Abstract
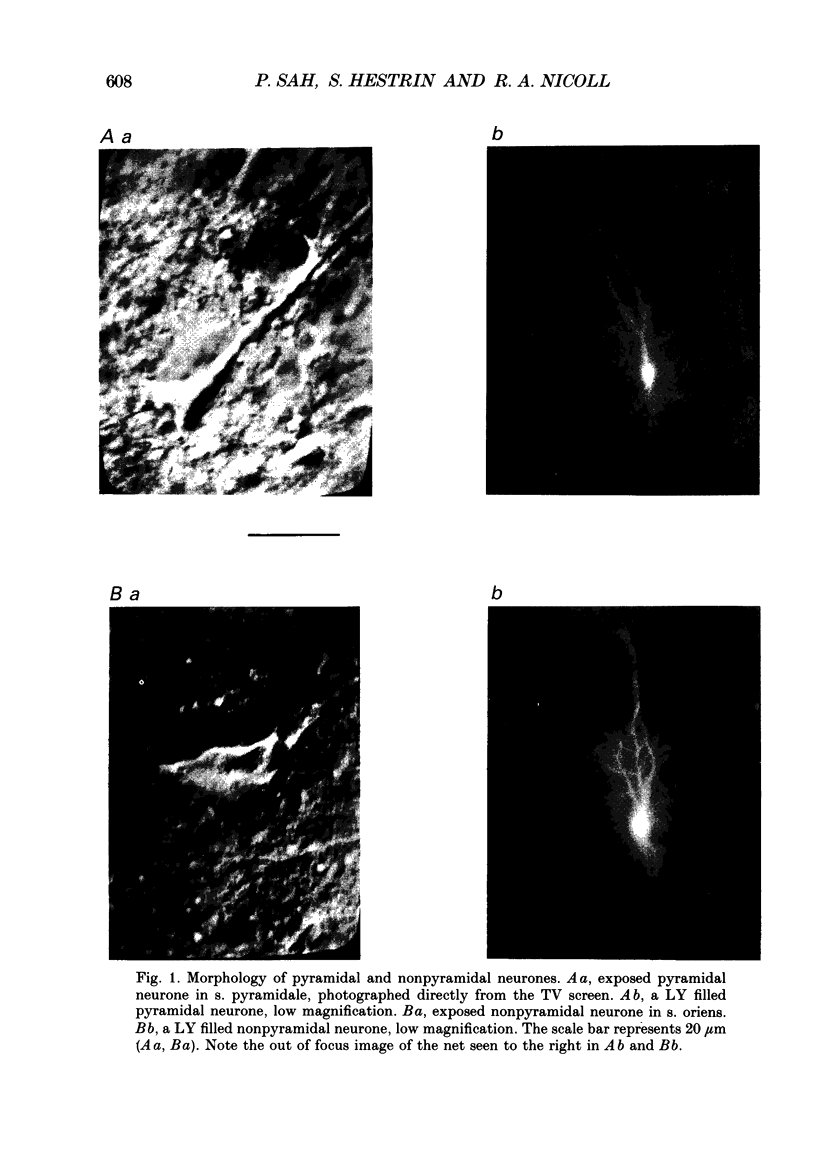
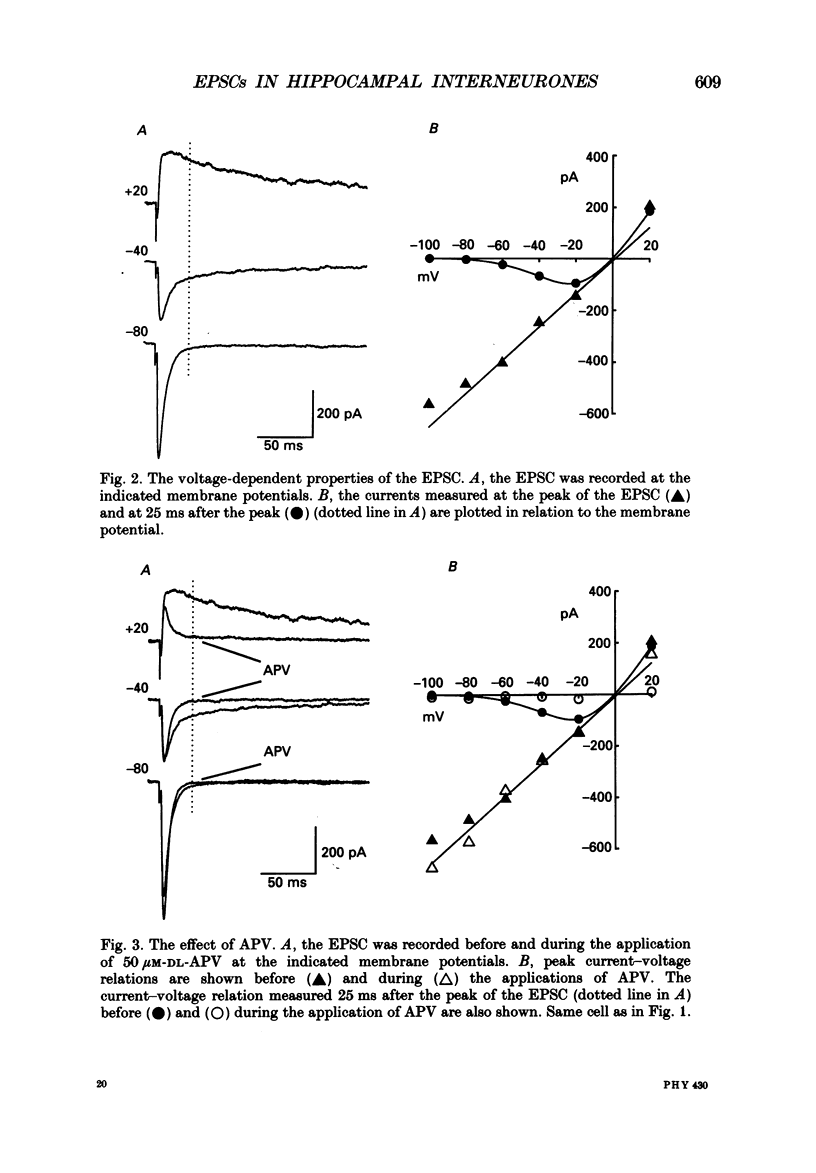
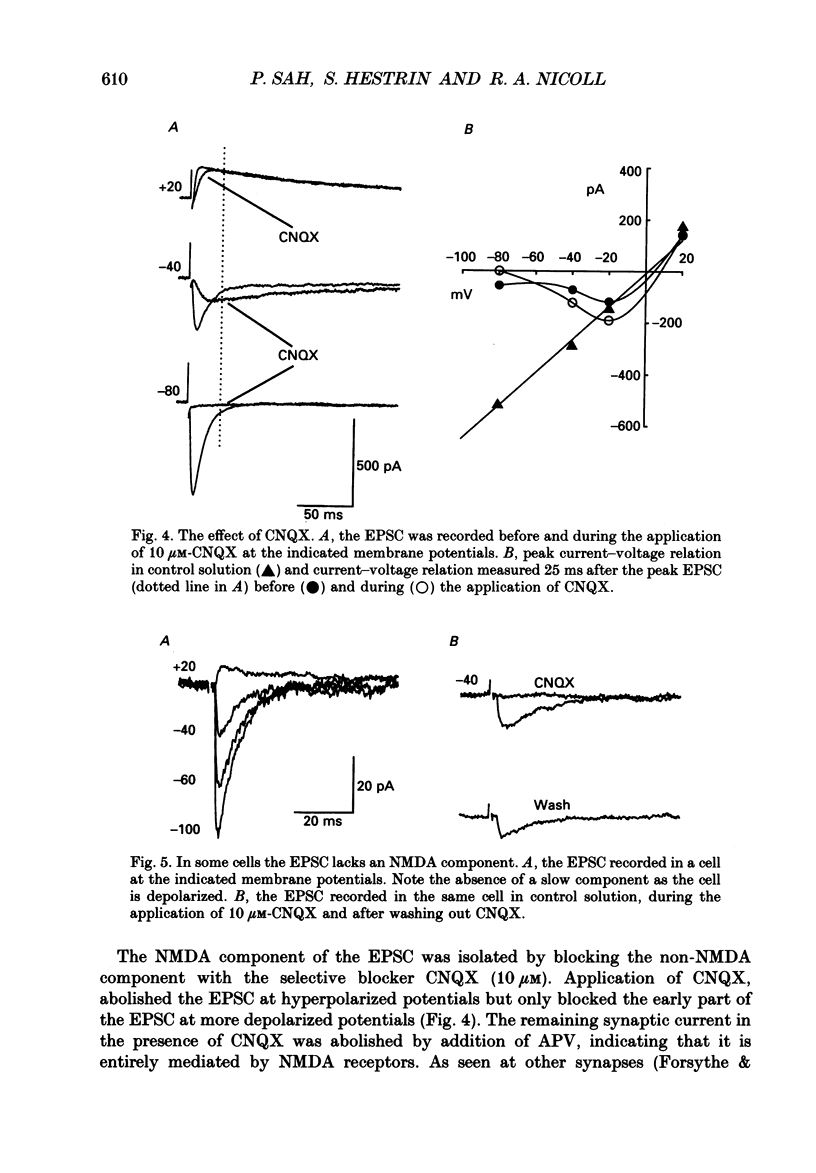
1. We studied excitatory synaptic currents activated by stimulation of Schaffer collateral-commissural fibres and recorded from interneurones in the CA1 region of hippocampal slices using whole-cell techniques. 2. Interneurones were identified by their location outside the cell layer and their morphology as seen with differential interference contrast (DIC) microscopy and by filling with Lucifer Yellow (LY). 3. The excitatory postsynaptic current (EPSC) had a fast, voltage-insensitive component and a slow component which had a region of negative slope resistance between -70 and -40 mV. The slow voltage-dependent component was abolished by the N-methyl-D-aspartate (NMDA) receptor antagonist (DL-2-amino-5-phosphonovalerate (APV) 50 microM) which had little effect on the fast component. Conversely, the fast component was abolished by the non-NMDA receptor antagonist 6-cyano-7-nitoquinoxaline-2,3-dione (CNQX; 10 microM), which had no effect on the slow component. 4. The rise time of the fast component ranged from 1 to 3 ms and the decay time constant ranged from 3 to 15 ms. The rise time of the slow component ranged from 5 to 11 ms and the decay time constant ranged from 50 to 100 ms. 5. It is concluded that although the morphology of the excitatory synapses onto interneurones differs considerably from those onto pyramidal cells, their electrophysiological and pharmacological properties are very similar.
