Abstract
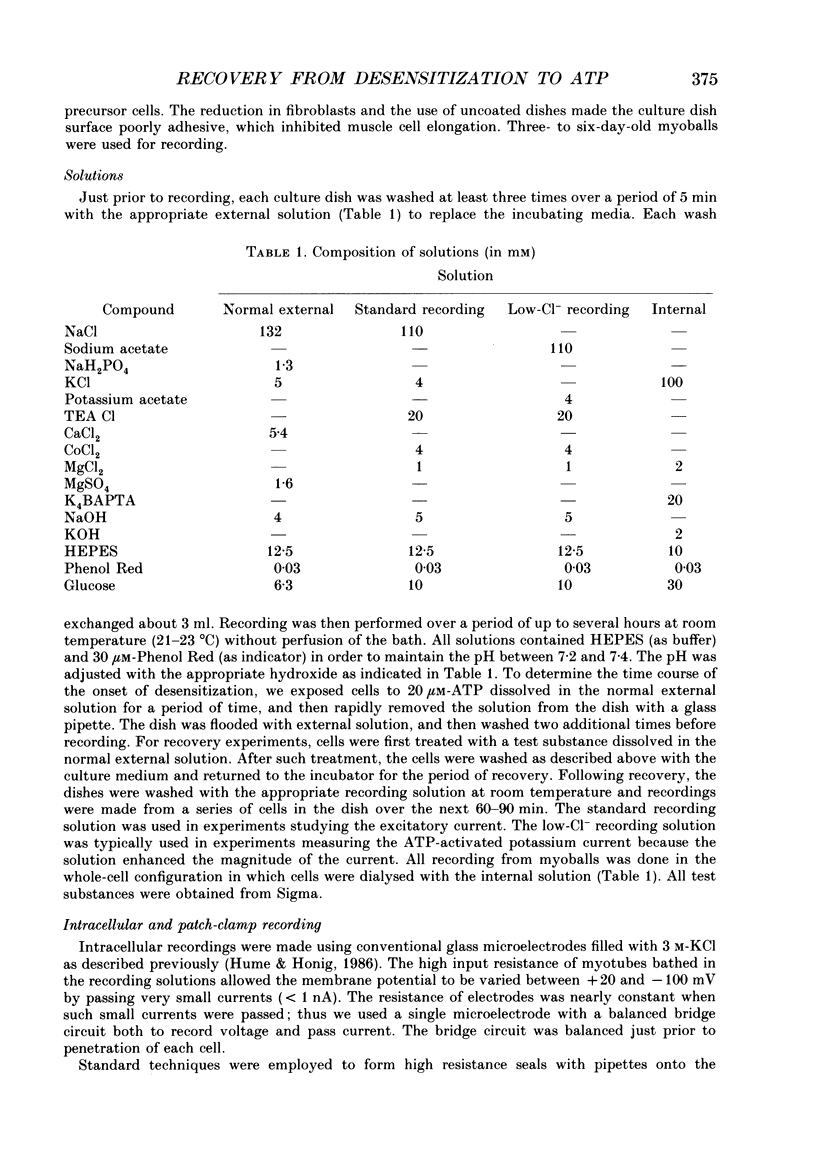
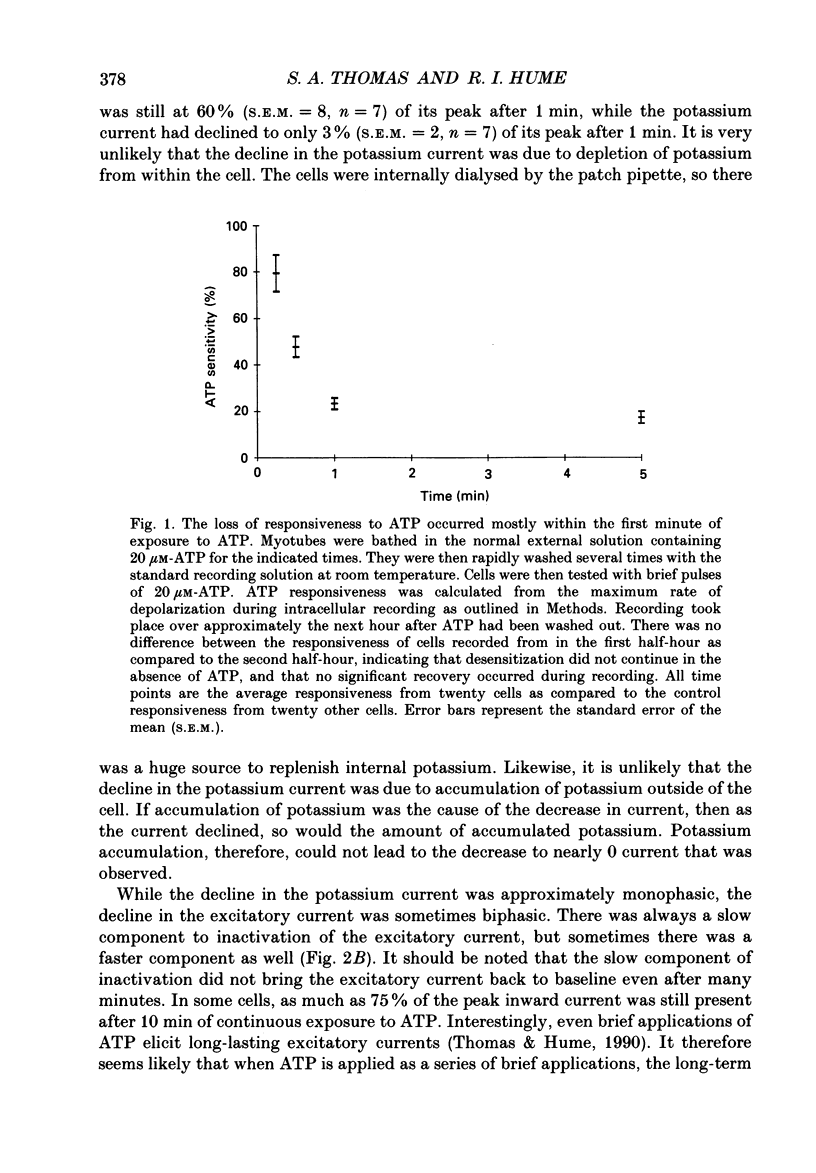
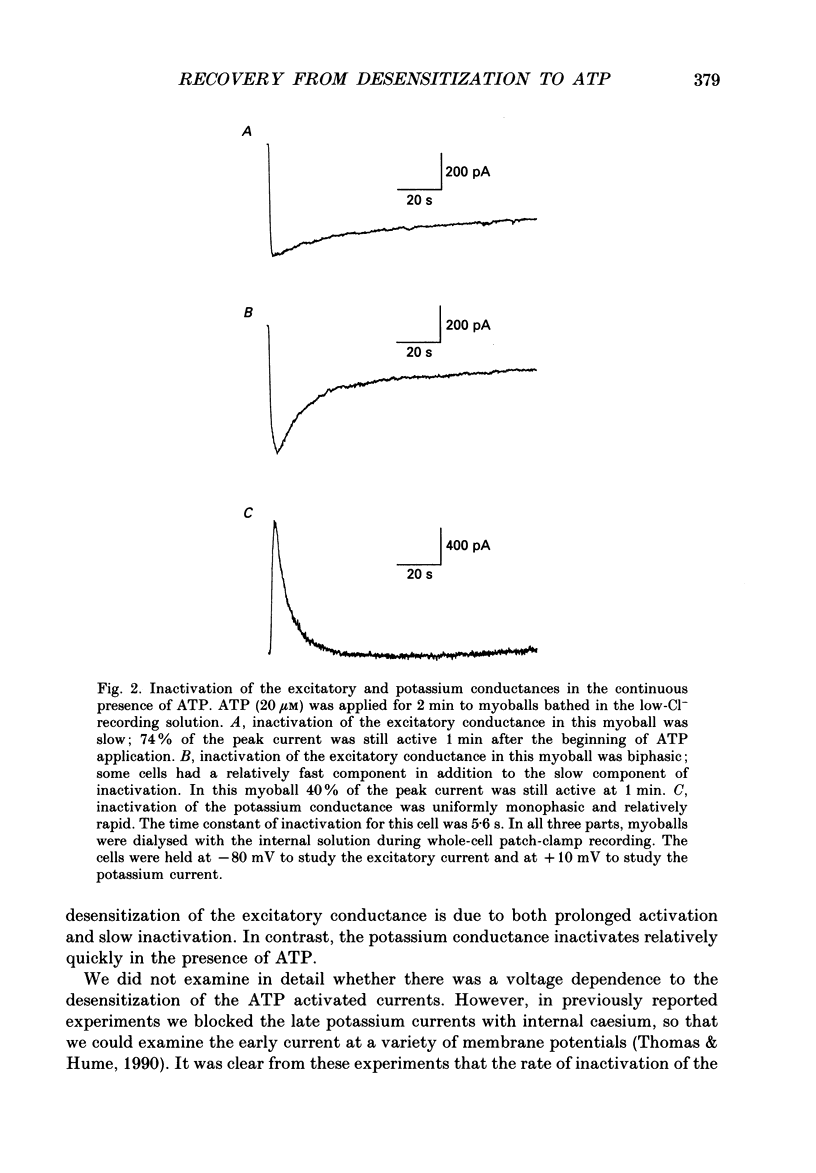
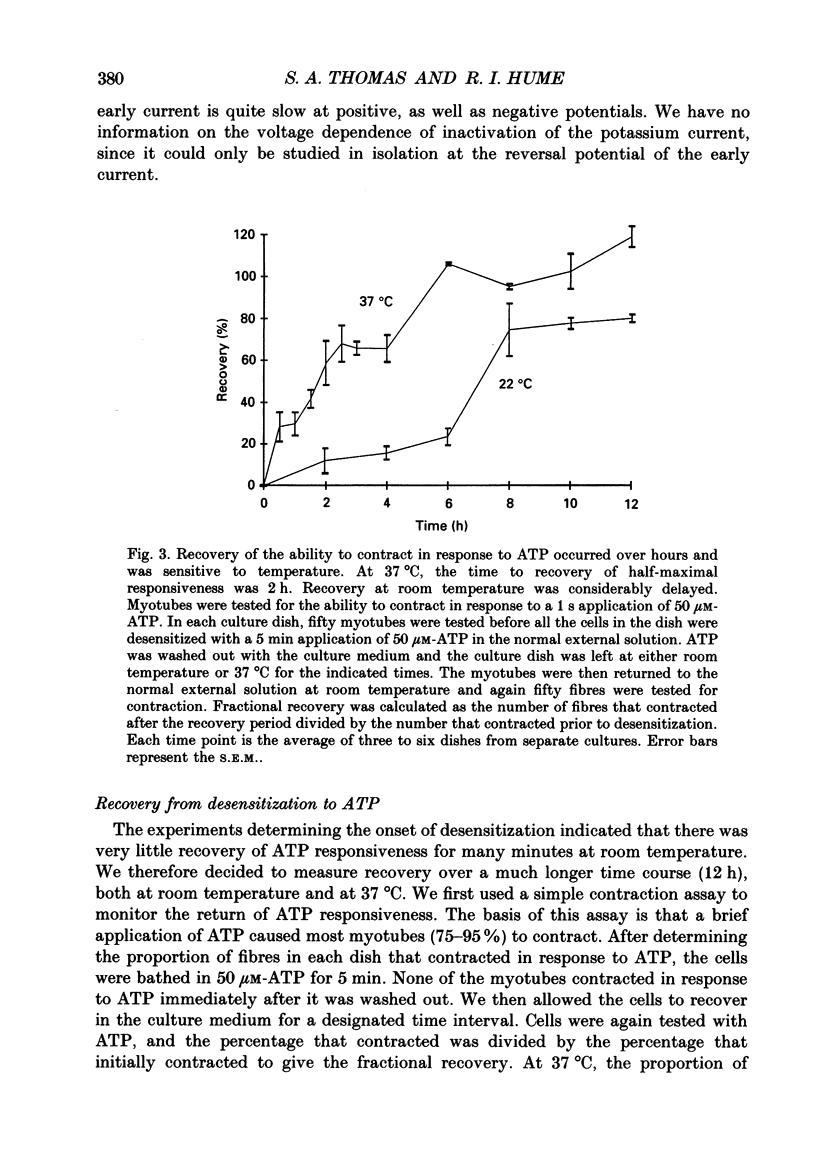
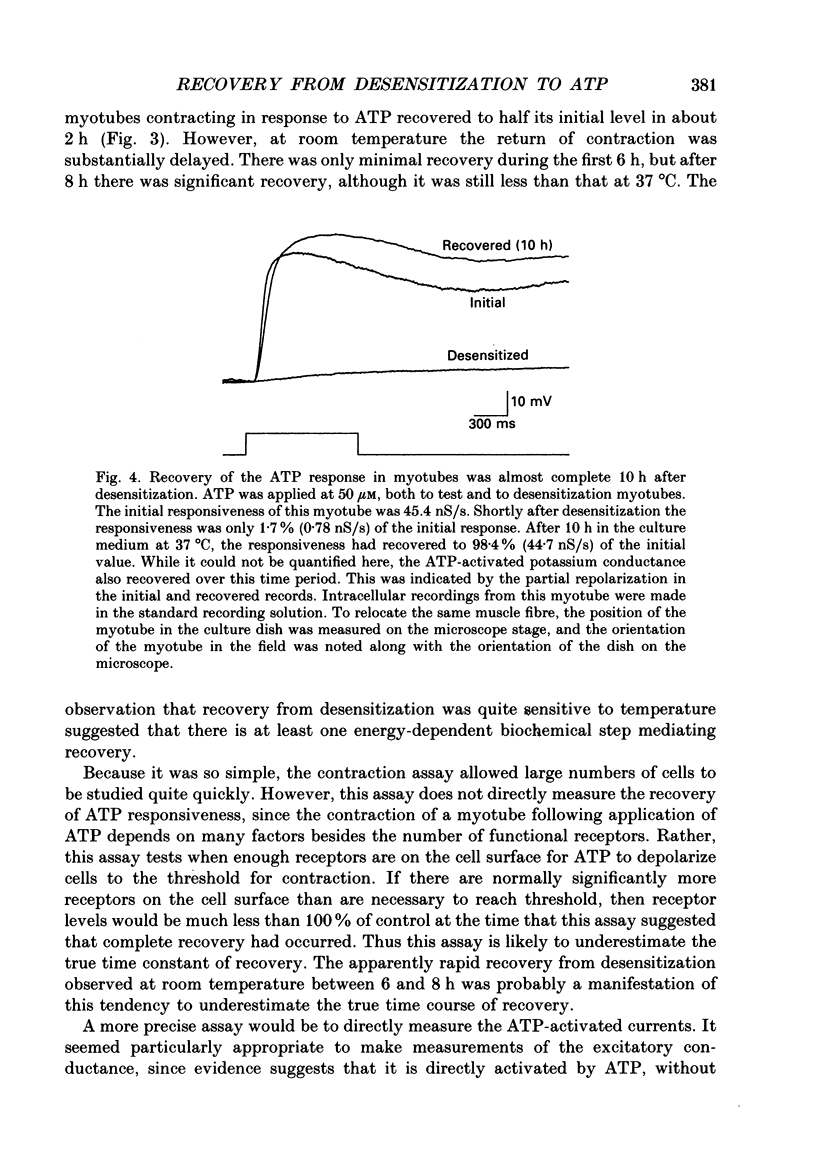
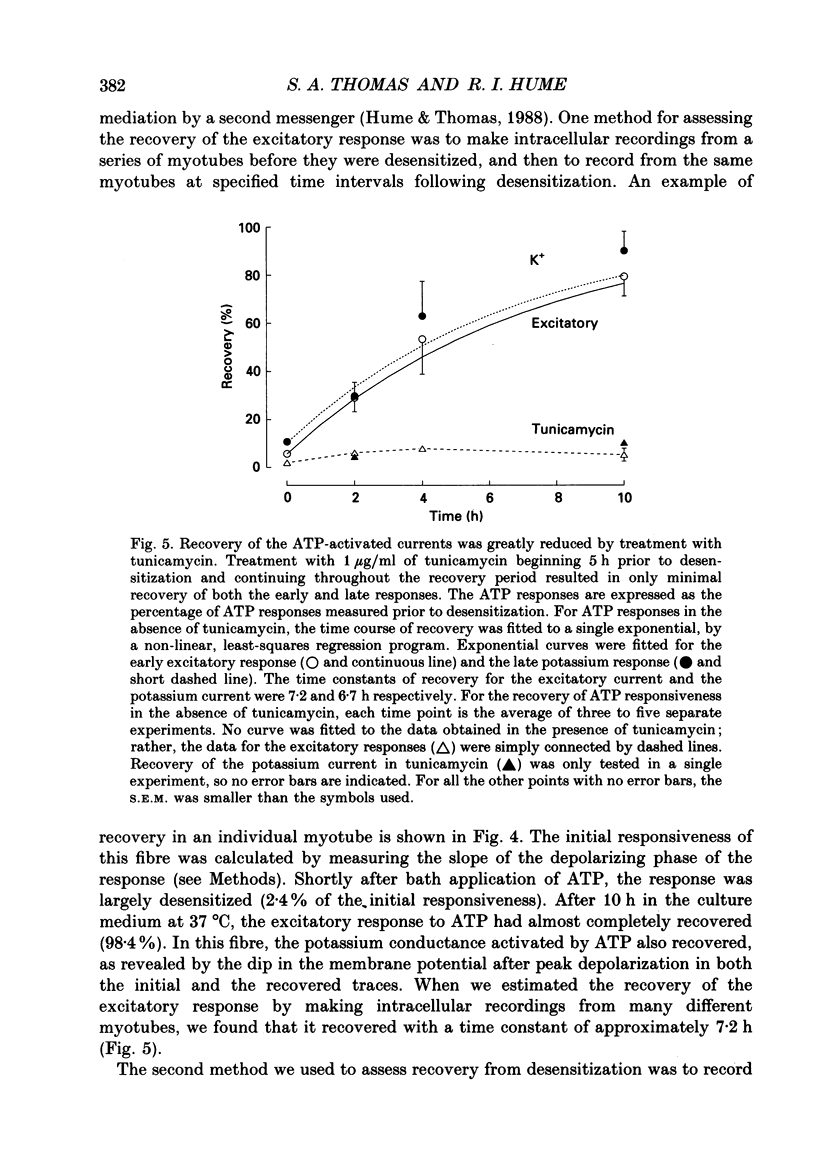
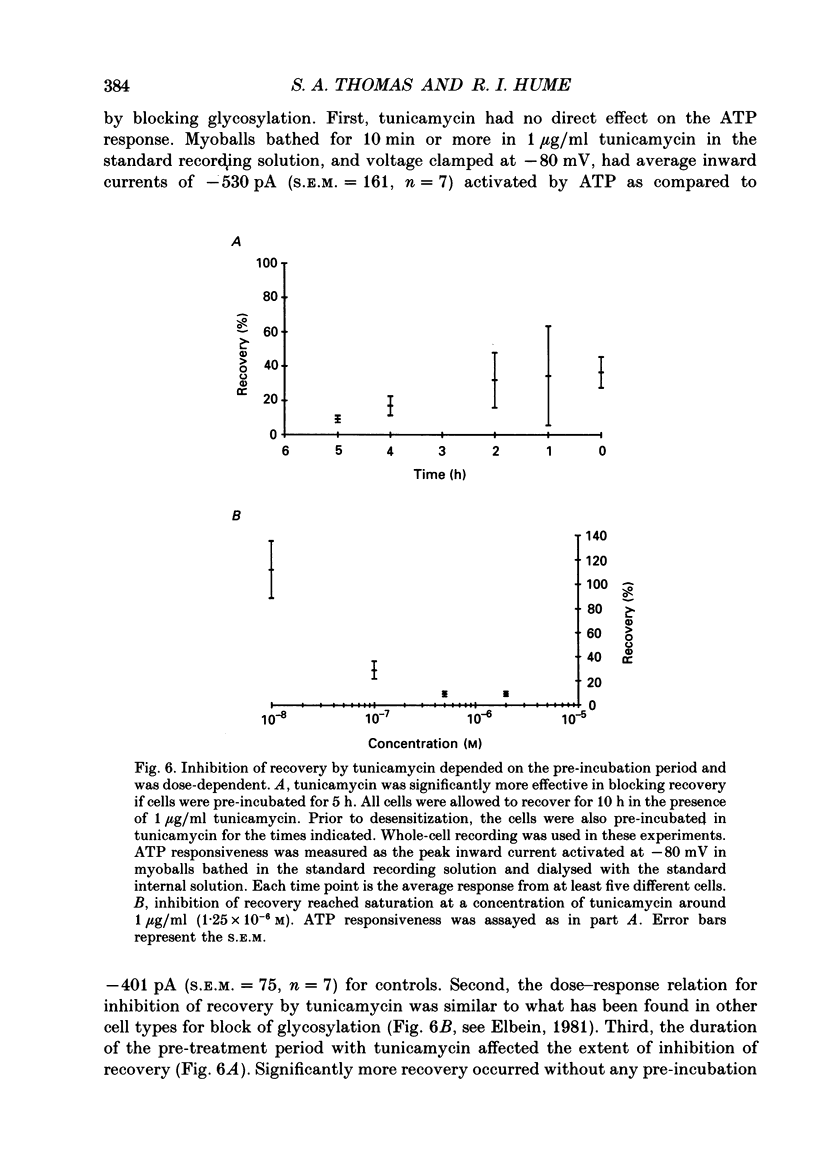
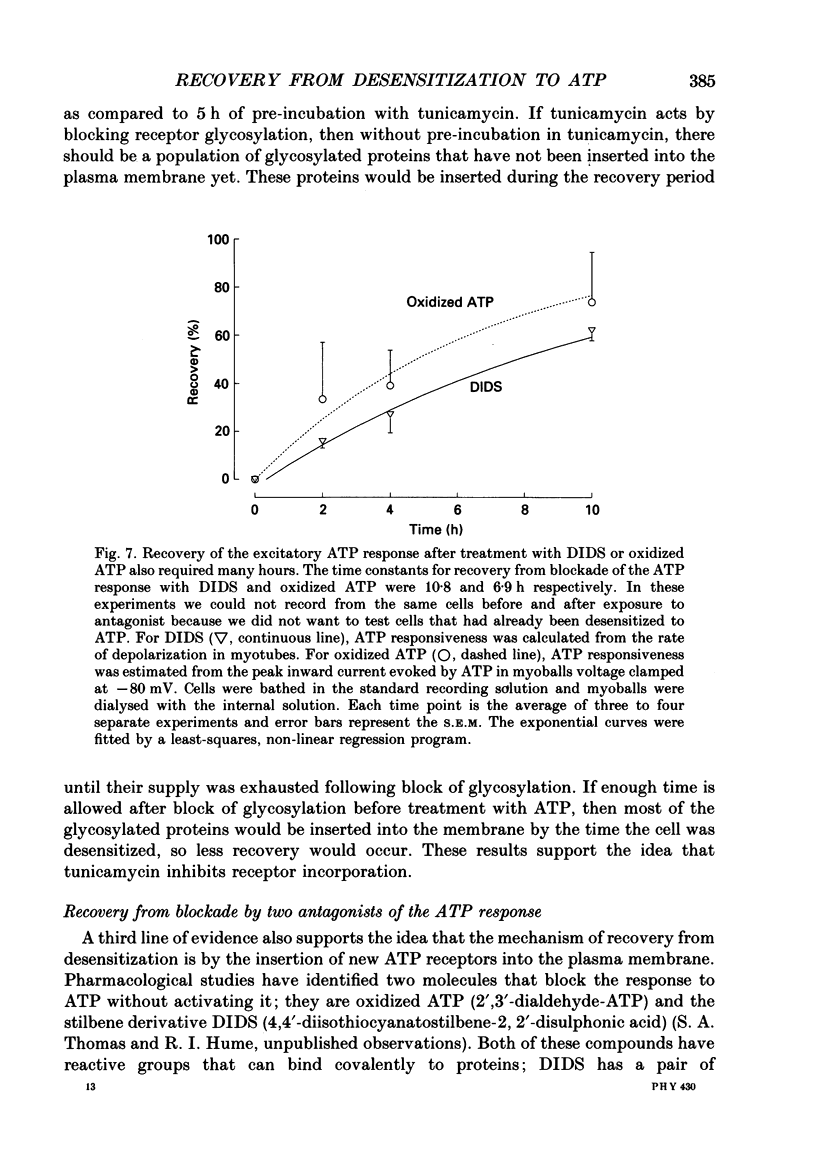
1. In developing chick skeletal muscle, extracellular adenosine 5'-triphosphate (ATP) elicits an early excitatory conductance increase followed by a late potassium conductance increase. Both of these