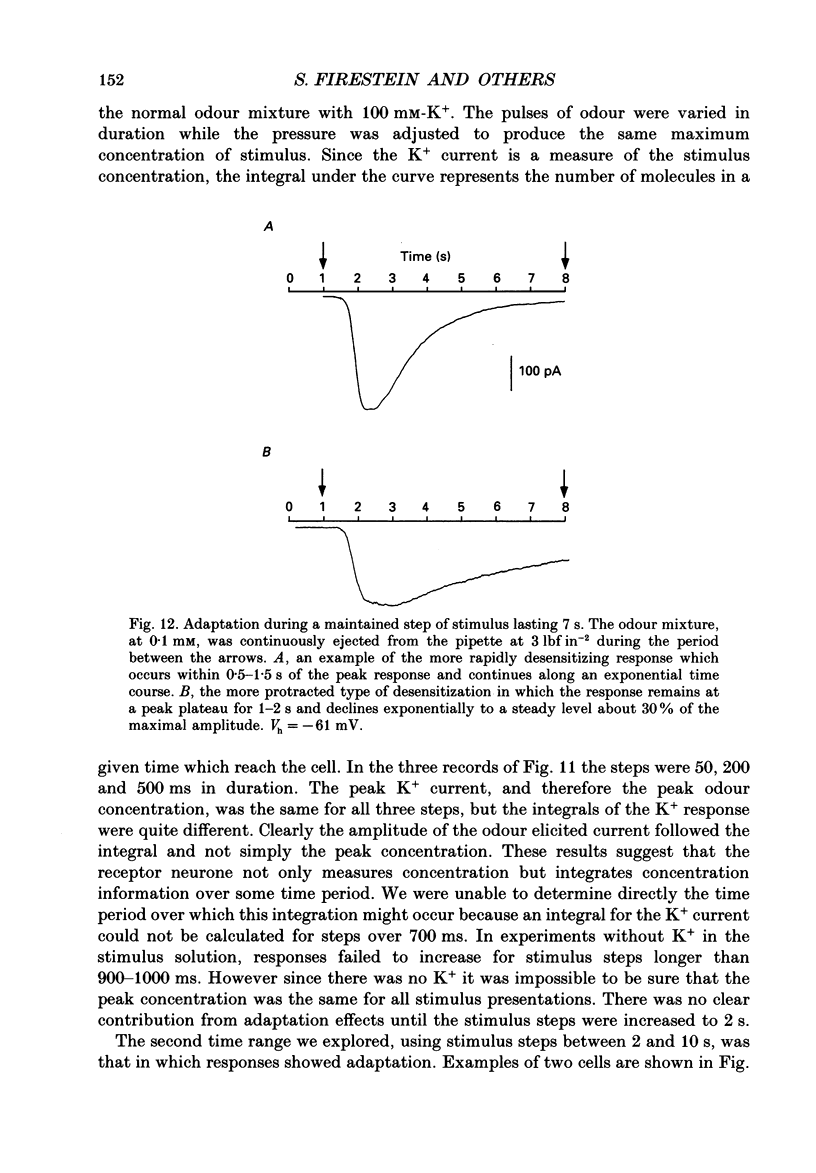
Abstract
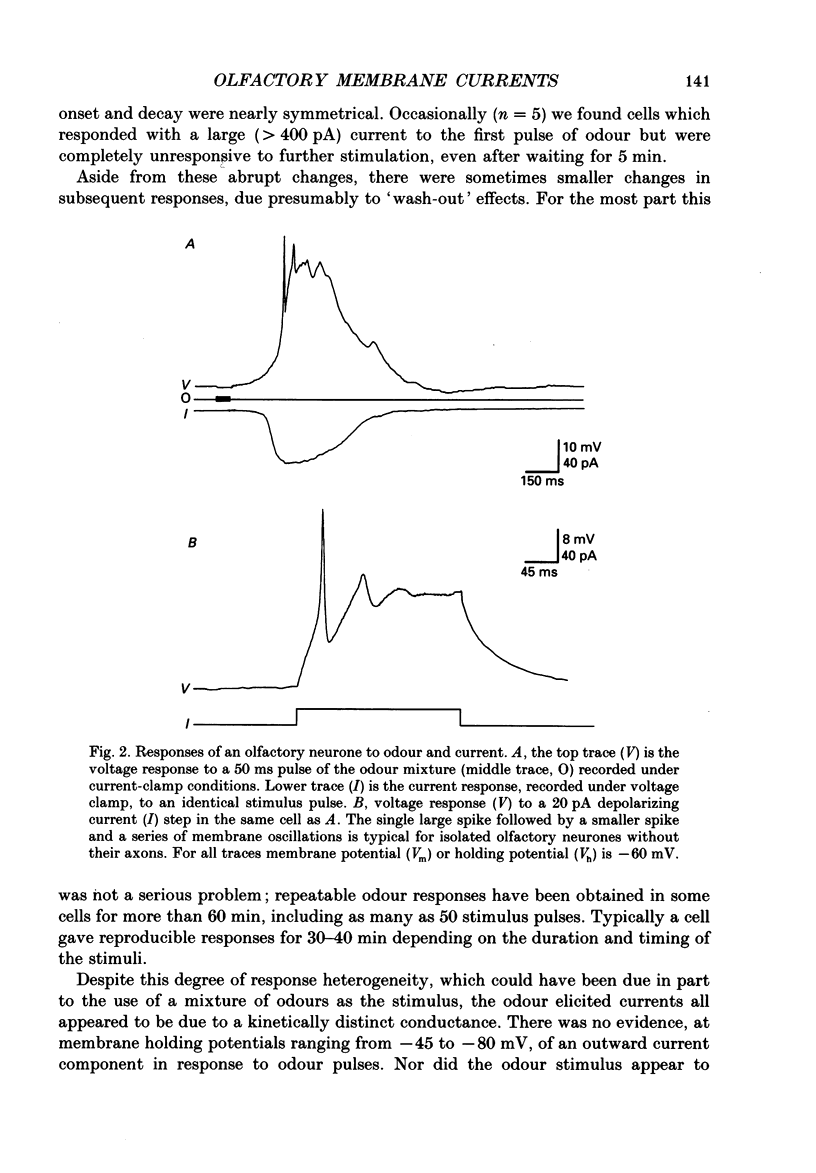
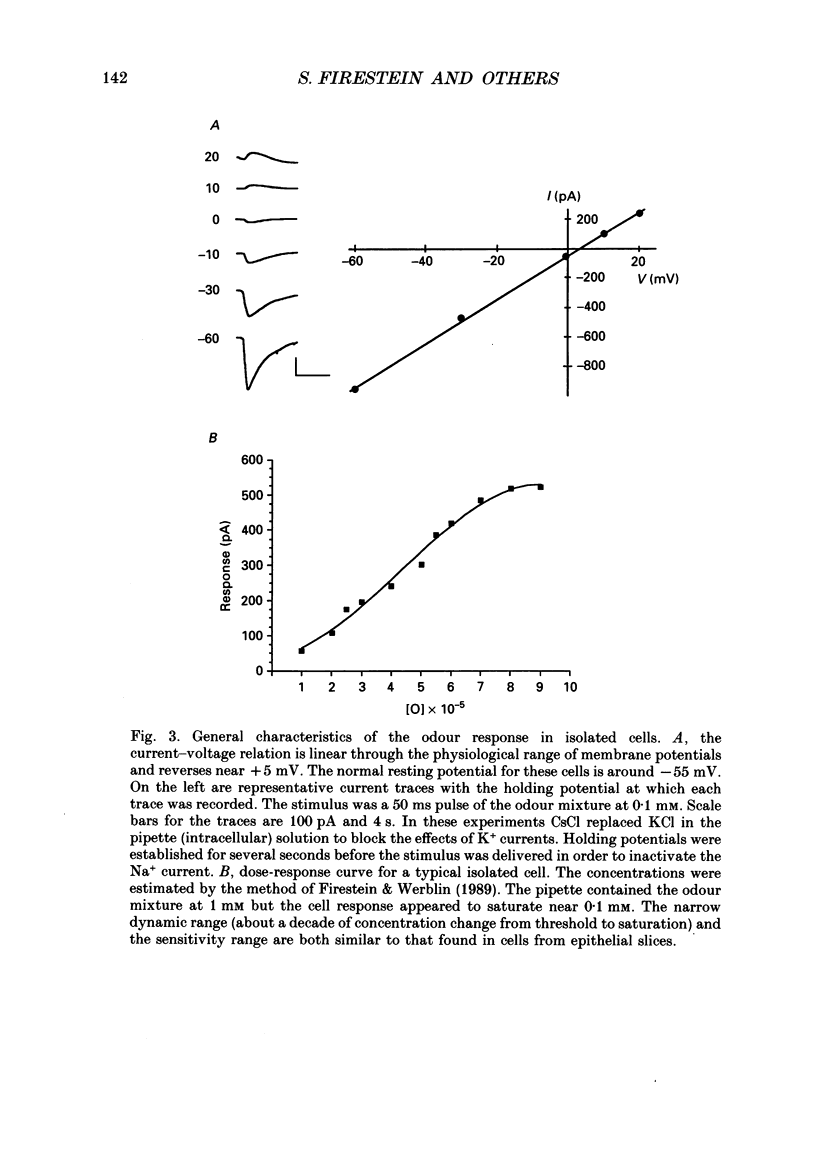
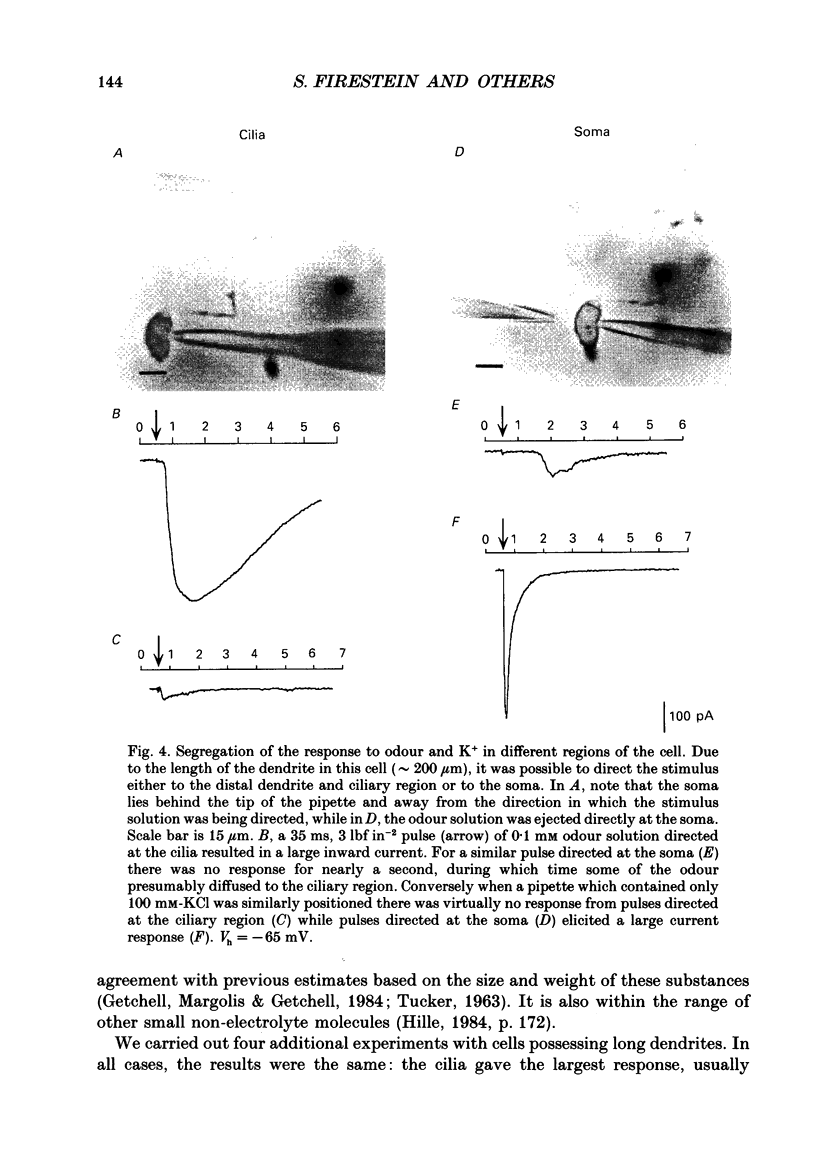
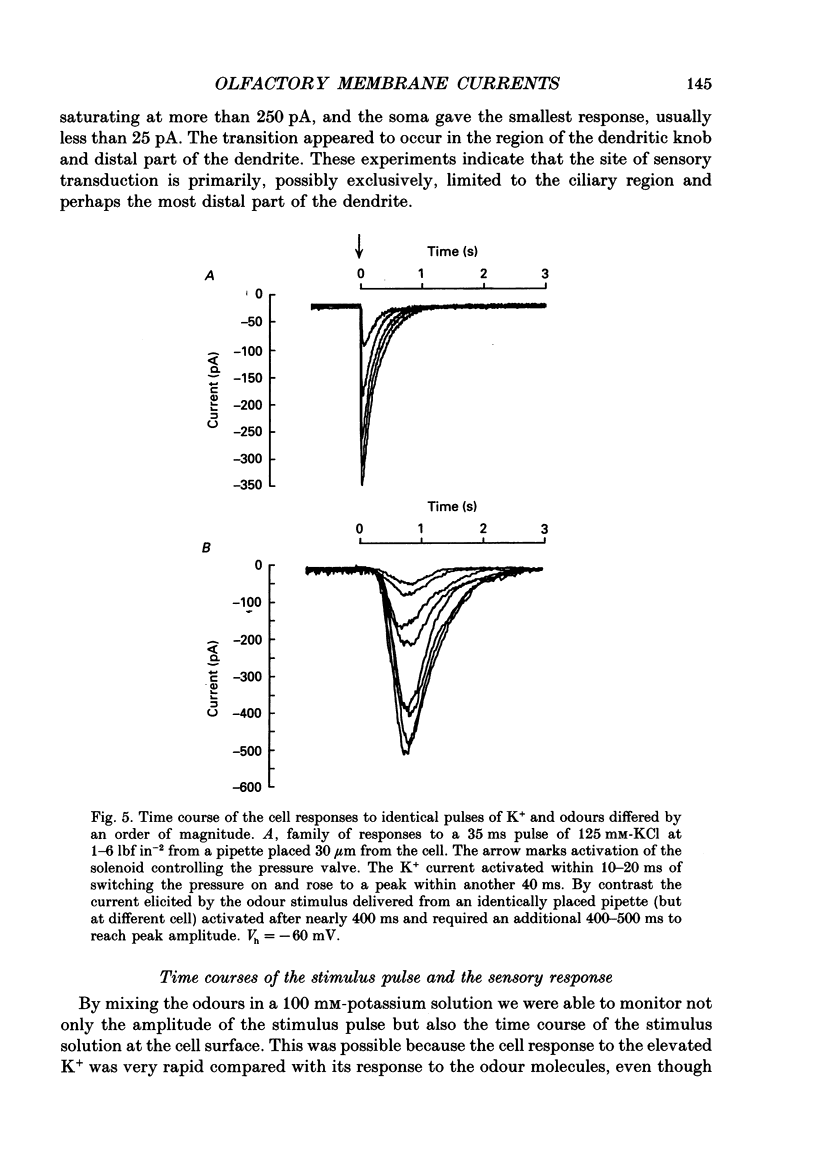
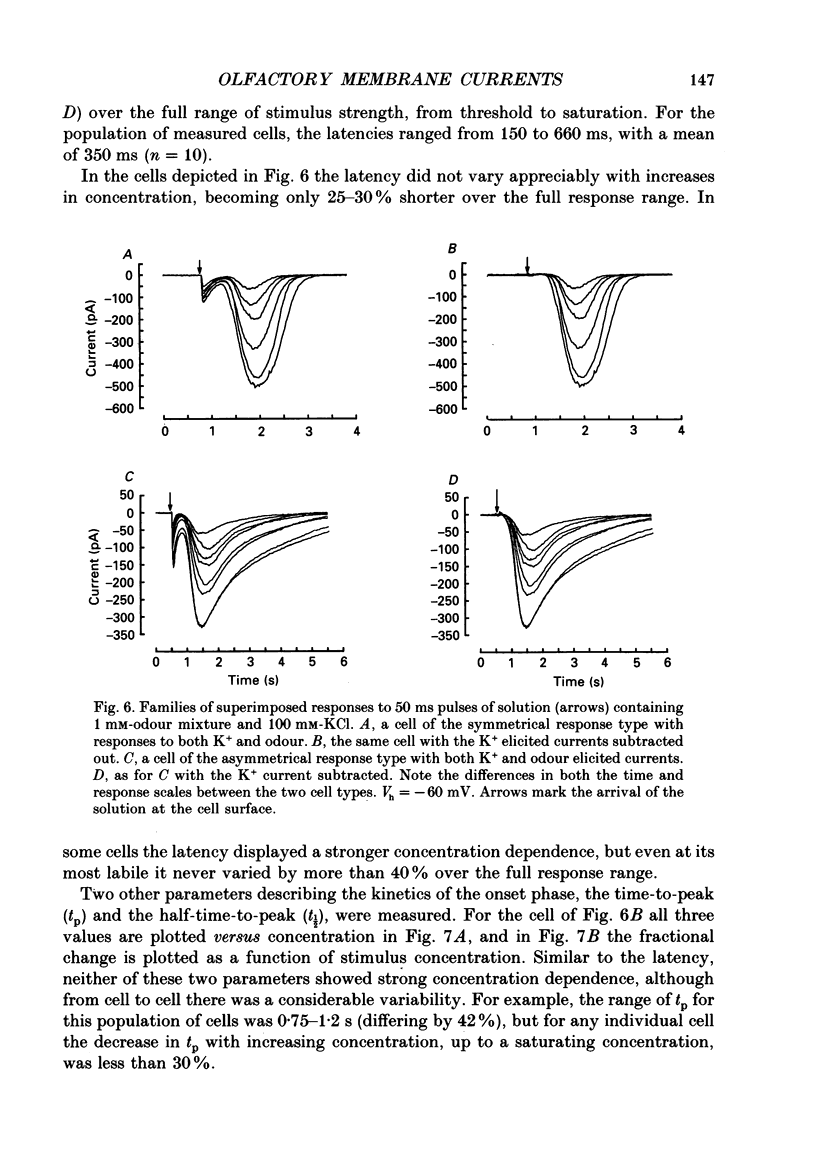
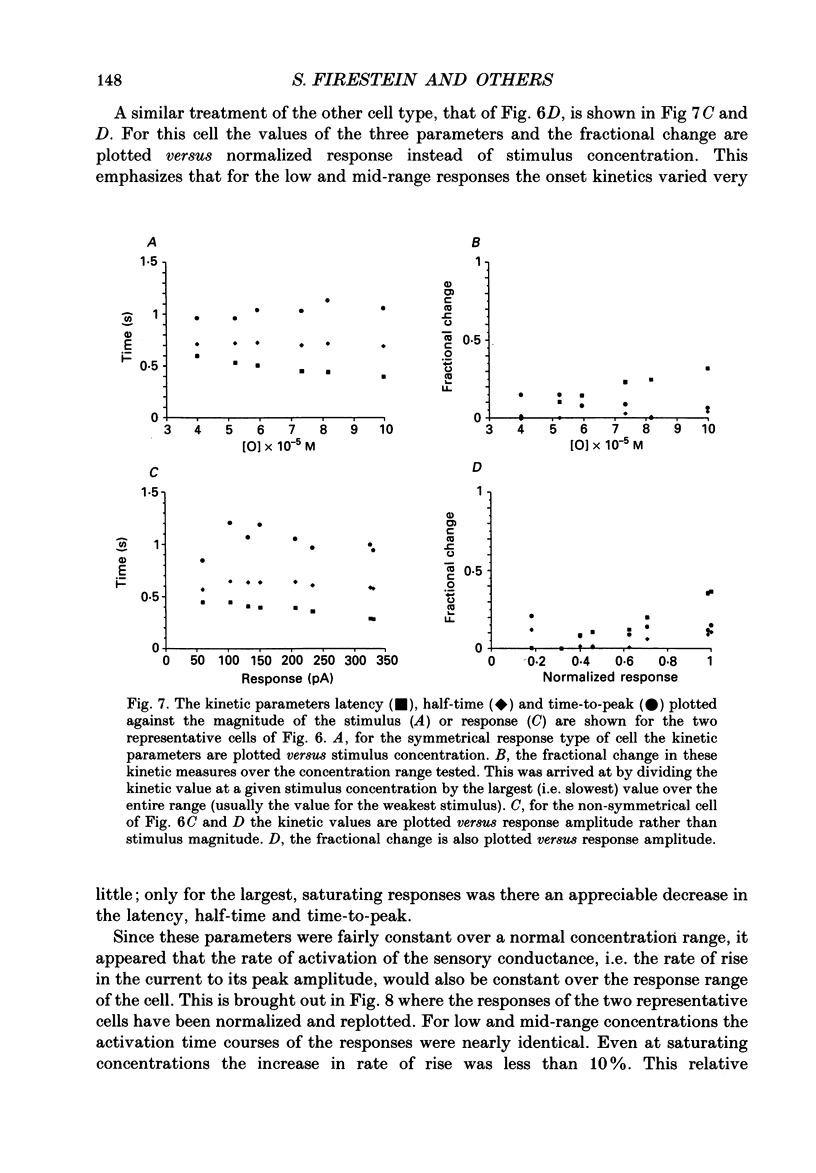
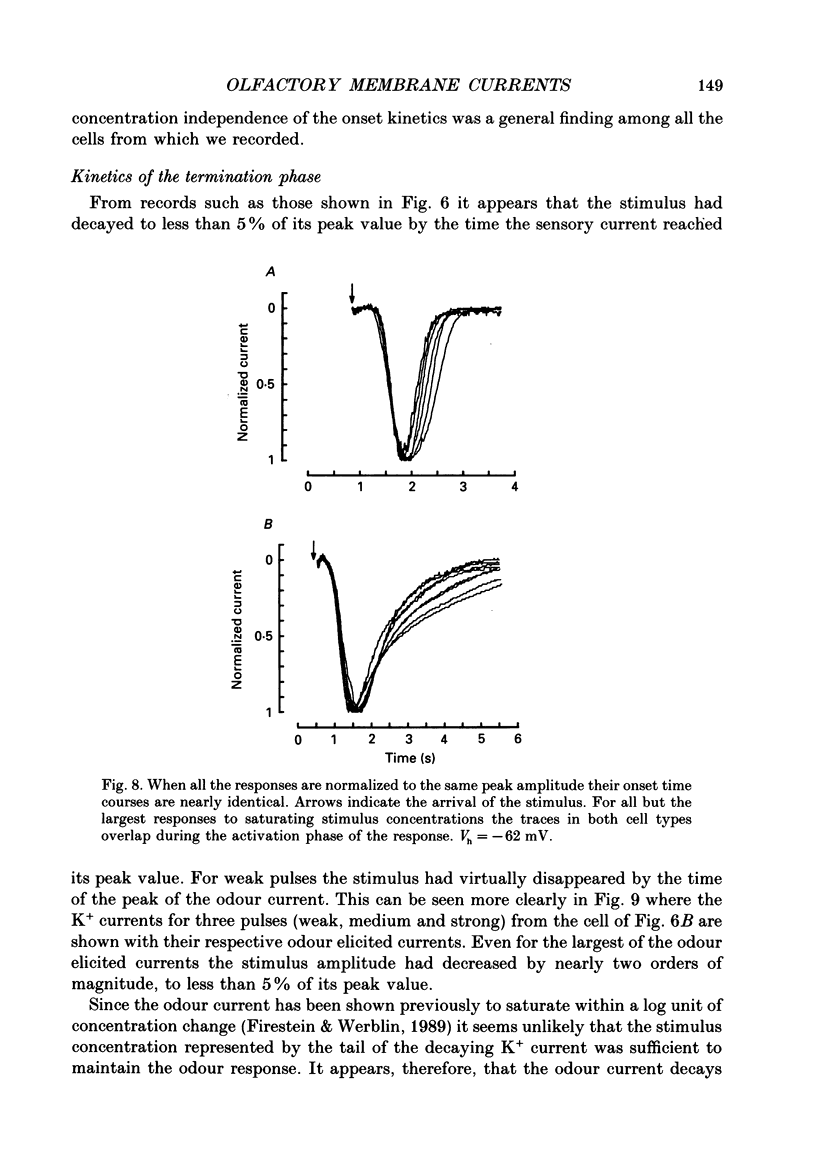
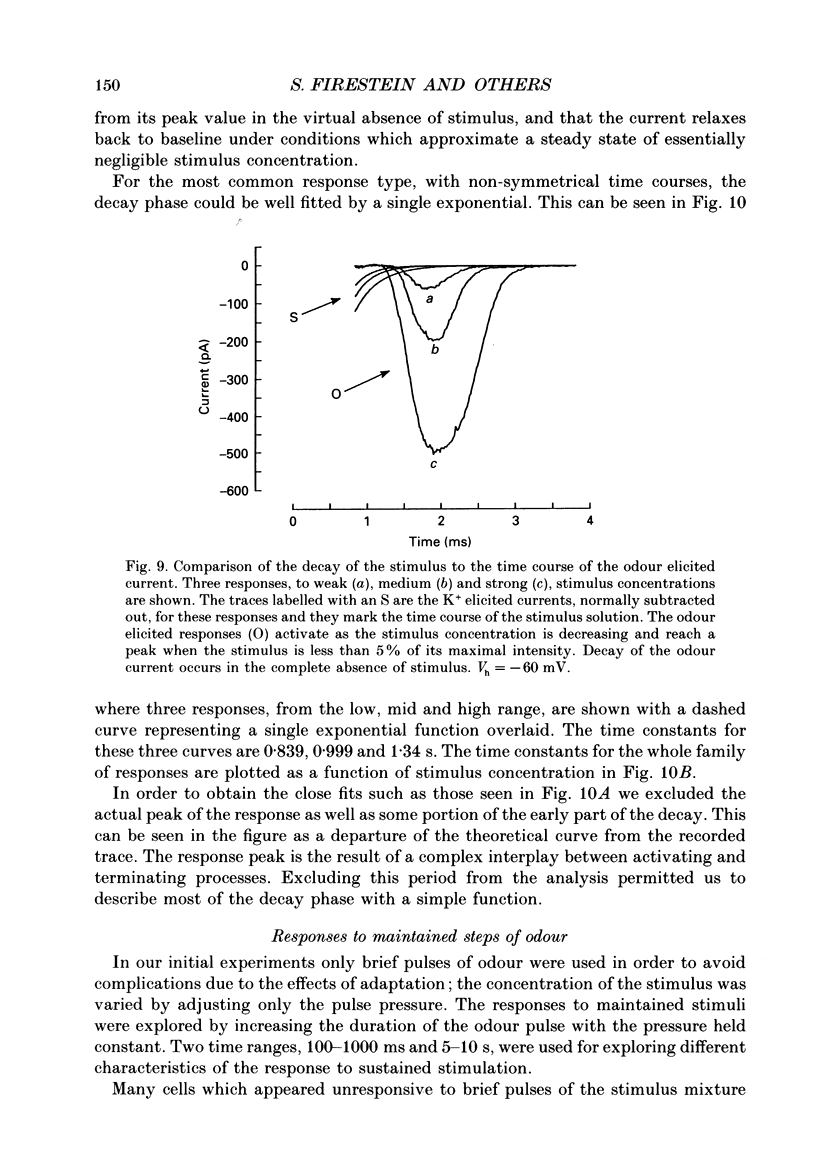
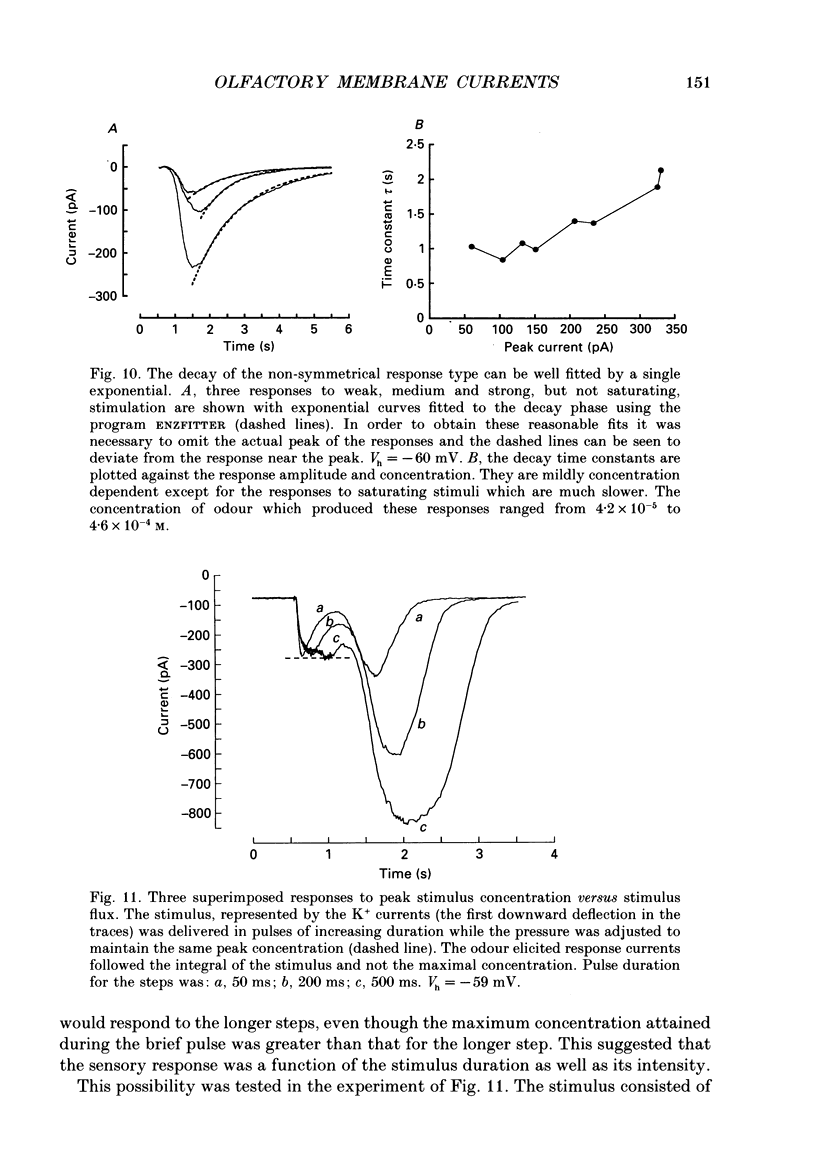
1. Odour elicited currents in freshly isolated olfactory receptor neurones were analysed using the whole-cell patch-clamp technique. Brief pulses (35-50 ms) and steps (100 ms-5 s) of odour solution were delivered by pressure ejection from a nearby micropipette. 2. Pulses of odour solution directed at the cell induced an inward depolarizing current of 50-750 pA leading to the generation of action potentials. The I-V relation for this current was linear over the range -60-(+)20 mV and showed a reversal potential of +5 mV. The magnitude of the current increased with stimulus strength, for a given pulse duration, over approximately one decade of concentration change. 