nature. 1987 Feb 5;325(6104):525–528. doi: 10.1038/325525a0. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. On the multiple-conductance single channels activated by excitatory amino acids in large cerebellar neurones of the rat. J Physiol. 1989 Aug;415:555–582. doi: 10.1113/jphysiol.1989.sp017736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani J. A. Ion-channel entrances influence permeation. Net charge, size, shape, and binding considerations. Biophys J. 1986 Mar;49(3):607–618. doi: 10.1016/S0006-3495(86)83688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M., Hollenberg S. M. Zinc fingers: gilt by association. Cell. 1988 Jan 15;52(1):1–3. doi: 10.1016/0092-8674(88)90522-3. [DOI] [PubMed] [Google Scholar]
- Forsythe I. D., Westbrook G. L., Mayer M. L. Modulation of excitatory synaptic transmission by glycine and zinc in cultures of mouse hippocampal neurons. J Neurosci. 1988 Oct;8(10):3733–3741. doi: 10.1523/JNEUROSCI.08-10-03733.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie P. B., Brenneman D. E., Neale E. A. Morphological and biochemical differences expressed in separate dissociated cell cultures of dorsal and ventral halves of the mouse spinal cord. Brain Res. 1987 Sep 15;420(2):313–323. doi: 10.1016/0006-8993(87)91252-2. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hollmann M., O'Shea-Greenfield A., Rogers S. W., Heinemann S. Cloning by functional expression of a member of the glutamate receptor family. Nature. 1989 Dec 7;342(6250):643–648. doi: 10.1038/342643a0. [DOI] [PubMed] [Google Scholar]
- Howell G. A., Welch M. G., Frederickson C. J. Stimulation-induced uptake and release of zinc in hippocampal slices. Nature. 1984 Apr 19;308(5961):736–738. doi: 10.1038/308736a0. [DOI] [PubMed] [Google Scholar]
- Huettner J. E., Bean B. P. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. A quantitative description of NMDA receptor-channel kinetic behavior. J Neurosci. 1990 Jun;10(6):1830–1837. doi: 10.1523/JNEUROSCI.10-06-01830.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987 Feb 5;325(6104):522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kiskin N. I., Krishtal O. A., Tsyndrenko AYa, Akaike N. Are sulfhydryl groups essential for function of the glutamate-operated receptor-ionophore complex? Neurosci Lett. 1986 May 23;66(3):305–310. doi: 10.1016/0304-3940(86)90036-4. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr, Westbrook G. L. Modulation of excitatory amino acid receptors by group IIB metal cations in cultured mouse hippocampal neurones. J Physiol. 1989 Aug;415:329–350. doi: 10.1113/jphysiol.1989.sp017724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J Physiol. 1987 Dec;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Vyklický L., Jr Sites of antagonist action on N-methyl-D-aspartic acid receptors studied using fluctuation analysis and a rapid perfusion technique. J Neurophysiol. 1988 Aug;60(2):645–663. doi: 10.1152/jn.1988.60.2.645. [DOI] [PubMed] [Google Scholar]
- Miller C. Competition for block of a Ca2(+)-activated K+ channel by charybdotoxin and tetraethylammonium. Neuron. 1988 Dec;1(10):1003–1006. doi: 10.1016/0896-6273(88)90157-2. [DOI] [PubMed] [Google Scholar]
- Moczydlowski E., Garber S. S., Miller C. Batrachotoxin-activated Na+ channels in planar lipid bilayers. Competition of tetrodotoxin block by Na+. J Gen Physiol. 1984 Nov;84(5):665–686. doi: 10.1085/jgp.84.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan J. B., Michel J. Identification and characterization of an N-methyl-D-aspartate-specific L-[3H]glutamate recognition site in synaptic plasma membranes. J Neurochem. 1987 Jun;48(6):1699–1708. doi: 10.1111/j.1471-4159.1987.tb05726.x. [DOI] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. The charge carried by single-channel currents of rat cultured muscle cells in the presence of local anaesthetics. J Physiol. 1983 Jun;339:663–678. doi: 10.1113/jphysiol.1983.sp014741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Patrick J., Boulter J., Goldman D., Gardner P., Heinemann S. Molecular biology of nicotinic acetylcholine receptors. Ann N Y Acad Sci. 1987;505:194–207. doi: 10.1111/j.1749-6632.1987.tb51292.x. [DOI] [PubMed] [Google Scholar]
- Peters S., Koh J., Choi D. W. Zinc selectively blocks the action of N-methyl-D-aspartate on cortical neurons. Science. 1987 May 1;236(4801):589–593. doi: 10.1126/science.2883728. [DOI] [PubMed] [Google Scholar]
- Pritchett D. B., Sontheimer H., Shivers B. D., Ymer S., Kettenmann H., Schofield P. R., Seeburg P. H. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989 Apr 13;338(6216):582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Prod'hom B., Pietrobon D., Hess P. Interactions of protons with single open L-type calcium channels. Location of protonation site and dependence of proton-induced current fluctuations on concentration and species of permeant ion. J Gen Physiol. 1989 Jul;94(1):23–42. doi: 10.1085/jgp.94.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Clausell J., Danscher G. Intravesicular localization of zinc in rat telencephalic boutons. A histochemical study. Brain Res. 1985 Jun 24;337(1):91–98. doi: 10.1016/0006-8993(85)91612-9. [DOI] [PubMed] [Google Scholar]
- Reynolds I. J., Miller R. J. Tricyclic antidepressants block N-methyl-D-aspartate receptors: similarities to the action of zinc. Br J Pharmacol. 1988 Sep;95(1):95–102. doi: 10.1111/j.1476-5381.1988.tb16552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sernagor E., Kuhn D., Vyklicky L., Jr, Mayer M. L. Open channel block of NMDA receptor responses evoked by tricyclic antidepressants. Neuron. 1989 Mar;2(3):1221–1227. doi: 10.1016/0896-6273(89)90306-1. [DOI] [PubMed] [Google Scholar]
- Traynelis S. F., Cull-Candy S. G. Proton inhibition of N-methyl-D-aspartate receptors in cerebellar neurons. Nature. 1990 May 24;345(6273):347–350. doi: 10.1038/345347a0. [DOI] [PubMed] [Google Scholar]
- Westbrook G. L., Mayer M. L. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature. 1987 Aug 13;328(6131):640–643. doi: 10.1038/328640a0. [DOI] [PubMed] [Google Scholar]


