Abstract
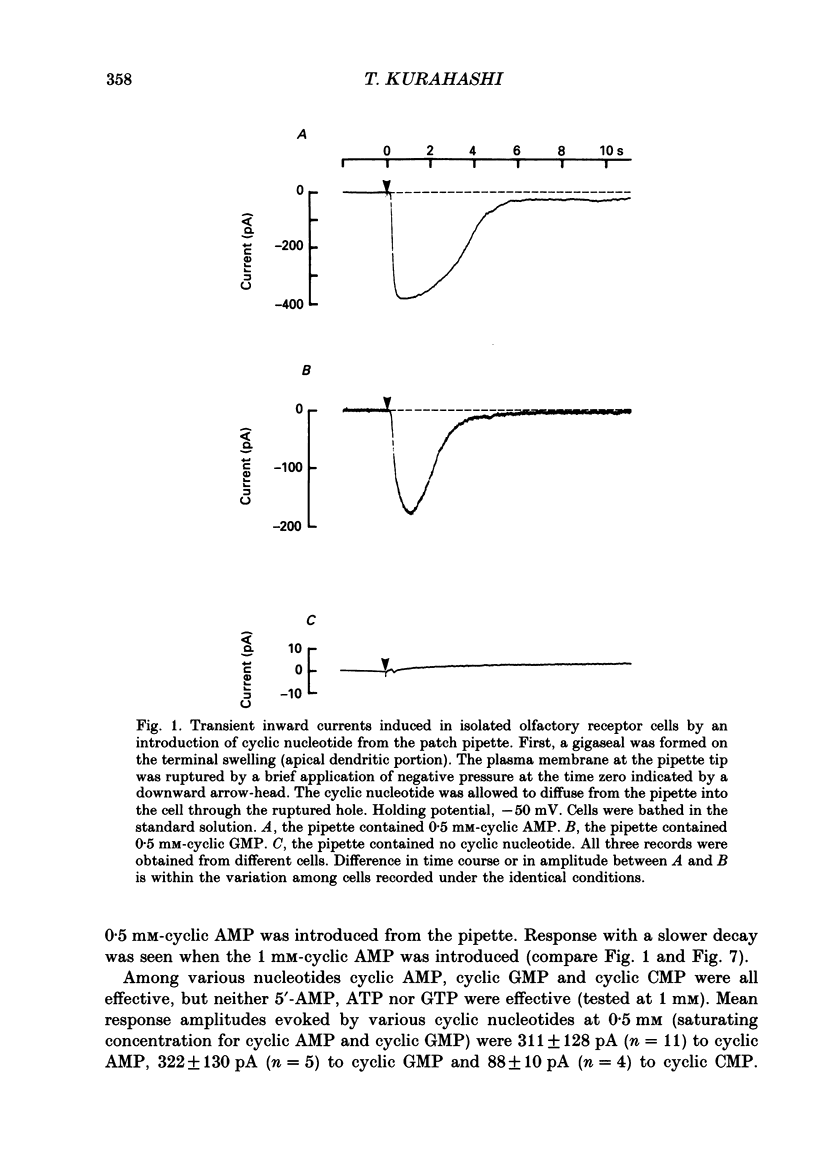
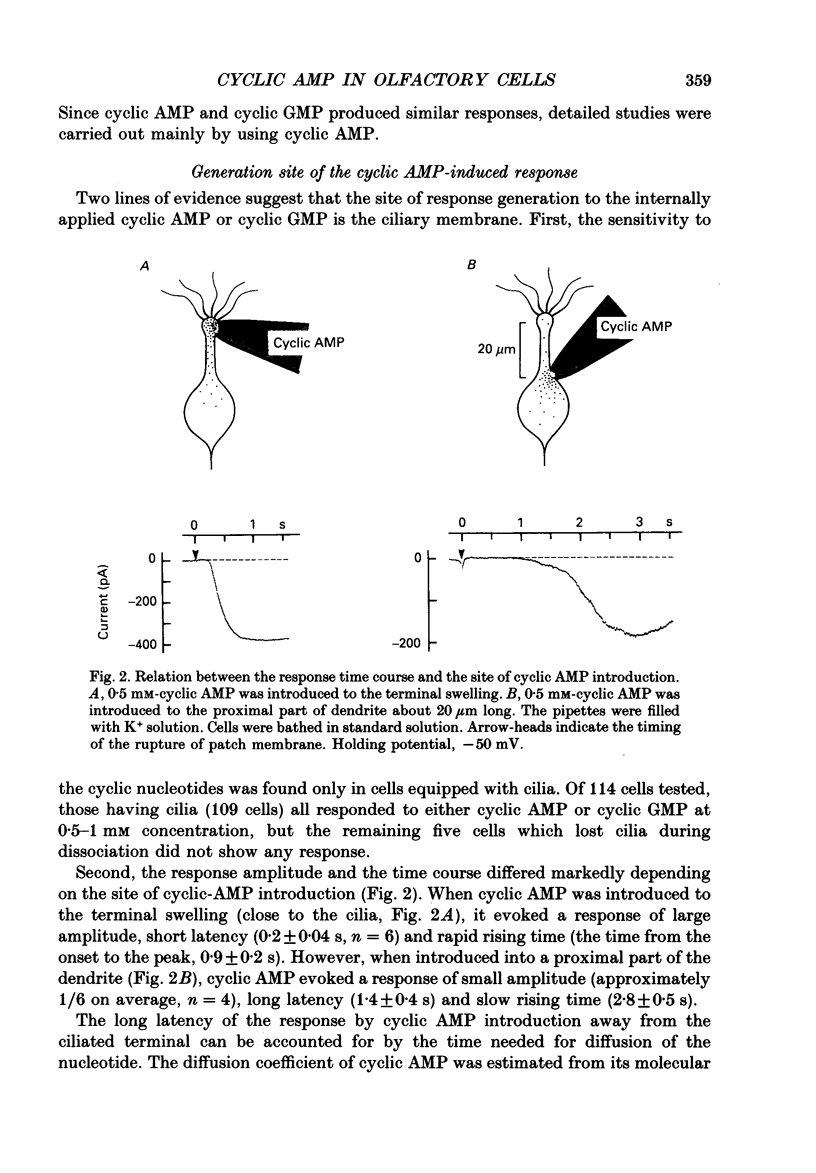
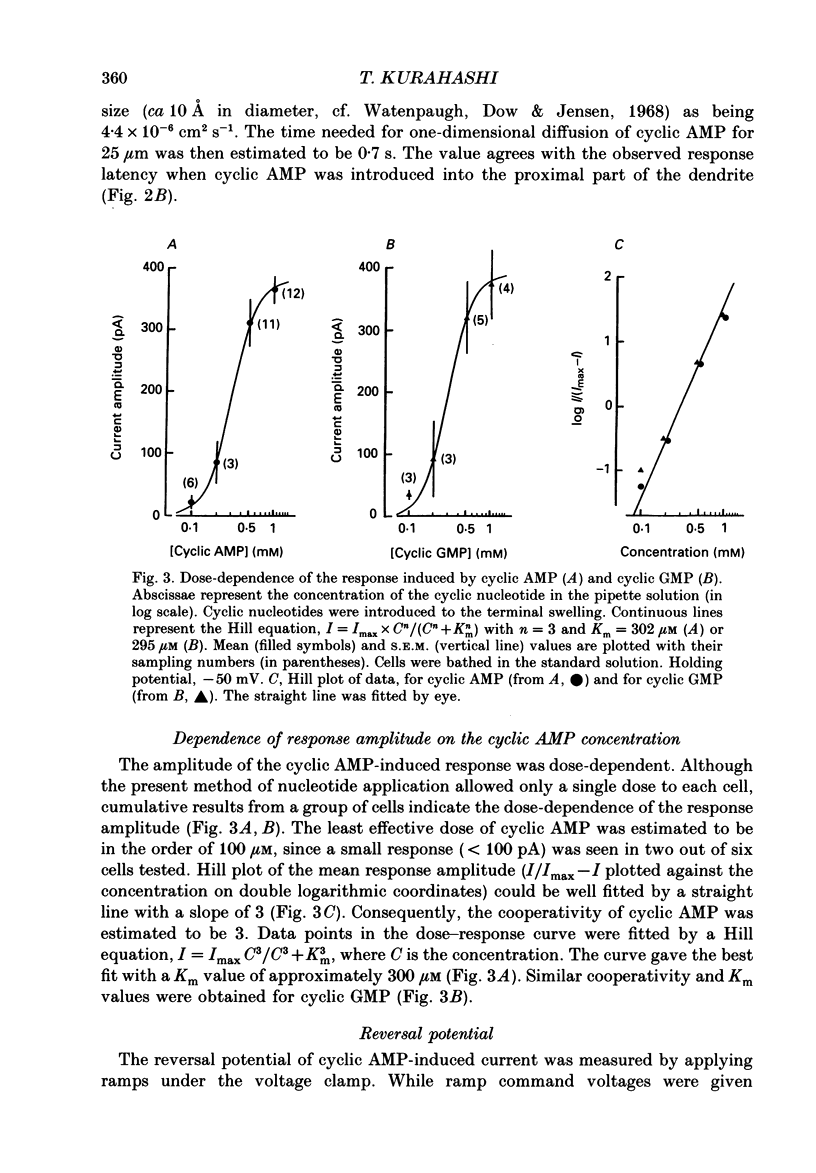
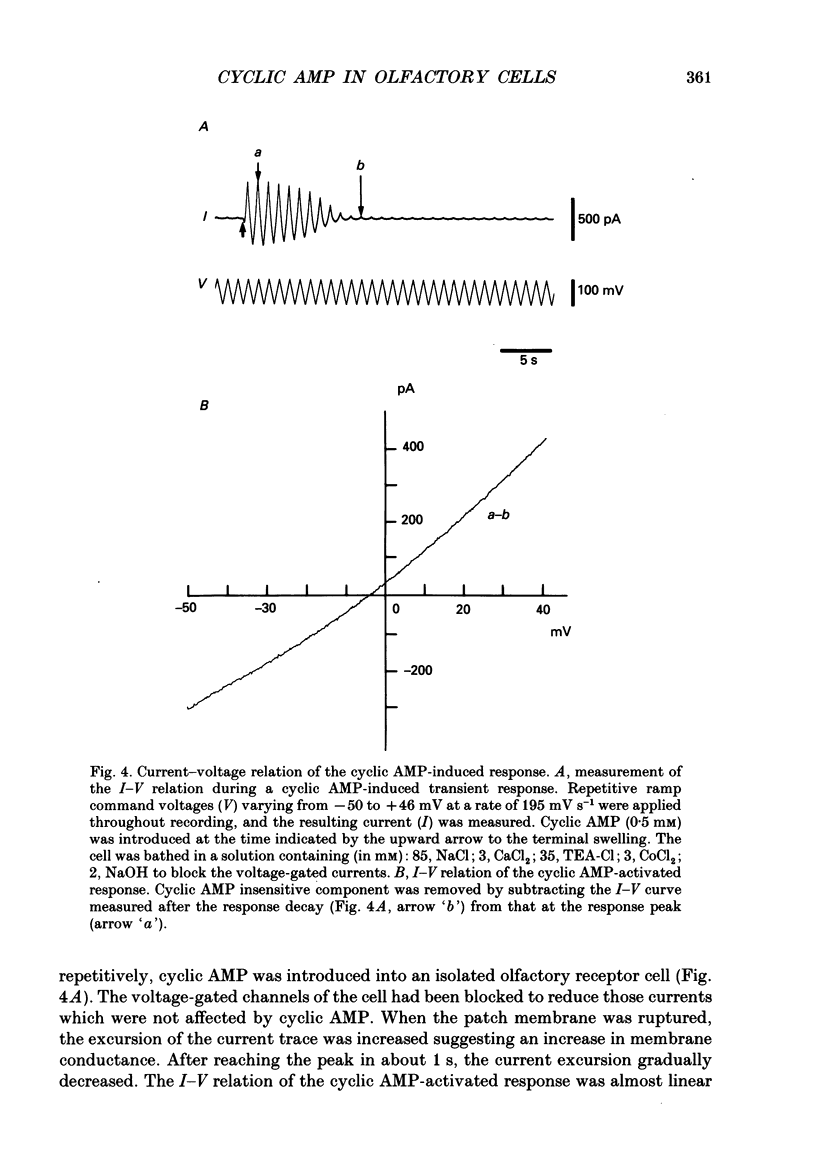
1. Responses induced by intracellular cyclic nucleotides were analysed in isolated olfactory receptor cells of the newt under a voltage-clamp condition by using the patch pipette in a whole-cell recording configuration. Cyclic nucleotides were applied by diffusion from the patch pipette. 2. Introduction of either cyclic AMP or cyclic GMP caused a transient inward current in cells held at -50 mV. The response amplitude was dose-dependent with the Hill coefficient of 3 and half-saturating concentration of 300 microM (concentration in the pipette) for both cyclic AMP and cyclic GMP. Cyclic CMP was less effective than those two nucleotides. 3. The response to intracellular cyclic AMP was seen in all cilia-bearing cells, but not in cells which lost the cilia during dissociation. The response latency was shorter when cyclic AMP was introduced into the ciliated terminal swelling (ca 0.2 s) rather than into the cell body (ca 1.4 s). These results suggest that the sensitivity to intracellular cyclic AMP is confined to the cilia. 