3. Pulses of odour solution focally delivered to the cilia elicited a large response, but those directed toward the soma did not. Conversely pulses of K+ solution at the cilia failed to evoke any response while those directed at the dendrite and soma elicited an inward clamp current. This provides direct evidence that odour sensitivity is localized mainly to the cilia and possibly the distal dendrite. 4. The odour elicited current activated with a long latency of 150-600 ms after the odour solution arrived at the cell. This latency, as well as the time-to-peak and the rise half-time, were relatively independent of stimulus concentration, changing less than 25% over the entire concentration range of stimulus sensitivity. These observations are consistent with the participation of a second messenger system in olfactory transduction. 5. For brief stimulus pulses less than 100 ms, the stimulus diffused away before the odour response current reached its peak value, so that the peak and decay of the odour response occurred in the absence of significant odour stimulus. The time course of the current decay was fitted by a single exponential with a time constant that was concentration dependent, varying from 0.8 to 1.3 s. 6. For longer steps of stimulus presentation, up to 1 s, the magnitude of the response current became a function of the duration of the pulse as well as the stimulus concentration, indicating that the transduction process involved an integrating step. This is consistent with the idea that the odour elicited current is the result of the summation of many smaller unitary events. From responses to weak stimulation an integration period of 700-1000 ms was calculated. 7. During prolonged steps of maintained stimulus presentation (greater than 5 s) the odour elicited current was transient.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader C. R., Macleish P. R., Schwartz E. A. A voltage-clamp study of the light response in solitary rods of the tiger salamander. J Physiol. 1979 Nov;296:1–26. doi: 10.1113/jphysiol.1979.sp012988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973 Oct;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Nunn B. J., Schnapf J. L. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J Physiol. 1984 Dec;357:575–607. doi: 10.1113/jphysiol.1984.sp015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein S., Werblin F. S. Gated currents in isolated olfactory receptor neurons of the larval tiger salamander. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6292–6296. doi: 10.1073/pnas.84.17.6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein S., Werblin F. Odor-induced membrane currents in vertebrate-olfactory receptor neurons. Science. 1989 Apr 7;244(4900):79–82. doi: 10.1126/science.2704991. [DOI] [PubMed] [Google Scholar]
- Getchell T. V., Margolis F. L., Getchell M. L. Perireceptor and receptor events in vertebrate olfaction. Prog Neurobiol. 1984;23(4):317–345. doi: 10.1016/0301-0082(84)90008-x. [DOI] [PubMed] [Google Scholar]
- Getchell T. V., Shepherd G. M. Adaptive properties of olfactory receptors analysed with odour pulses of varying durations. J Physiol. 1978 Sep;282:541–560. doi: 10.1113/jphysiol.1978.sp012480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getchell T. V., Shepherd G. M. Responses of olfactory receptor cells to step pulses of odour at different concentrations in the salamander. J Physiol. 1978 Sep;282:521–540. doi: 10.1113/jphysiol.1978.sp012479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth A. J. Extracellular current flow and the site of transduction by vertebrate hair cells. J Neurosci. 1982 Jan;2(1):1–10. doi: 10.1523/JNEUROSCI.02-01-00001.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth A. J., Lewis R. S. Kinetic analysis of voltage- and ion-dependent conductances in saccular hair cells of the bull-frog, Rana catesbeiana. J Physiol. 1988 Jun;400:237–274. doi: 10.1113/jphysiol.1988.sp017119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. T., Reed R. R. Golf: an olfactory neuron specific-G protein involved in odorant signal transduction. Science. 1989 May 19;244(4906):790–795. doi: 10.1126/science.2499043. [DOI] [PubMed] [Google Scholar]
- Kurahashi T. Activation by odorants of cation-selective conductance in the olfactory receptor cell isolated from the newt. J Physiol. 