Abstract
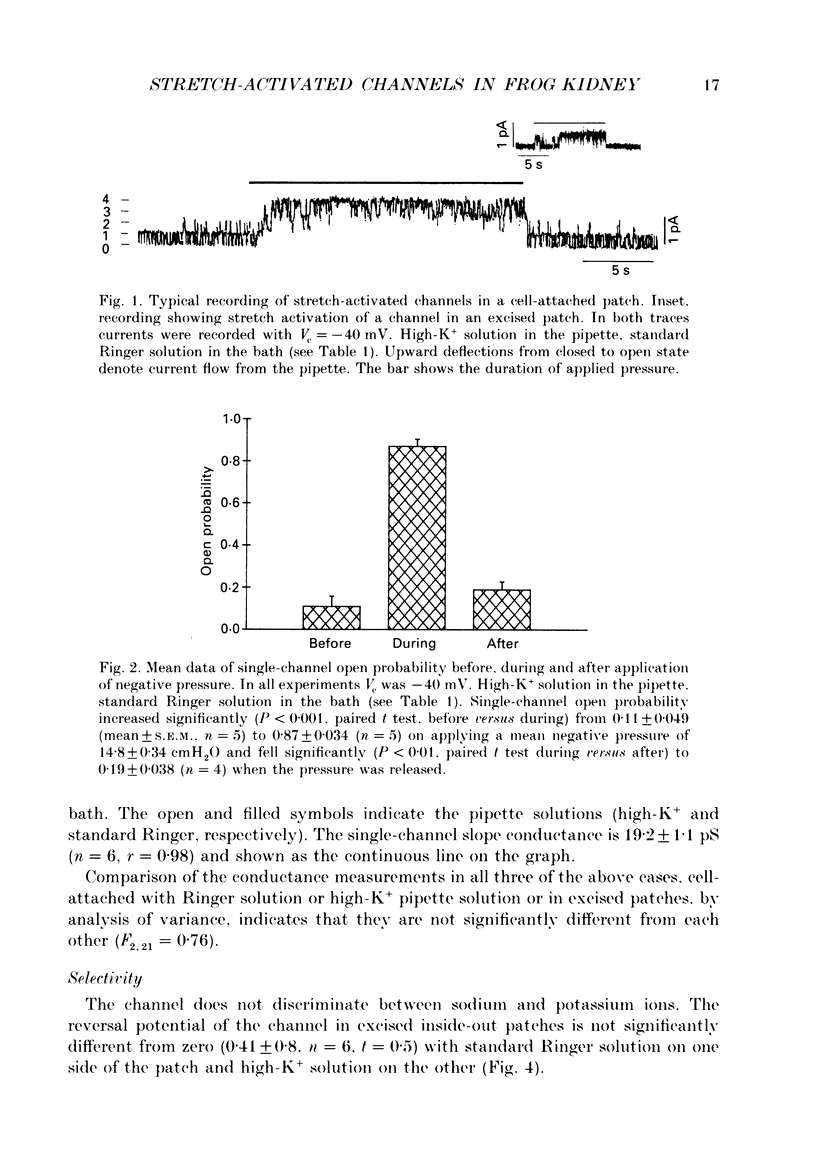
1. Single stretch-activated channels have been studied in cell-attached and excised patches from single early distal tubule (diluting segment) cells of Rana temporaria. 2. The channels can be reversibly activated, in both cell-attached and excised patches, by the application of negative pressure to the pipette causing mechanical stretching of the cell membrane. In cell-attached patches, application of 14.8 cmH2O negative pressure to the patch pipette increased reversibly the open probably from 0.11 to 0.87. 3. The channel conductance in the cell-attached configuration with standard Ringer solution in the pipette is 21.3 pS. 4. The channel is non-specific. In excised inside-out patches ion substitution experiments show that the channel does not discriminate between sodium and potassium ions, nor does it appear to select for cations over anions. 5. The channel is voltage sensitive such that depolarizing the cell opens the channel. The open probability at the resting membrane potential, 0.89, was reduced to 0.26 at a hyperpolarizing potential of 100 mV (holding pressure of -20.1 cmH2O or -206 Pa). 6. The sensitivity of the channel to mechanical stretching suggests that the channel may be involved in cell volume regulation.
