Abstract
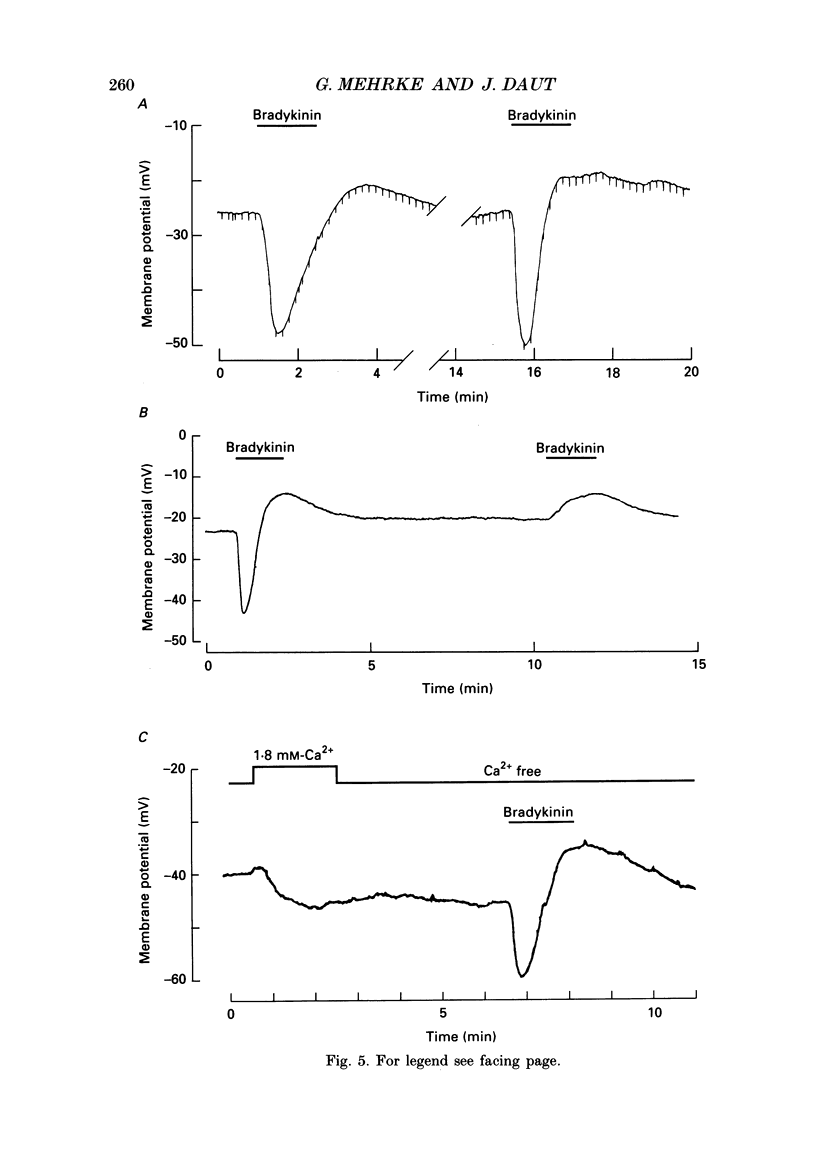
1. Primary cultures of coronary endothelial cells were obtained by enzymatic dispersion of isolated guinea-pig hearts and separation of different cardiac cell types by density gradient centrifugation. The cells were grown to confluency and the membrane potential of the monolayer was recorded using the whole-cell-clamp mode of the patch-clamp technique. 2. When superfused with physiological salt solution at 37 degrees C the average resting potential of the monolayers was -35 +/- 9 mV. A 2 min application of bradykinin (0.1-20 nM) induced a transient hyperpolarization of up to 40 mV (median 33 mV), which was followed by a sustained depolarization of up to 28 mV (median 10 mV). The average duration of the hyperpolarization, measured midway between the resting potential and peak negativity, was 48 s with 20 nM-bradykinin. 3. The concentration of bradykinin producing a half-maximal hyperpolarization was 2.5 nM. When high concentrations of bradykinin (greater than 10 nM) were applied for several minutes voltage oscillations of low amplitude with periodicity of 2-3 min were observed. 4. The peak of the hyperpolarization depended on the extracellular potassium concentration ([K+]o). The limiting slope of the relation between membrane potential and log [K+]o was 52 mV per 10-fold change in [K+]o. With 50 mM [K+]o the hyperpolarization was abolished and with 100 mM [K+]o the hyperpolarization turned into a depolarization. 5. After removal of external Ca2+ the first transient hyperpolarization elicited by bradykinin had the same amplitude as under control conditions, but its duration was reduced to about 72%. The second application of bradykinin in Ca2(+)-free solution produced only a depolarization. The hyperpolarizing response to bradykinin could be re-primed by exposing the preparation to Ca2(+)-containing solution for 2 min. 6. The transient hyperpolarization elicited by 4 nM-bradykinin could be inhibited by d-tubocurarine, a blocker of Ca2(+)-activated potassium channels. On average, 1 mM-tubocurarine reduced the hyperpolarization by 49 +/- 18%. Apamin (10 microM) reduced the hyperpolarization by 15 +/- 11%. 7. ATP (1 microM) produced a hyperpolarization of similar amplitude to that produced by bradykinin, but of shorter duration (average 29 s), and a very small (less than 5 mV) or no sustained depolarization. Histamine (10 microM) produced an even shorter transient hyperpolarization, followed by a depolarization of up to 15 mV. 8. Most of the monolayers of coronary endothelial cells responded to adenosine in a similar way as to bradykinin.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Barakeh J., Laskey R., Van Breemen C. Ion channels and regulation of intracellular calcium in vascular endothelial cells. FASEB J. 1989 Oct;3(12):2389–2400. doi: 10.1096/fasebj.3.12.2477294. [DOI] [PubMed] [Google Scholar]