1989 Dec;419:177–192. doi: 10.1113/jphysiol.1989.sp017868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancet D. Vertebrate olfactory reception. Annu Rev Neurosci. 1986;9:329–355. doi: 10.1146/annurev.ne.09.030186.001553. [DOI] [PubMed] [Google Scholar]
- Levitzki A. From epinephrine to cyclic AMP. Science. 1988 Aug 12;241(4867):800–806. doi: 10.1126/science.2841758. [DOI] [PubMed] [Google Scholar]
- Lewis R. S., Hudspeth A. J. Voltage- and ion-dependent conductances in solitary vertebrate hair cells. Nature. 1983 Aug 11;304(5926):538–541. doi: 10.1038/304538a0. [DOI] [PubMed] [Google Scholar]
- Masukawa L. M., Hedlund B., Shepherd G. M. Electrophysiological properties of identified cells in the in vitro olfactory epithelium of the tiger salamander. J Neurosci. 1985 Jan;5(1):128–135. doi: 10.1523/JNEUROSCI.05-01-00128.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menco B. P. Qualitative and quantitative freeze-fracture studies on olfactory and nasal respiratory epithelial surfaces of frog, ox, rat, and dog. II. Cell apices, cilia, and microvilli. Cell Tissue Res. 1980;211(1):5–29. doi: 10.1007/BF00233719. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Gold G. H. A cyclic nucleotide-gated conductance in olfactory receptor cilia. 1987 Jan 29-Feb 4Nature. 325(6103):442–444. doi: 10.1038/325442a0. [DOI] [PubMed] [Google Scholar]
- Pace U., Hanski E., Salomon Y., Lancet D. Odorant-sensitive adenylate cyclase may mediate olfactory reception. Nature. 1985 Jul 18;316(6025):255–258. doi: 10.1038/316255a0. [DOI] [PubMed] [Google Scholar]
- Peachey L. D. The sarcoplasmic reticulum and transverse tubules of the frog's sartorius. J Cell Biol. 1965 Jun;25(3 Suppl):209–231. doi: 10.1083/jcb.25.3.209. [DOI] [PubMed] [Google Scholar]
- Rafols J. A., Getchell T. V. Morphological relations between the receptor neurons, sustentacular cells and Schwann cells in the olfactory mucosa of the salamander. Anat Rec. 1983 May;206(1):87–101. doi: 10.1002/ar.1092060111. [DOI] [PubMed] [Google Scholar]
- Sicard G., Holley A. Receptor cell responses to odorants: similarities and differences among odorants. Brain Res. 1984 Feb 6;292(2):283–296. doi: 10.1016/0006-8993(84)90764-9. [DOI] [PubMed] [Google Scholar]
- Simmons P. A., Getchell T. V. Neurogenesis in olfactory epithelium: loss and recovery of transepithelial voltage transients following olfactory nerve section. J Neurophysiol. 1981 Mar;45(3):516–528. doi: 10.1152/jn.1981.45.3.516. [DOI] [PubMed] [Google Scholar]
- Sklar P. B., Anholt R. R., Snyder S. H. The odorant-sensitive adenylate cyclase of olfactory receptor cells. Differential stimulation by distinct classes of odorants. J Biol Chem. 1986 Nov 25;261(33):15538–15543. [PubMed] [Google Scholar]
- TUCKER D. Physical variables in the olfactory stimulation process. J Gen Physiol. 1963 Jan;46:453–489. doi: 10.1085/jgp.46.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotier D. A patch-clamp analysis of membrane currents in salamander olfactory receptor cells. Pflugers Arch. 1986 Dec;407(6):589–595. doi: 10.1007/BF00582636. [DOI] [PubMed] [Google Scholar]
- Trotier D., MacLeod P. Intracellular recordings from salamander olfactory receptor cells. Brain Res. 1983 Jun 6;268(2):225–237. doi: 10.1016/0006-8993(83)90488-2. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Baylor D. A. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]