Full text
PDF



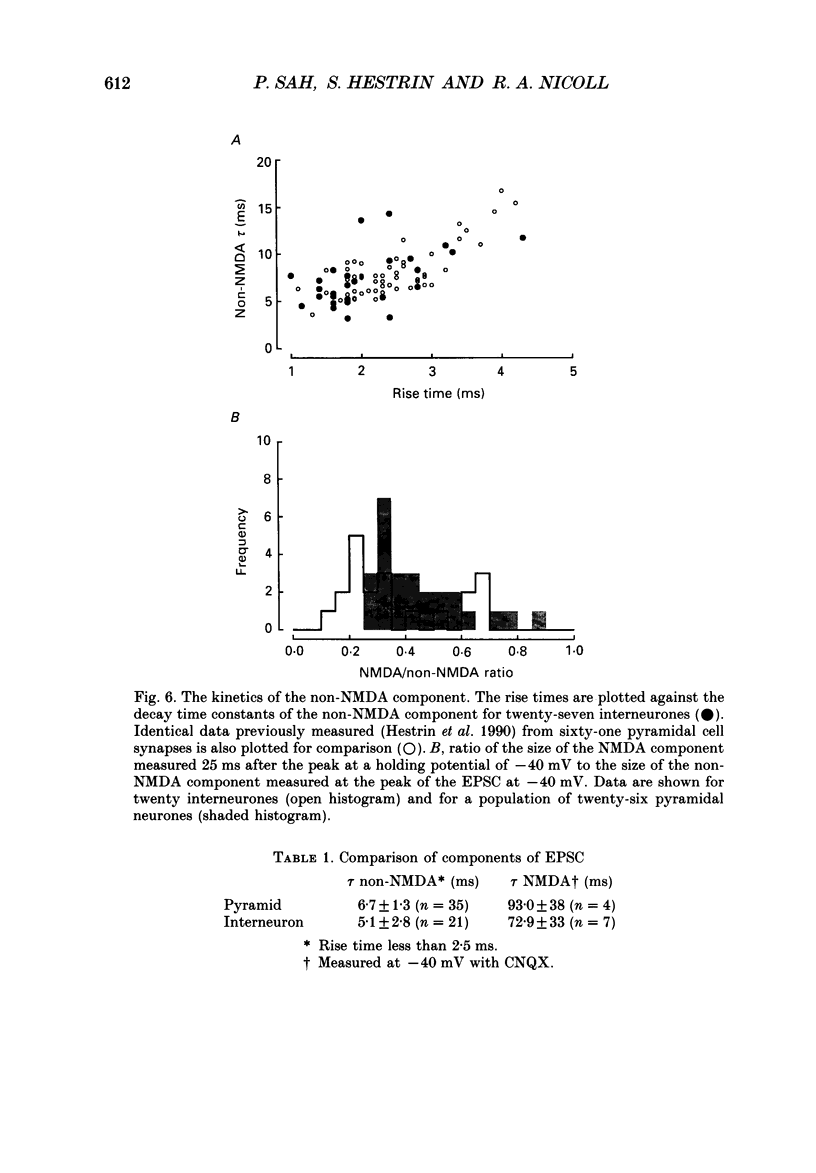




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham W. C., Gustafsson B., Wigström H. Long-term potentiation involves enhanced synaptic excitation relative to synaptic inhibition in guinea-pig hippocampus. J Physiol. 1987 Dec;394:367–380. doi: 10.1113/jphysiol.1987.sp016875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen M., Lambert J. D., Jensen M. S. Effects of new non-N-methyl-D-aspartate antagonists on synaptic transmission in the in vitro rat hippocampus. J Physiol. 1989 Jul;414:317–336. doi: 10.1113/jphysiol.1989.sp017690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G., Eidelberg E. Direct afferent excitation and long-term potentiation of hippocampal interneurons. J Neurophysiol. 1982 Sep;48(3):597–607. doi: 10.1152/jn.1982.48.3.597. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Herron C. E., Lester R. A. Frequency-dependent N-methyl-D-aspartate receptor-mediated synaptic transmission in rat hippocampus. J Physiol. 1988 May;399:301–312. doi: 10.1113/jphysiol.1988.sp017081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Herron C. E., Lester R. A. Synaptic activation of N-methyl-D-aspartate receptors in the Schaffer collateral-commissural pathway of rat hippocampus. J Physiol. 1988 May;399:283–300. doi: 10.1113/jphysiol.1988.sp017080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N., Roberts A. Dual-component amino-acid-mediated synaptic potentials: excitatory drive for swimming in Xenopus embryos. J Physiol. 1985 Jun;363:35–59. doi: 10.1113/jphysiol.1985.sp015694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S. N., Collingridge G. L. Role of excitatory amino acid receptors in synaptic transmission in area CA1 of rat hippocampus. Proc R Soc Lond B Biol Sci. 1989 May 22;236(1285):373–384. doi: 10.1098/rspb.1989.0028. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B., Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989 Sep;414(5):600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Forsythe I. D., Westbrook G. L. Slow excitatory postsynaptic currents mediated by N-methyl-D-aspartate receptors on cultured mouse central neurones. J Physiol. 1988 Feb;396:515–533. doi: 10.1113/jphysiol.1988.sp016975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher J. P., Hasuo H. Bicuculline- and phaclofen-sensitive components of N-methyl-D-aspartate-induced hyperpolarizations in rat dorsolateral septal nucleus neurones. J Physiol. 1989 Nov;418:367–377. doi: 10.1113/jphysiol.1989.sp017846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble E., Koch C. The dynamics of free calcium in dendritic spines in response to repetitive synaptic input. Science. 1987 Jun 5;236(4806):1311–1315. doi: 10.1126/science.3495885. [DOI] [PubMed] [Google Scholar]