Full text
PDF



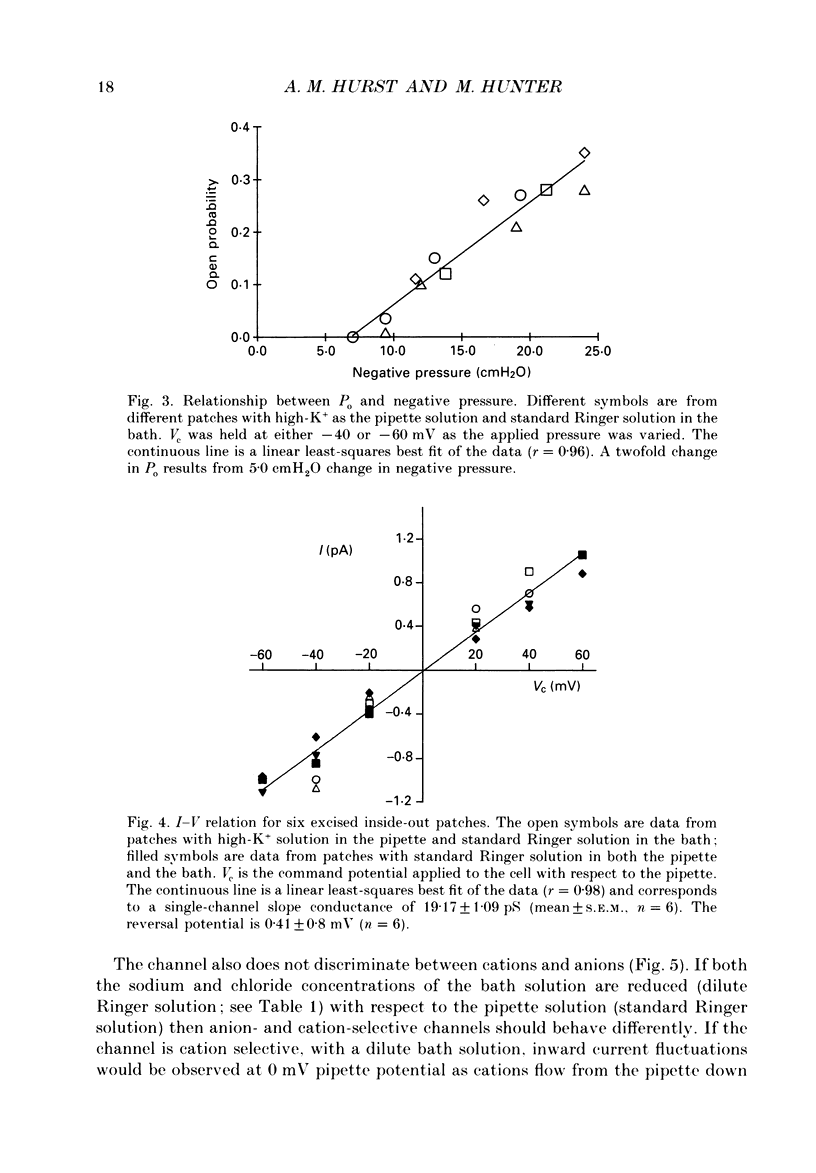
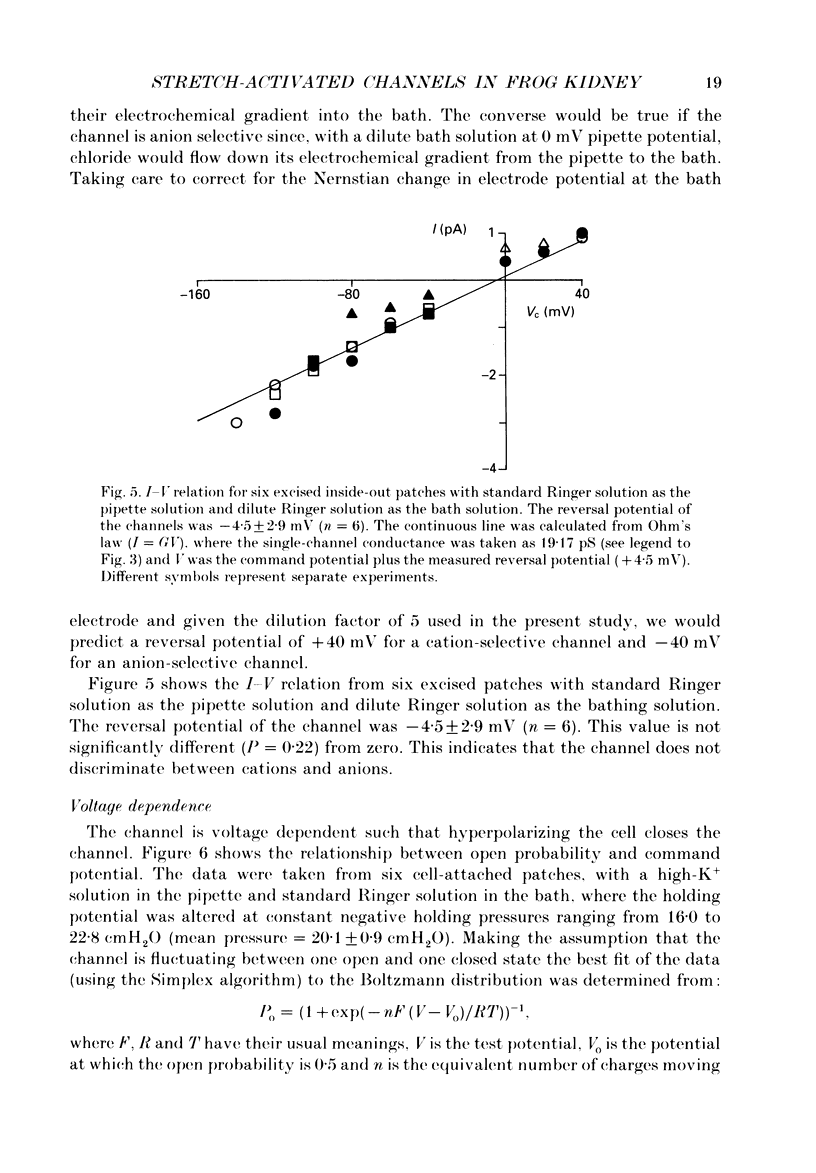
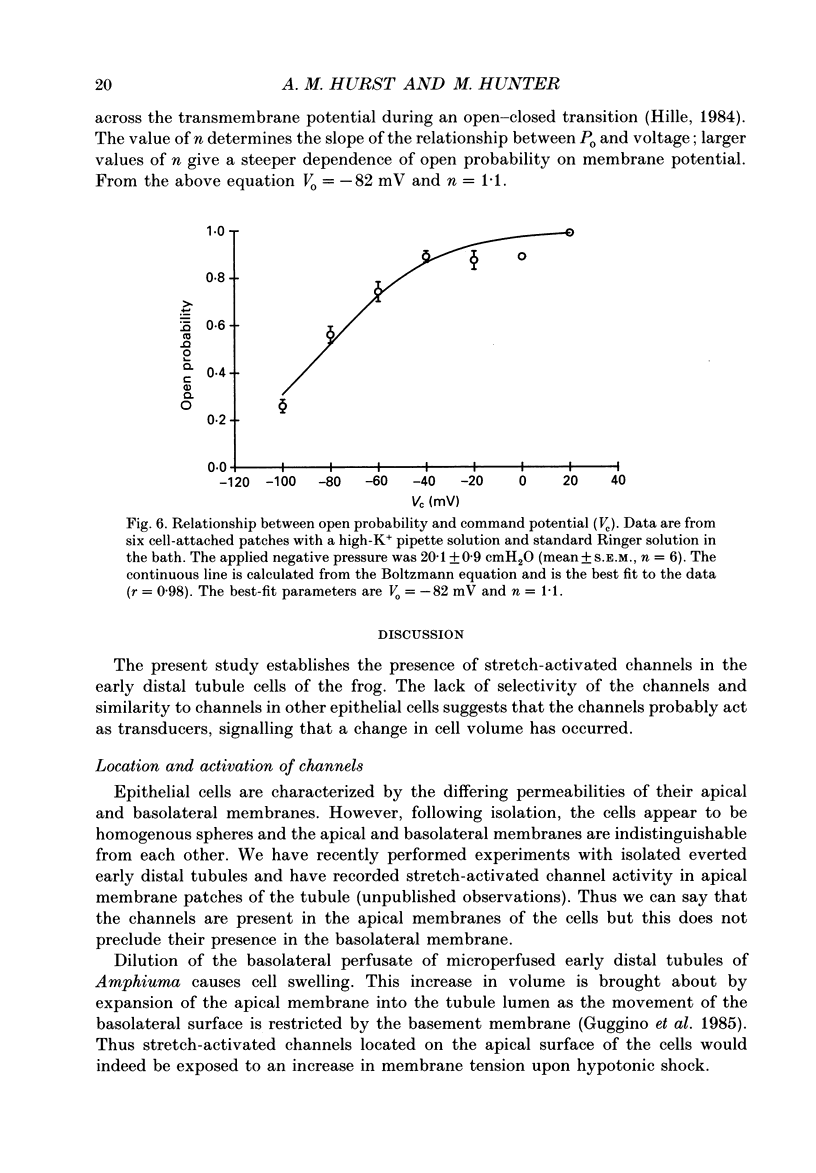




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christensen O. Mediation of cell volume regulation by Ca2+ influx through stretch-activated channels. Nature. 1987 Nov 5;330(6143):66–68. doi: 10.1038/330066a0. [DOI] [PubMed] [Google Scholar]
- Dellasega M., Grantham J. J. Regulation of renal tubule cell volume in hypotonic media. Am J Physiol. 1973 Jun;224(6):1288–1294. doi: 10.1152/ajplegacy.1973.224.6.1288. [DOI] [PubMed] [Google Scholar]
- Guggino W. B., Oberleithner H., Giebisch G. Relationship between cell volume and ion transport in the early distal tubule of the Amphiuma kidney. J Gen Physiol. 1985 Jul;86(1):31–58. doi: 10.1085/jgp.86.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guharay F., Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol. 1984 Jul;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert S. C., Sun A. Hypotonic cell volume regulation in mouse medullary thick ascending limb: effects of ADH. Am J Physiol. 1988 Nov;255(5 Pt 2):F962–F969. doi: 10.1152/ajprenal.1988.255.5.F962. [DOI] [PubMed] [Google Scholar]
- Hoffmann E. K., Simonsen L. O. Membrane mechanisms in volume and pH regulation in vertebrate cells. Physiol Rev. 1989 Apr;69(2):315–382. doi: 10.1152/physrev.1989.69.2.315. [DOI] [PubMed] [Google Scholar]
- Kirk K. L., DiBona D. R., Schafer J. A. Regulatory volume decrease in perfused proximal nephron: evidence for a dumping of cell K+. Am J Physiol. 1987 May;252(5 Pt 2):F933–F942. doi: 10.1152/ajprenal.1987.252.5.F933. [DOI] [PubMed] [Google Scholar]