- Alkon D. L., Rasmussen H. A spatial-temporal model of cell activation. Science. 1988 Feb 26;239(4843):998–1005. doi: 10.1126/science.2830669. [DOI] [PubMed] [Google Scholar]
- Boeynaems J. M., Pirotton S., Van Coevorden A., Raspe E., Demolle D., Erneux C. P2-purinergic receptors in vascular endothelial cells: from concept to reality. J Recept Res. 1988;8(1-4):121–132. doi: 10.3109/10799898809048982. [DOI] [PubMed] [Google Scholar]
- Bolton T. B., Clapp L. H. Endothelial-dependent relaxant actions of carbachol and substance P in arterial smooth muscle. Br J Pharmacol. 1986 Apr;87(4):713–723. doi: 10.1111/j.1476-5381.1986.tb14589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayden J. E., Wellman G. C. Endothelium-dependent dilation of feline cerebral arteries: role of membrane potential and cyclic nucleotides. J Cereb Blood Flow Metab. 1989 Jun;9(3):256–263. doi: 10.1038/jcbfm.1989.42. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Higashida H. Inositol 1,4,5-trisphosphate and diacylglycerol mimic bradykinin effects on mouse neuroblastoma x rat glioma hybrid cells. J Physiol. 1988 Mar;397:185–207. doi: 10.1113/jphysiol.1988.sp016995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Higashida H. Membrane current responses of NG108-15 mouse neuroblastoma x rat glioma hybrid cells to bradykinin. J Physiol. 1988 Mar;397:167–184. doi: 10.1113/jphysiol.1988.sp016994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R., Fichtner H., Lückhoff A., Kohlhardt M. Hyperpolarization and increased free calcium in acetylcholine-stimulated endothelial cells. Am J Physiol. 1988 Oct;255(4 Pt 2):H965–H969. doi: 10.1152/ajpheart.1988.255.4.H965. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Sage S. O. Bradykinin-evoked changes in cytosolic calcium and membrane currents in cultured bovine pulmonary artery endothelial cells. J Physiol. 1989 Dec;419:555–568. doi: 10.1113/jphysiol.1989.sp017886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Suzuki H. Some electrical properties of the endothelium-dependent hyperpolarization recorded from rat arterial smooth muscle cells. J Physiol. 1989 Mar;410:91–106. doi: 10.1113/jphysiol.1989.sp017522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daut J., Maier-Rudolph W., von Beckerath N., Mehrke G., Günther K., Goedel-Meinen L. Hypoxic dilation of coronary arteries is mediated by ATP-sensitive potassium channels. Science. 1990 Mar 16;247(4948):1341–1344. doi: 10.1126/science.2107575. [DOI] [PubMed] [Google Scholar]
- Daut J., Mehrke G., Nees S., Newman W. H. Passive electrical properties and electrogenic sodium transport of cultured guinea-pig coronary endothelial cells. J Physiol. 1988 Aug;402:237–254. doi: 10.1113/jphysiol.1988.sp017202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derian C. K., Moskowitz M. A. Polyphosphoinositide hydrolysis in endothelial cells and carotid artery segments. Bradykinin-2 receptor stimulation is calcium-independent. J Biol Chem. 1986 Mar 15;261(8):3831–3837. [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Newby A. C., Lewis M. J., Henderson A. H. Production of endothelium derived relaxant factor is dependent on oxidative phosphorylation and extracellular calcium. Cardiovasc Res. 1986 Jan;20(1):7–12. doi: 10.1093/cvr/20.1.7. [DOI] [PubMed] [Google Scholar]