responses desensitize profoundly. Intracellular recordings and whole-cell voltage-clamp recordings were made in order to examine the time course and mechanism of desensitization and the recovery from desensitization. 2. Most of the loss of responsiveness to ATP occurred during the first minute of exposure to ATP. For the excitatory conductance, the loss of responsiveness to ATP resulted in part from long-lasting activation of the ATP-sensitive channels and in part from entrance into an inactive (non-conducting) state. In contrast, desensitization of the potassium conductance was entirely the result of a relatively fast transition to an inactive state. 3. Recovery from desensitization took many hours for both responses and was quite sensitive to temperature. 4. Recovery from desensitization for both responses was prevented by preincubation with the glycosylation inhibitor, tunicamycin. Several lines of evidence suggest that tunicamycin treatment blocked the delivery of new ATP receptors to the cell surface. 5. The recovery of the early response to ATP following exposure to two non-competitive inhibitors of the ATP response was also examined. These two compounds are thought to covalently modify the receptor. After exposure to either of these inhibitors, responsiveness to ATP returned over a time course that was similar to the time course of recovery from desensitization. 6. These results indicate that, following activation, ATP receptors do not become available for reactivation, and that recovery from desensitization is due to the insertion of newly synthesized receptors into the plasma membrane.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Feltz A., Trautmann A. Desensitization at the frog neuromuscular junction: a biphasic process. J Physiol. 1982 Jan;322:257–272. doi: 10.1113/jphysiol.1982.sp014036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hickman S., Kulczycki A., Jr, Lynch R. G., Kornfeld S. Studies of the mechanism of tunicamycin in hibition of IgA and IgE secretion by plasma cells. J Biol Chem. 1977 Jun 25;252(12):4402–4408. [PubMed] [Google Scholar]
- Hume R. I., Honig M. G. Excitatory action of ATP on embryonic chick muscle. J Neurosci. 1986 Mar;6(3):681–690. doi: 10.1523/JNEUROSCI.06-03-00681.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume R. I., Thomas S. A. Multiple actions of adenosine 5'-triphosphate on chick skeletal muscle. J Physiol. 1988 Dec;406:503–524. doi: 10.1113/jphysiol.1988.sp017393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israël M., Lesbats B., Meunier F. M., Stinnakre J. Postsynaptic release of adenosine triphosphate induced by single impulse transmitter action. Proc R Soc Lond B Biol Sci. 1976 Jun 30;193(1113):461–468. doi: 10.1098/rspb.1976.0058. [DOI] [PubMed] [Google Scholar]
- Neve K. A., Molinoff P. B. Turnover of beta 1- and beta 2-adrenergic receptors after down-regulation or irreversible blockade. Mol Pharmacol. 1986 Aug;30(2):104–111. [PubMed] [Google Scholar]
- Thomas S. A., Hume R. I. Permeation of both cations and anions through a single class of ATP-activated ion channels in developing chick skeletal muscle. J Gen Physiol. 1990 Apr;95(4):569–590. doi: 10.1085/jgp.95.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]