4. The cyclic AMP-induced current was transient (half decay time, ca 2.3s) despite the fact that cyclic AMP was continuously loaded from the patch pipette. The response time course was controlled by Ca2+; the reduction of external Ca2+ concentration (replaced with Mg2+) or loading the cell with 50 mM-EGTA prolonged the cyclic AMP-induced responses. The Ca2(+)-induced suppression was reversible. 5. The reversal potential of the cyclic AMP-induced transient current was -4.8 +/- 3.8 mV, and that of the current re-induced by Ca2+ removal was 1.5 +/- 2.1 mV, suggesting that both currents flowed through the same ionic channel. The channel permeates all alkali metal ions with the permeability ratios of PLi:PNa:PK:PRb:PCs = 0.93:1:0.93:0.91:0.72, but not Cl- or choline ions. 6. These results demonstrate that the cyclic AMP-induced response and the odorant-induced response of the isolated olfactory cell have nearly identical characteristics. The present study supports the notion that cyclic AMP is the internal messenger mediating olfactory transduction.
Full text
PDF



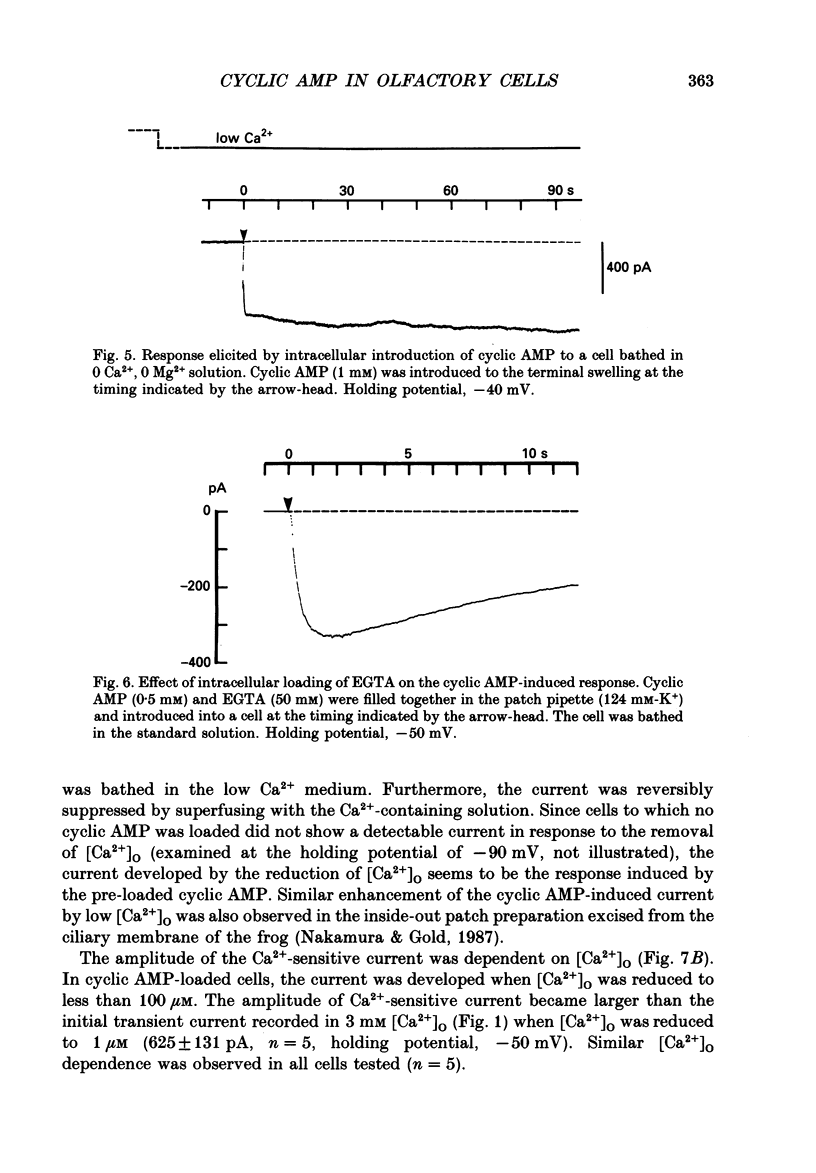
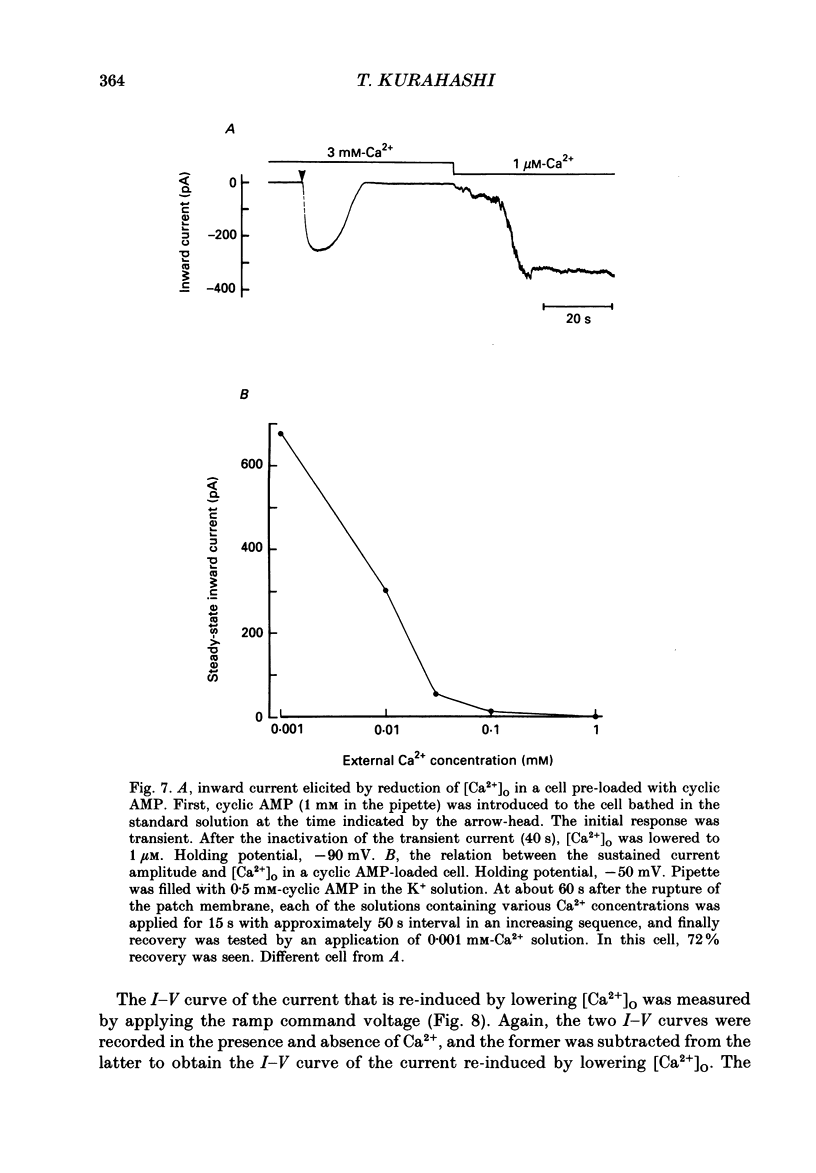





Selected References
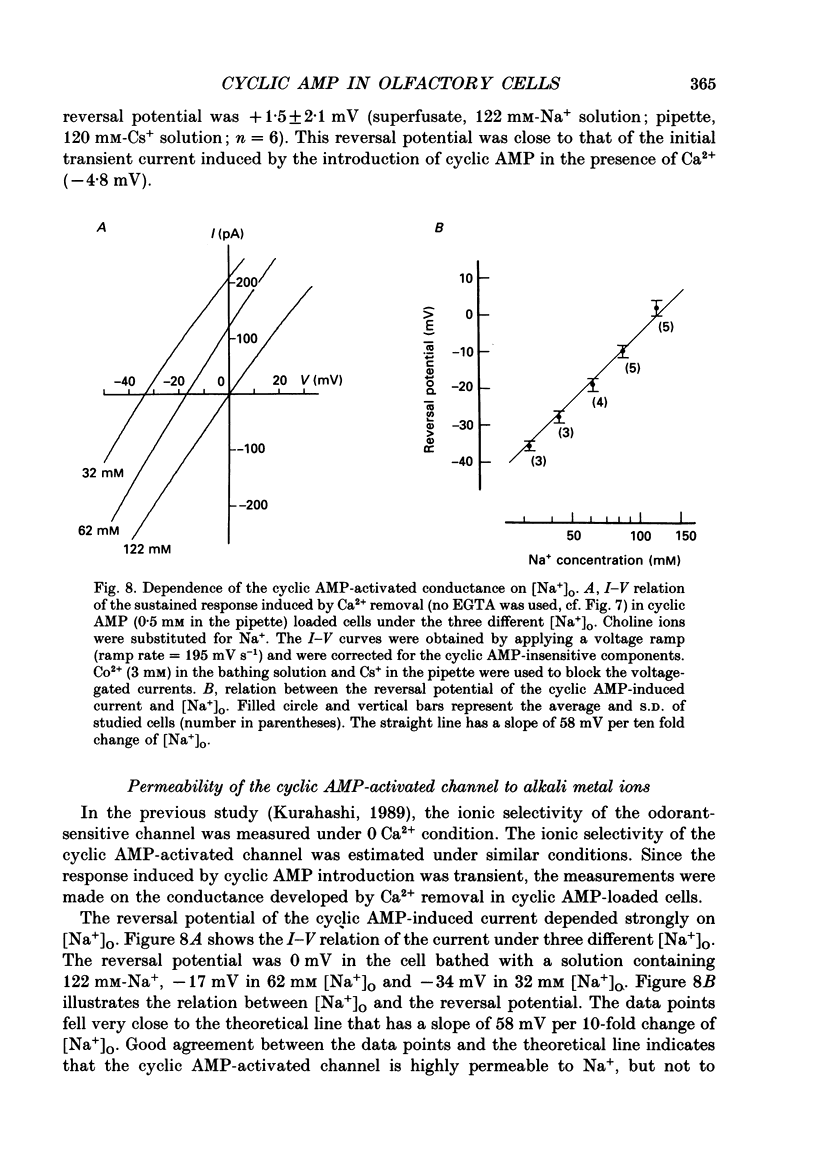
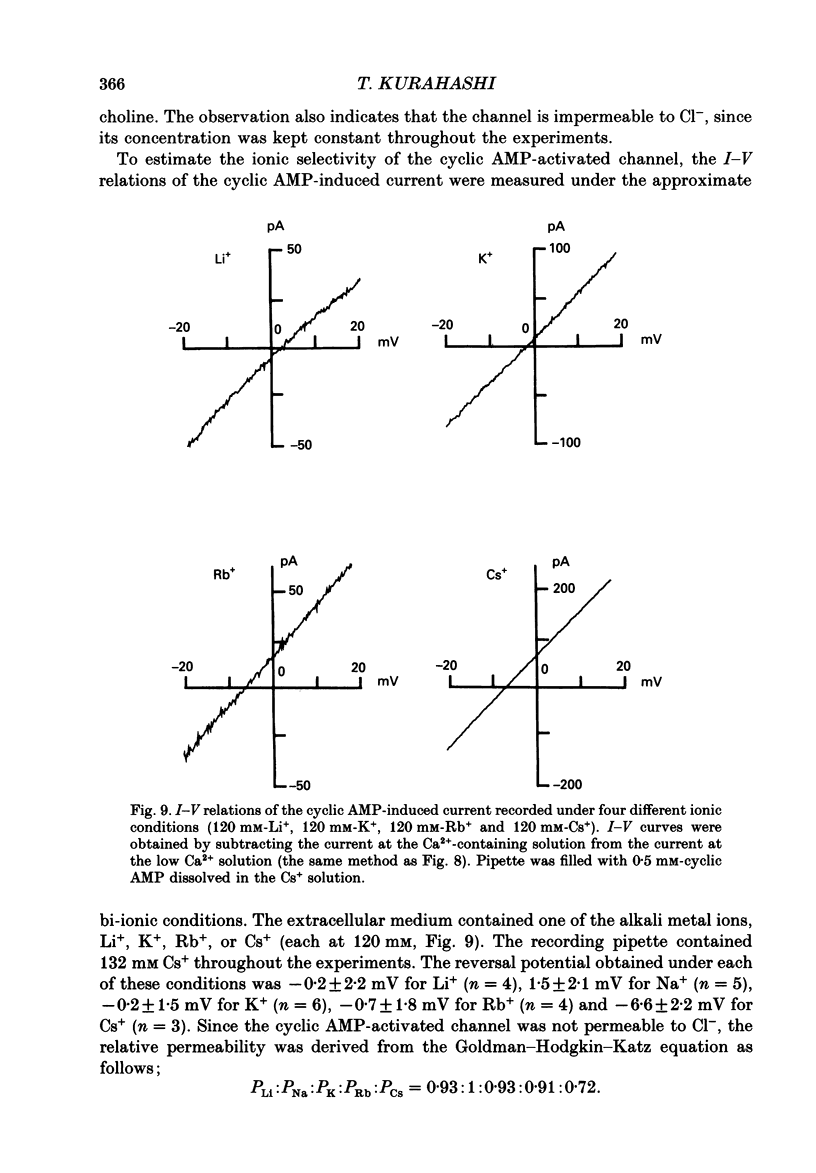
These references are in PubMed. This may not be the complete list of references from this article.
- Adamek G. D., Gesteland R. C., Mair R. G., Oakley B. Transduction physiology of olfactory receptor cilia. Brain Res. 1984 Sep 17;310(1):87–97. doi: 10.1016/0006-8993(84)90012-x. [DOI] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Firestein S., Werblin F. Odor-induced membrane currents in vertebrate-olfactory receptor neurons. Science. 1989 Apr 7;244(4900):79–82. doi: 10.1126/science.2704991. [DOI] [PubMed] [Google Scholar]
- Getchell T. V. Analysis of intracellular recordings from salamander olfactory epithelium. Brain Res. 1977 Mar 11;123(2):275–286. doi: 10.1016/0006-8993(77)90479-6. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Haynes L. W., Kay A. R., Yau K. W. Single cyclic GMP-activated channel activity in excised patches of rod outer segment membrane. Nature. 1986 May 1;321(6065):66–70. doi: 10.1038/321066a0. [DOI] [PubMed] [Google Scholar]