- Hallam T. J., Jacob R., Merritt J. E. Evidence that agonists stimulate bivalent-cation influx into human endothelial cells. Biochem J. 1988 Oct 1;255(1):179–184. doi: 10.1042/bj2550179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam T. J., Jacob R., Merritt J. E. Influx of bivalent cations can be independent of receptor stimulation in human endothelial cells. Biochem J. 1989 Apr 1;259(1):125–129. doi: 10.1042/bj2590125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J. Biological actions and properties of endothelium-derived nitric oxide formed and released from artery and vein. Circ Res. 1989 Jul;65(1):1–21. doi: 10.1161/01.res.65.1.1. [DOI] [PubMed] [Google Scholar]
- Jacob R. Agonist-stimulated divalent cation entry into single cultured human umbilical vein endothelial cells. J Physiol. 1990 Feb;421:55–77. doi: 10.1113/jphysiol.1990.sp017933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R., Merritt J. E., Hallam T. J., Rink T. J. Repetitive spikes in cytoplasmic calcium evoked by histamine in human endothelial cells. Nature. 1988 Sep 1;335(6185):40–45. doi: 10.1038/335040a0. [DOI] [PubMed] [Google Scholar]
- Johns A., Lategan T. W., Lodge N. J., Ryan U. S., Van Breemen C., Adams D. J. Calcium entry through receptor-operated channels in bovine pulmonary artery endothelial cells. Tissue Cell. 1987;19(6):733–745. doi: 10.1016/0040-8166(87)90015-2. [DOI] [PubMed] [Google Scholar]
- Katsuki S., Arnold W., Mittal C., Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res. 1977 Feb;3(1):23–35. [PubMed] [Google Scholar]
- Lambert T. L., Kent R. S., Whorton A. R. Bradykinin stimulation of inositol polyphosphate production in porcine aortic endothelial cells. J Biol Chem. 1986 Nov 15;261(32):15288–15293. [PubMed] [Google Scholar]
- Lückhoff A., Zeh R., Busse R. Desensitization of the bradykinin-induced rise in intracellular free calcium in cultured endothelial cells. Pflugers Arch. 1988 Oct;412(6):654–658. doi: 10.1007/BF00583768. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Standen N. B., Brayden J. E., Worley J. F., 3rd Noradrenaline contracts arteries by activating voltage-dependent calcium channels. Nature. 1988 Nov 24;336(6197):382–385. doi: 10.1038/336382a0. [DOI] [PubMed] [Google Scholar]
- Olesen S. P., Davies P. F., Clapham D. E. Muscarinic-activated K+ current in bovine aortic endothelial cells. Circ Res. 1988 Jun;62(6):1059–1064. doi: 10.1161/01.res.62.6.1059. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pennefather P., Lancaster B., Adams P. R., Nicoll R. A. Two distinct Ca-dependent K currents in bullfrog sympathetic ganglion cells. Proc Natl Acad Sci U S A. 1985 May;82(9):3040–3044. doi: 10.1073/pnas.82.9.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner R., Neher E. The role of calcium in stimulus-secretion coupling in excitable and non-excitable cells. J Exp Biol. 1988 Sep;139:329–345. doi: 10.1242/jeb.139.1.329. [DOI] [PubMed] [Google Scholar]