- Lopes A. G., Guggino W. B. Volume regulation in the early proximal tubule of the Necturus kidney. J Membr Biol. 1987;97(2):117–125. doi: 10.1007/BF01869418. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Guggino W., Giebisch G. Resistance properties of the diluting segment of Amphiuma kidney: influence of potassium adaptation. J Membr Biol. 1985;88(2):139–147. doi: 10.1007/BF01868428. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Lang F., Wang W., Giebisch G. Effects of inhibition of chloride transport on intracellular sodium activity in distal amphibian nephron. Pflugers Arch. 1982 Jul;394(1):55–60. doi: 10.1007/BF01108308. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Schmidt B., Dietl P. Fusion of renal epithelial cells: a model for studying cellular mechanisms of ion transport. Proc Natl Acad Sci U S A. 1986 May;83(10):3547–3551. doi: 10.1073/pnas.83.10.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberleithner H., Weigt M., Westphale H. J., Wang W. Aldosterone activates Na+/H+ exchange and raises cytoplasmic pH in target cells of the amphibian kidney. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1464–1468. doi: 10.1073/pnas.84.5.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackin H., Palmer L. G. Basolateral potassium channels in renal proximal tubule. Am J Physiol. 1987 Sep;253(3 Pt 2):F476–F487. doi: 10.1152/ajprenal.1987.253.3.F476. [DOI] [PubMed] [Google Scholar]