- Haynes L., Yau K. W. Cyclic GMP-sensitive conductance in outer segment membrane of catfish cones. Nature. 1985 Sep 5;317(6032):61–64. doi: 10.1038/317061a0. [DOI] [PubMed] [Google Scholar]
- Karpen J. W., Zimmerman A. L., Stryer L., Baylor D. A. Molecular mechanics of the cyclic-GMP-activated channel of retinal rods. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 1):325–332. doi: 10.1101/sqb.1988.053.01.039. [DOI] [PubMed] [Google Scholar]
- Kurahashi T. Activation by odorants of cation-selective conductance in the olfactory receptor cell isolated from the newt. J Physiol. 1989 Dec;419:177–192. doi: 10.1113/jphysiol.1989.sp017868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi T., Shibuya T. Ca2(+)-dependent adaptive properties in the solitary olfactory receptor cell of the newt. Brain Res. 1990 May 7;515(1-2):261–268. doi: 10.1016/0006-8993(90)90605-b. [DOI] [PubMed] [Google Scholar]
- Lowe G., Nakamura T., Gold G. H. Adenylate cyclase mediates olfactory transduction for a wide variety of odorants. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5641–5645. doi: 10.1073/pnas.86.14.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews D. F. Response patterns of single neurons in the tortoise olfactory epithelium and olfactory bulb. J Gen Physiol. 1972 Aug;60(2):166–180. doi: 10.1085/jgp.60.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G. Comparison of the light-sensitive and cyclic GMP-sensitive conductances of the rod photoreceptor: noise characteristics. J Neurosci. 1986 Sep;6(9):2521–2526. doi: 10.1523/JNEUROSCI.06-09-02521.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G., Watanabe S. Activation of single ion channels from toad retinal rod inner segments by cyclic GMP: concentration dependence. J Physiol. 1988 Sep;403:389–405. doi: 10.1113/jphysiol.1988.sp017255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H. R., Murphy R. L., Fain G. L., Lamb T. D. Photoreceptor light adaptation is mediated by cytoplasmic calcium concentration. Nature. 1988 Jul 7;334(6177):67–69. doi: 10.1038/334067a0. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Gold G. H. A cyclic nucleotide-gated conductance in olfactory receptor cilia. 1987 Jan 29-Feb 4Nature. 