- Pirotton S., Raspe E., Demolle D., Erneux C., Boeynaems J. M. Involvement of inositol 1,4,5-trisphosphate and calcium in the action of adenine nucleotides on aortic endothelial cells. J Biol Chem. 1987 Dec 25;262(36):17461–17466. [PubMed] [Google Scholar]
- Rhodin J. A. The ultrastructure of mammalian arterioles and precapillary sphincters. J Ultrastruct Res. 1967 Apr;18(1):181–223. doi: 10.1016/s0022-5320(67)80239-9. [DOI] [PubMed] [Google Scholar]
- Rickwood D., Ford T., Graham J. Nycodenz: a new nonionic iodinated gradient medium. Anal Biochem. 1982 Jun;123(1):23–31. doi: 10.1016/0003-2697(82)90618-2. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Jacob R. Calcium oscillations in non-excitable cells. Trends Neurosci. 1989 Feb;12(2):43–46. doi: 10.1016/0166-2236(89)90133-1. [DOI] [PubMed] [Google Scholar]
- Sage S. O., Adams D. J., van Breemen C. Synchronized oscillations in cytoplasmic free calcium concentration in confluent bradykinin-stimulated bovine pulmonary artery endothelial cell monolayers. J Biol Chem. 1989 Jan 5;264(1):6–9. [PubMed] [Google Scholar]
- Sauve R., Parent L., Simoneau C., Roy G. External ATP triggers a biphasic activation process of a calcium-dependent K+ channel in cultured bovine aortic endothelial cells. Pflugers Arch. 1988 Oct;412(5):469–481. doi: 10.1007/BF00582535. [DOI] [PubMed] [Google Scholar]
- Schilling W. P., Rajan L., Strobl-Jager E. Characterization of the bradykinin-stimulated calcium influx pathway of cultured vascular endothelial cells. Saturability, selectivity, and kinetics. J Biol Chem. 1989 Aug 5;264(22):12838–12848. [PubMed] [Google Scholar]
- Schilling W. P., Ritchie A. K., Navarro L. T., Eskin S. G. Bradykinin-stimulated calcium influx in cultured bovine aortic endothelial cells. Am J Physiol. 1988 Aug;255(2 Pt 2):H219–H227. doi: 10.1152/ajpheart.1988.255.2.H219. [DOI] [PubMed] [Google Scholar]
- Spagnoli L. G., Villaschi S., Neri L., Palmieri G. Gap junctions in myo-endothelial bridges of rabbit carotid arteries. Experientia. 1982 Jan 15;38(1):124–125. doi: 10.1007/BF01944566. [DOI] [PubMed] [Google Scholar]
- Taylor S. G., Weston A. H. Endothelium-derived hyperpolarizing factor: a new endogenous inhibitor from the vascular endothelium. Trends Pharmacol Sci. 1988 Aug;9(8):272–274. doi: 10.1016/0165-6147(88)90003-x. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M., Rubanyi G. M., Miller V. M., Houston D. S. Modulation of vascular smooth muscle contraction by the endothelium. Annu Rev Physiol. 1986;48:307–320. doi: 10.1146/annurev.ph.48.030186.001515. [DOI] [PubMed] [Google Scholar]
- Voyta J. C., Via D. P., Butterfield C. E., Zetter B. R. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984 Dec;99(6):2034–2040. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]