- Haas H. L., Rose G. Long-term potentiation of excitatory synaptic transmission in the rat hippocampus: the role of inhibitory processes. J Physiol. 1982 Aug;329:541–552. doi: 10.1113/jphysiol.1982.sp014318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestrin S., Nicoll R. A., Perkel D. J., Sah P. Analysis of excitatory synaptic action in pyramidal cells using whole-cell recording from rat hippocampal slices. J Physiol. 1990 Mar;422:203–225. doi: 10.1113/jphysiol.1990.sp017980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Baughman R. W. NMDA- and non-NMDA-receptor components of excitatory synaptic potentials recorded from cells in layer V of rat visual cortex. J Neurosci. 1988 Sep;8(9):3522–3534. doi: 10.1523/JNEUROSCI.08-09-03522.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles W. D., Schwartzkroin P. A. Local circuit synaptic interactions in hippocampal brain slices. J Neurosci. 1981 Mar;1(3):318–322. doi: 10.1523/JNEUROSCI.01-03-00318.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C., Poggio T. A theoretical analysis of electrical properties of spines. Proc R Soc Lond B Biol Sci. 1983 Jul 22;218(1213):455–477. doi: 10.1098/rspb.1983.0051. [DOI] [PubMed] [Google Scholar]
- Kunkel D. D., Schwartzkroin P. A. Ultrastructural characterization and GAD co-localization of somatostatin-like immunoreactive neurons in CA1 of rabbit hippocampus. Synapse. 1988;2(4):371–381. doi: 10.1002/syn.890020404. [DOI] [PubMed] [Google Scholar]
- Lacaille J. C., Mueller A. L., Kunkel D. D., Schwartzkroin P. A. Local circuit interactions between oriens/alveus interneurons and CA1 pyramidal cells in hippocampal slices: electrophysiology and morphology. J Neurosci. 1987 Jul;7(7):1979–1993. doi: 10.1523/JNEUROSCI.07-07-01979.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaille J. C., Schwartzkroin P. A. Stratum lacunosum-moleculare interneurons of hippocampal CA1 region. I. Intracellular response characteristics, synaptic responses, and morphology. J Neurosci. 1988 Apr;8(4):1400–1410. doi: 10.1523/JNEUROSCI.08-04-01400.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaille J. C., Schwartzkroin P. A. Stratum lacunosum-moleculare interneurons of hippocampal CA1 region. II. Intrasomatic and intradendritic recordings of local circuit synaptic interactions. J Neurosci. 1988 Apr;8(4):1411–1424. doi: 10.1523/JNEUROSCI.08-04-01411.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy W. B., Steward O. Temporal contiguity requirements for long-term associative potentiation/depression in the hippocampus. Neuroscience. 1983 Apr;8(4):791–797. doi: 10.1016/0306-4522(83)90010-6. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Kauer J. A., Perkel D. J., Nicoll R. A. The impact of postsynaptic calcium on synaptic transmission--its role in long-term potentiation. Trends Neurosci. 1989 Nov;12(11):444–450. doi: 10.1016/0166-2236(89)90094-5. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Miller J. P., Rall W., Rinzel J. Synaptic amplification by active membrane in dendritic spines. Brain Res. 1985 Jan 28;325(1-2):325–330. doi: 10.1016/0006-8993(85)90333-6. [DOI] [PubMed] [Google Scholar]