325(6103):442–444. doi: 10.1038/325442a0. [DOI] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Calcium and light adaptation in retinal rods and cones. Nature. 1988 Jul 7;334(6177):69–71. doi: 10.1038/334069a0. [DOI] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Guanosine 3',5'-cyclic monophosphate-activated conductance studied in a truncated rod outer segment of the toad. J Physiol. 1988 Jan;395:731–753. doi: 10.1113/jphysiol.1988.sp016943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace U., Hanski E., Salomon Y., Lancet D. Odorant-sensitive adenylate cyclase may mediate olfactory reception. Nature. 1985 Jul 18;316(6025):255–258. doi: 10.1038/316255a0. [DOI] [PubMed] [Google Scholar]
- Pace U., Lancet D. Olfactory GTP-binding protein: signal-transducing polypeptide of vertebrate chemosensory neurons. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4947–4951. doi: 10.1073/pnas.83.13.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeuffer E., Mollner S., Lancet D., Pfeuffer T. Olfactory adenylyl cyclase. Identification and purification of a novel enzyme form. J Biol Chem. 1989 Nov 5;264(31):18803–18807. [PubMed] [Google Scholar]
- Rhein L. D., Cagan R. H. Biochemical studies of olfaction: isolation, characterization, and odorant binding activity of cilia from rainbow trout olfactory rosettes. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4412–4416. doi: 10.1073/pnas.77.8.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson P. R., Kawamura S., Abramson B., Bownds M. D. Control of the cyclic GMP phosphodiesterase of frog photoreceptor membranes. J Gen Physiol. 1980 Nov;76(5):631–645. doi: 10.1085/jgp.76.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley S. G., Robinson C. J., Dickinson K., Aujla R., Dodd G. H. Olfactory adenylate cyclase of the rat. Stimulation by odorants and inhibition by Ca2+. Biochem J. 1986 Dec 1;240(2):605–607. doi: 10.1042/bj2400605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard G., Holley A. Receptor cell responses to odorants: similarities and differences among odorants. Brain Res. 1984 Feb 6;292(2):283–296. doi: 10.1016/0006-8993(84)90764-9. [DOI] [PubMed] [Google Scholar]
- Sklar P. B., Anholt R. R., Snyder S. H. The odorant-sensitive adenylate cyclase of olfactory receptor cells. Differential stimulation by distinct classes of odorants. J Biol Chem. 1986 Nov 25;261(33):15538–15543. [PubMed] [Google Scholar]
- Suzuki S., Tachibana M., Kaneko A. Effects of glycine and GABA on isolated bipolar cells of the mouse retina. J Physiol. 1990 Feb;421:645–662. doi: 10.1113/jphysiol.1990.sp017967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotier D. A patch-clamp analysis of membrane currents in salamander olfactory receptor cells. Pflugers Arch. 1986 Dec;407(6):589–595. doi: 10.1007/BF00582636. [DOI] [PubMed] [Google Scholar]
- Trotier D., MacLeod P. Intracellular recordings from salamander olfactory receptor cells. Brain Res. 1983 Jun 6;268(2):225–237. doi: 10.1016/0006-8993(83)90488-2. [DOI] [PubMed] [Google Scholar]
- Watenpaugh K., Dow J., Jensen L. H., Furberg S. Crystal and molecular structure of adenosine 3',5'-cyclic phosphate. Science. 1968 Jan 12;159(3811):206–207. doi: 10.1126/science.159.3811.206. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. L., Baylor D. A. Cyclic GMP-sensitive conductance of retinal rods consists of aqueous pores. Nature. 1986 May 1;321(6065):70–72. doi: 10.1038/321070a0. [DOI] [PubMed] [Google Scholar]