- Stanton B., Biemesderfer D., Stetson D., Kashgarian M., Giebisch G. Cellular ultrastructure of Amphiuma distal nephron: effects of exposure to potassium. Am J Physiol. 1984 Sep;247(3 Pt 1):C204–C216. doi: 10.1152/ajpcell.1984.247.3.C204. [DOI] [PubMed] [Google Scholar]
- Ubl J., Murer H., Kolb H. A. Hypotonic shock evokes opening of Ca2+-activated K channels in opossum kidney cells. Pflugers Arch. 1988 Oct;412(5):551–553. doi: 10.1007/BF00582547. [DOI] [PubMed] [Google Scholar]
- Ubl J., Murer H., Kolb H. A. Ion channels activated by osmotic and mechanical stress in membranes of opossum kidney cells. J Membr Biol. 1988 Sep;104(3):223–232. doi: 10.1007/BF01872324. [DOI] [PubMed] [Google Scholar]
- Ubl J., Murer H., Kolb H. A. Simultaneous recording of cell volume, membrane current and membrane potential: effect of hypotonic shock. Pflugers Arch. 1989 Dec;415(3):381–383. doi: 10.1007/BF00370891. [DOI] [PubMed] [Google Scholar]
- Völkl H., Lang F. Electrophysiology of cell volume regulation in proximal tubules of the mouse kidney. Pflugers Arch. 1988 May;411(5):514–519. doi: 10.1007/BF00582372. [DOI] [PubMed] [Google Scholar]
- Völkl H., Lang F. Ionic requirement for regulatory cell volume decrease in renal straight proximal tubules. Pflugers Arch. 1988 Jul;412(1-2):1–6. doi: 10.1007/BF00583723. [DOI] [PubMed] [Google Scholar]
- Welling P. A., Linshaw M. A. Importance of anion in hypotonic volume regulation of rabbit proximal straight tubule. Am J Physiol. 1988 Nov;255(5 Pt 2):F853–F860. doi: 10.1152/ajprenal.1988.255.5.F853. [DOI] [PubMed] [Google Scholar]