- Minkwitz H. G. Zur Entwicklung der Neuronenstruktur des Hippocampus während der prä- und postnatalen Ontogenese der Albinoratte. II. Mitteilung: Neurohistologische Darstellung der Entwicklung von Interneuronen und des Zusammenhanges lang- und kurzaxoniger Neurone. J Hirnforsch. 1976;17(3):233–253. [PubMed] [Google Scholar]
- Minkwitz H. G. Zur Entwicklung der Neuronenstruktur des Hippocampus während der prä- und postnatalen Ontogenese der Albinoratte. III. Mitteilung: Morphometrische Erfassung der ontogenetischen Veränderungen in Dendritenstruktur und Spinebesatz an Pyramidenneuronen (CA1) des Hippocampus. J Hirnforsch. 1976;17(3):255–275. [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Perkel D. H., Perkel D. J. Dendritic spines: role of active membrane in modulating synaptic efficacy. Brain Res. 1985 Jan 28;325(1-2):331–335. doi: 10.1016/0006-8993(85)90334-8. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Mathers L. H. Physiological and morphological identification of a nonpyramidal hippocampal cell type. Brain Res. 1978 Nov 17;157(1):1–10. doi: 10.1016/0006-8993(78)90991-5. [DOI] [PubMed] [Google Scholar]
- Segev I., Rall W. Computational study of an excitable dendritic spine. J Neurophysiol. 1988 Aug;60(2):499–523. doi: 10.1152/jn.1988.60.2.499. [DOI] [PubMed] [Google Scholar]
- Seress L., Ribak C. E. A combined Golgi-electron microscopic study of non-pyramidal neurons in the CA 1 area of the hippocampus. J Neurocytol. 1985 Oct;14(5):717–730. doi: 10.1007/BF01170824. [DOI] [PubMed] [Google Scholar]
- Somogyi P., Hodgson A. J., Smith A. D., Nunzi M. G., Gorio A., Wu J. Y. Different populations of GABAergic neurons in the visual cortex and hippocampus of cat contain somatostatin- or cholecystokinin-immunoreactive material. J Neurosci. 1984 Oct;4(10):2590–2603. doi: 10.1523/JNEUROSCI.04-10-02590.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube J. S., Schwartzkroin P. A. Intracellular recording from hippocampal CA1 interneurons before and after development of long-term potentiation. Brain Res. 1987 Sep 1;419(1-2):32–38. doi: 10.1016/0006-8993(87)90565-8. [DOI] [PubMed] [Google Scholar]
- Thomson A. M. A magnesium-sensitive post-synaptic potential in rat cerebral cortex resembles neuronal responses to N-methylaspartate. J Physiol. 1986 Jan;370:531–549. doi: 10.1113/jphysiol.1986.sp015949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens J. Electrically coupled but chemically isolated synapses: dendritic spines and calcium in a rule for synaptic modification. Prog Neurobiol. 1988;31(6):507–528. doi: 10.1016/0301-0082(88)90013-5. [DOI] [PubMed] [Google Scholar]
- Wigström H., Gustafsson B. On long-lasting potentiation in the hippocampus: a proposed mechanism for its dependence on coincident pre- and postsynaptic activity. Acta Physiol Scand. 1985 Apr;123(4):519–522. doi: 10.1111/j.1748-1716.1985.tb07621.x. [DOI] [PubMed] [Google Scholar]